Abstract
Background:
Exercise-related studies do not agree on changes in blood parameters regarding anemia and serum immunoglobulin concentration as adaptive immunity.Objectives:
The current study aimed to investigate exercise-induced variations in hematological and immunological parameters in response to one bout of intensive judo exercise in adolescent elite judoka.Methods:
Venipuncture was performed on ten adolescent male Iranian elite judoka (age: 15.60 ± 0.69 years; body mass index: 24.15 ± 2.80 kg/m2) before and immediately after one bout of intensive judo protocol. Erythrocyte variables [red blood cell count (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), red distribution width (RDW), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC)], platelets variables [platelet count (PLT), platelet distribution width (PDW), mean platelet volume (MPV), plateletcrit (PCT)], cellular immune variables [white blood cell (WBC), neutrophil (NEU), lymphocyte (LYM)], and humoral immune variables (IgM, IgA, IgG, IgE) were measured. Data were analyzed using Wilcoxon and paired-samples t-test, and P < 0.05 was considered significant.Results:
After the plasma volume was corrected using the Dill and Costill equation, the results showed that RBC, HGB, HCT, MCHC, IgA, and IgM decreased significantly immediately after exercise. However, MCH, RDW, PLT, PDW, MPV, PCT, WBC, LYM, NEU, IgG, and IgE did not change significantly.Conclusions:
According to the findings, it can be concluded that intensive judo exercise results in transient anemia and suppression of humoral immune response among adolescent elite judoka.Keywords
Judo Cellular Immunity Humoral Immune Platelets Erythrocyte Adolescent
1. Background
Coaches and sport team physicians are interested in maintaining the health of their athletes during training and competition sessions. Epidemiological evidence suggests that heavy acute or chronic bouts of strenuous exercise correspond with an increased incidence of upper respiratory tract infections (URTI) that can negatively affect the health and performance of athletes (1). Previous research has particularly confirmed that intense exercise required in competitive sports is related to a threefold higher incidence of URTI (2). This increased risk of infection reflects altered or suppressed immunity. To elucidate what exercise-induced changes occur, researchers have assayed different aspects of the immune function (3). Exercise immunologists have long been interested in the relationship between infection and exercise; however, the nature of this relationship remains underdetermined. Immune responses to pathogens are mainly examined using the relative plasma level of antibodies. Leukocytosis in athletes might be due to an inflammatory response induced by tissue injury (4). In addition, red blood cells (RBC), hemoglobin (HGB), and hematocrit (HCT) have been considered as screening markers for anemia (5). Other hematological parameters related to immunity and exercise are circulating immunoglobulins (4).
An antibody is a large, Y-shaped glycoprotein belonging to the immunoglobulin superfamily, which is mostly secreted by differentiated B cells called plasma cells (6). The membrane-bound form of immunoglobulin is attached to the surface of a B cell, referred to as the B cell receptor, which facilitates the activation of B cells and their subsequent differentiation into memory B cells. Moreover, soluble antibodies are secreted from the cell to be free in the blood plasma and tissue fluids (6). Five major antibody classes have been identified in placental mammals: IgA, IgD, IgE, IgG, and IgM (7). Among these, IgM is the first immunoglobulin expressed during B cell development and is associated with a primary immune response. IgA is found in high concentrations in mucosal surfaces and secretions, including the saliva, while IgD is found at very low levels in the serum that may be attributed to short half-life. With the lowest serum concentration and the shortest half-life, IgE is a very potent immunoglobulin and participates in hypersensitivity and allergic response (7). Finally, with the most prolonged serum half-life of all types, IgG is the predominant immunoglobulin isotype that is found in all body fluids and participates in all immune responses (8). It has been reported that a high level of this immunoglobulin suggests an increase in the intensity of immune system activities and indicates an inflammatory response (9).
As stimulants, stress, and physical activity induce changes in the immune system. In reality, the acute immune response to exercise depends on the intensity and duration of effort (6). It has been postulated that during moderate- and vigorous-intensity aerobic exercise bouts of less than 60 min, both the antipathogen activity of tissue macrophages and recirculation of immunoglobulins are enhanced (6). Reports indicate, moreover, that the number of leukocytes, immature neutrophils, mature neutrophils, monocytes, and serum IgG are increased, whereas lymphocytes, serum IgA, and IgM do not change after 100-km ultra-marathon under cold conditions in runners (4). Moreover, McKune et al. (3) report that although total IgG increases immediately, IgD and IgM significantly reduce immediately and 24 hours after a 90-km ultra-marathon race in experienced ultra-endurance runners. Besides, significant increases in RBC, HGB, white blood cells (WBC), and their main subpopulations (i.e., monocytes, neutrophils, and lymphocytes) have been observed after short-term Wingate test in football players (10). In contrast, neither a 30-minute aerobic exercise on a treadmill and the 30-second anaerobic Wingate test have had a significant effect on IgG, IgM, and IgA in elite volleyball players (11). However, after two and five days of aerobic exercise, the IgA, IgG, and IgM levels are reported to increase significantly in athletes as compared to pre-exercise levels (11). Also, serum IgA level does not change after four sets of bench press and squat at an intensity corresponding to 85% of one repetition maximum with varying rest intervals (60-, 90- or 120-second) in young resistance-trained men (12). Finally, no significant changes have been observed in absolute concentration and secretory IgA rate immediately after a kickboxing match (13).
Because of the combative nature of the sport, elite judo athletes are required to possess great physical prowess as well as sophisticated techniques for successful performance (14). Hence, judo athletes incorporate intensified physical and technical training into their pre-competition training program. It has been shown that strenuous bouts of exercise affect critical physiological functions in elite athletes (15). In this regard, Tsai and colleagues (14) reported that prolonged intensive training, in combination with rapid weight loss, affects mucosal immunity negatively and increases the chances of URTI development in elite male and female athletes. To the best of our knowledge, the effects of intensive judo on hematological and immunoglobulin concentrations have not yet been well examined in adolescent elite judo players.
2. Objectives
the current study aimed to investigate exercise-induced changes in hematological and immunological parameters in response to one bout of intensive judo in adolescent elite judoka.
3. Methods
3.1. Participants
The research protocol was approved by the Institutional Review Board (University of Bojnord, Iran) prior to subject enrollment. Ten adolescent male Iranian elite judoka (aged 15.60 ± 0.69 years) were recruited from Bojnord city (North Khorasan Province) to participate in this present experimental study. Identification card information was the basis for determining the age of the players. The study took place in the off-season of competitions. The participant’s history of judo was 5.70 ± 1.70 years. The judo athletes were examined by a physician two weeks before the protocol was started, and there were no reports of URTI symptoms in the subjects (10). The subjects had a history of winning medals in national and international competitions. All players were in the first grade and had a black belt degree (first dan). No subjects had a history of chronic respiratory disease (3) or a history of hematologic diseases (16). In addition, they had no history of smoking or using drugs, steroid hormones, and nutritional supplements (10, 16). The procedures of the study were explained to the subjects and their parents before the study. Written informed consent was obtained from each subject’s parent, which was approved by the institutional ethical committee for human use (IR.IAU.BOJNOURD.REC.1398.013). The procedures were in accordance with the 1975 Declaration of Helsinki, as revised in 1996. Finally, the subjects were encouraged to participate voluntarily and were allowed to leave the study, even without any wrongs, resentment, or discontent (10).
3.2. Determination of Body Composition
The body composition of subjects was determined one-week prior to the testing day using a bioimpedance body composition analyzer (IOI 353 model, Danilsmc Co, South Korea) in the Islamic Azad University (Bojnourd branch). For this purpose, the participants were asked to empty their bladder prior to testing, to stand on metal footpads in bare feet, and to grasp a pair of electrodes fixed on a handle. Details of the body composition of participants are reported in Table 1.
| Variables | Values |
|---|---|
| Weight (kg) | 77.38 ± 14.49 |
| Height (cm) | 176.70 ± 9.98 |
| BMI (kg/m2) | 24.15 ± 2.80 |
| WHR | 0.78 ± 0.03 |
| PBF (%) | 17.93 ± 4.39 |
| MBF (kg) | 14.31 ± 6.14 |
| LBM (kg) | 64.77 ± 10.24 |
| SLM (kg) | 59.97 ± 9.15 |
| TBW (%) | 46.62 ± 7.38 |
| BMR (kcal) | 1763.2 ± 187.13 |
| TEE (kcal) | 2715.1 ± 288.21 |
| MF left arm (kg) | 0.93 ± 0.37 |
| MF right arm (kg) | 0.92 ± 0.36 |
| MF trunk (kg) | 7.34 ± 3.17 |
| MF left leg (kg) | 2.47 ± 1.21 |
| MF right leg (kg) | 2.56 ± 1.11 |
| SLM left arm (kg) | 3.94 ± 0.65 |
| SLM right arm (kg) | 3.93 ± 0.65 |
| SLM trunk (kg) | 29.83 ± 4.32 |
| SLM left leg (kg) | 11.11 ± 1.72 |
| SLM right leg (kg) | 11.14 ± 1.83 |
3.3. Intensive Judo Protocol
The current study took place under the supervision of a qualified and experienced judo coach at Bojnord Judo Home (Alidokht Sports Complex, Bojnord). The study began at 11 a.m. and lasted for about 1.5 hours. Three days before the judo protocol initiated, the subjects received glycogen loading and were prohibited from performing any exercise during the time. During the exercise, they were asked to take a brief rest if they were excessively fatigued. They had free access to water during the exercise. All the participants had a similar breakfast in the morning of the test. Acute judo exercise involved aerobic and anaerobic exercise, as well as concentric and eccentric types of movement. The protocol consisted of the following:
- Warm-up (15 minutes): Jogging and stretch exercise with sport-specific calisthenics such as shadowing techniques without opponents.
- Technique drills (45 minutes): The subjects performed typical judo skills at high-intensity and short duration. These drills consisted of standing (tachi-waza) and lying techniques (sutemi-waza), as shown in Table 2. Twenty seconds of inactive recovery was allocated between each set.
Acute Judo Exercise Protocol
| Technique | Details | ||
|---|---|---|---|
| Standing technique (tachi - waza) | Hand technique (te-waza) | Ippon seoi nage | 3 sets of 10 reps without throw + 3 sets of 5 reps with throw |
| Morote-seoi-nage | 3 sets of 10 reps without throw + 3 sets of 5 reps with throw | ||
| Tai otoshi | 3 sets of 10 reps without throw + 3 sets of 5 reps with throw | ||
| Waist technique (koshi-waza) | O goshi | 3 sets of 10 reps without throw + 3 sets of 5 reps with throw | |
| Koshi guruma | 3 sets of 10 reps without throw + 3 sets of 5 reps with throw | ||
| Ushiro goshi | 3 sets of 10 reps without throw + 3 sets of 5 reps with throw | ||
| Leg technique (ashi-waza) | Uchi mata | 3 sets of 10 reps without throw + 3 sets of 5 reps with throw | |
| Okuriashi harai | 3 sets of 10 reps without throw + 3 sets of 5 reps with throw | ||
| Hiza guruma | 3 sets of 10 reps without throw + 3 sets of 5 reps with throw | ||
| Lying technique (sutemi-waza) | Supine technique (ma-sutemi- waza) | Tomoe nage | 1 set of 10 reps with throw |
| Ura nage | 3 sets of 10 reps with throw | ||
| Tawara gaeshi | 3 sets of 10 reps with throw | ||
| Side sleeping technique (yoko- sutemi-waza) | Kata guruma | 3 sets of 10 reps with throw | |
| Sumi gaeshi | 3 sets of 10 reps with throw | ||
| Tani otoshi | 3 sets of 10 reps with throw | ||
- Iron man (10 minutes): The subjects were placed in 4 groups (3 athletes per group) with 1 subject in each group designated as the iron man. He continuously faced new participants every 60 seconds with a 30-second rest between each interval. Each judo player was the iron man at least once during this drill.
- Live matching (10 minutes): Each judo player was paired and competed twice with a partner of similar weight and ability. Every pair of judoka competed for one round, with each round lasting for 4 minutes and a 120-second rest between each competition.
- Cool-down (10 minutes): Jogging and stretching exercise.
3.4. Sample Collection and Biochemical Assays
Blood samples of 6 mL were collected from the participants’ antecubital vein by standard phlebotomy before and immediately after the exercise session. Postural changes can influence plasma volume; therefore, the procedures of blood collection in pre- and post-judo phases were identical (4). The 3 mL blood samples were collected in tubes containing ethylene diamine tetraacetic acid. The collected samples were stored under cold conditions and sent to Farzandian’s Pathobiology Laboratory (Bojnord, Iran). Erythrocyte variables (RBC, HGB, HCT, MCV, RDW, MCH, MCHC), platelets variables (PLT, PDW, MPV, PCT) and leukocyte variables (WBC, NEU, LYM) were assayed using a Sysmex automated hematology analyzer (K-1000 Model, Sysmex Corporation, Japan). The remaining samples were centrifuged (Universal Model, Behdad Corporation, Iran) for 15 min at 1500 rpm and 4ºC to remove cells and debris (3). The supernatants were stored at -80ºC until used.
We used the commercial ELISA kits to measure the protein contents of total IgA, total IgM, and total IgG in the serum according to the immunoturbidimetry method. In addition, serum IgE assay was carried out by an assay kit according to the chemiluminescence method. Finally, changes in plasma volume after acute judo exercise were calculated using the Dill and Costill equation (16).
3.5. Statistical Analyses
All statistical analyses were performed using the Statistical Package for the Social Sciences version 16.0 (IBM, Inc., Chicago, IL). Initially, the homogeneity of the samples was determined by the Shapiro-Wilk normality test. Then, the paired-sample t-test and Wilcoxon Signed Ranks test were used to compare the mean differences between pre- and post-tests in parametric and non-parametric variables, respectively. In addition, the level of significance was set at P < 0.05.
4. Results
In the context of hematological variables, RBC (z = 2.193, P = 0.025), HGB (z = 2.497, P = 0.010), HCT (t = 2.591, P = 0.029), and MCHC (t = 3.150, P = 0.012) significantly decreased, whereas MCV (t = 0.488, P = 0.637), MCH (t = 0.453, P = 0.661), and RDW (z = 1.336, P = 0.207) did not significantly change (Table 3). In addition, PLT (z = 357, P = 0.770), PDW (z = 494, P = 0.633), MPV (t = 0.380, P = 0.713), PCT (t = 1.575, P = 0.150) remained unchanged after exercise (Table 3). In regard to cellular immune variables, WBC (t = 0.118, P = 0.909), LYM (t = 1.554, P = 0.155), and NEU (t = 0.703, P = 0.501) showed no significant change (Table 3).
Statistically Significant Changes in Pre- and Post-Exercise Levels of Circulating Erythrocyte, Platelet, and Leukocyteleukocytes in Adolescent Male Elite Judokaa
| Hematologic Parameters | Pre-Exercise | Post-Exercise |
|---|---|---|
| RBC (106/μL) | 5.31 ± 0.56 | 4.90 ± 0.45b |
| Hgb (g/dL) | 15.24 ± 0.86 | 14.17 ± 0.93b |
| Hct (%) | 44.40 ± 3.29 | 43.07 ± 2.42b |
| MCV (fL) | 83.86 ± 4.34 | 84.15 ± 4.75 |
| MCH (pg) | 28.84 ± 1.86 | 28.97 ± 1.52 |
| MCHC (g/dL) | 34.37 ± 1.08 | 32.94 ± 1.35b |
| RDW (fL) | 11.92 ± 0.66 | 11.80 ± 0.80 |
| WBC (103/μL) | 6.93 ± 1.60 | 6.97 ± 1.49 |
| LYM (103/μL) | 2.49 ± 0.42 | 2.31 ± 0.53 |
| NEU (103/μL) | 3.92 ± 1.36 | 4.17 ± 1.34 |
| LYM (%) | 37.05 ± 8.14 | 34.13 ± 8.62 |
| NEU (%) | 55.42 ± 8.30 | 58.83 ± 9.15 |
| PLT (103/μL) | 262.10 ± 50.57 | 255.82 ± 52.64 |
| MPV (fL) | 7.09 ± 0.072 | 7.12 ± 0.67 |
| PDW (fL) | 8.71 ± 1.38 | 8.63 ± 1.51 |
| PCT (%) | 9.20 ± 3.88 | 9.79 ± 3.89 |
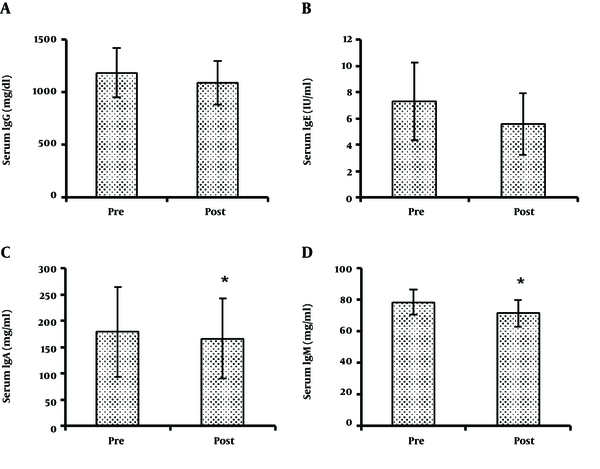
Finally, IgA (179.22 ± 85.27 and 166.29 ± 75.99 mg/dL for pre- and post-exercise, respectively) (t = 3.088, P = 0.015), and IgM (78.50 ± 7.93 and 71.51 ± 8.59 mg/dL for pre- and post-exercise, respectively) (t = 3.136, P = 0.012) significantly reduced, while IgG (1186 ± 235 and 1092 ± 209 mg/dL for pre- and post-exercise, respectively) (t = 2.178, P = 0.061) and IgE (7.33 ± 2.96 and 5.61 ± 2.34 IU/mL for pre- and post-exercise, respectively) (t = 2.060, P = 0.176) did not change significantly (Figure 1).
Statistically significant changes in pre- and post-exercise levels of circulating IgG (A), IgE (B), IgA (C), and IgM (D) in adolescent male elite judoka. The asterisk (*) indicates a significant difference from pre-exercise.
5. Discussion
The results of the present study showed that hematological indices of RBC, HGB, HCT, and MCHC decreased significantly after exercise, whereas MCH and RDW did not change in a significant manner. Consistent with the results of the present study, it has been shown that one session of resistance exercise significantly reduces RBC levels immediately after exercise (17), while no significant difference has been found in RBC, HGB, and HCT between athlete and non-athlete men before and after one session of resistance training (18). However, Ghanbari Niaki and Mohammadi (19) report that RAST anaerobic activity increases HGB and RBC levels. According to previous studies, changes in erythrocyte indices depend on the severity and type of activity performed and the readiness of individuals, so that in combat sports such as judo and high-intensity activities, a significant reduction in RBC has been observed due to increased intravascular hemolysis (20). In other words, reduced RBC during vigorous exercise can be attributed to intravascular hemolysis caused by mechanical trauma, osmotic/oxidative damage to RBC, or gastrointestinal bleeding (21-23). Some researchers have also maintained that the hemolysis caused by foot strike reduces HGB levels (24). In addition, some studies have shown that the hemolysis of older RBCs increases as they pass through capillaries during muscle contraction. Other research suggests that increased RBC deformity due to physical activity results in hemolysis and a decrease in HGB percentage (22, 24, 25). In addition, a review study has reported that intestinal blood loss, in addition to exercise-induced hemolysis, might be another contributor to lowering HGB levels (26). The physical conditions and readiness of the participants are considered as a reason for the decrease in blood concentration. Plasma volume has been reported to decrease during and after exercise; however, plasma volume increases following adaptation to exercise training. Furthermore, a decrease in RBC concentration may be due to fluid intake, high intensity, and the long duration of the exercise, and finally, body water loss induced by sweating (27, 28). There are two major challenges to regulating red blood cell volume during exercise. One is an increase in extracellular osmolality (plasma), and the other is acidosis, both of which are expressed during high-intensity exercise. Moreover, literature data on the alterations of RBC aggregation has shown a significant association between elevated blood lactate levels and erythrocyte rigidification. One of the limitations of the current study is that the lactate and other factors affecting erythrocyte density (such as oxidative or proteolytic enzymes released from granulocytes) have not been measured (28). Further research should take lactate and factors affecting erythrocyte density into account.
The results of the present study showed that none of the platelet-related markers changed as a result of one intensive judo activity session. Platelet response to physical activity depends on many factors, including intensity, duration of physical activity, and the level of readiness of the participants (18). Contradictory findings have been reported regarding the effect of exercise training on platelet indexes. In this regard, Alis et al. (29) showed that there was no significant difference between baseline levels of PLT, PDW, MPW, and PCT in healthy active and inactive men. Two other studies reported no change in PLT concentration in response to one bout of exercise activity (30, 31). Conversely, Kırbaş et al. (32) reported a significant difference in platelet indices between active and inactive students. Increased platelet concentrations have also been reported in trained athletes in response to half-marathon (21.1 km) and high-intensity aerobic interval training (approximately 80% VO2 peak) (33, 34). Research has shown that a temporary increase in platelet counts results from vigorous physical activity, leading to increased blood concentrations and platelet release from the liver, lungs, and, most importantly, the spleen (35). Also, an increase in epinephrine in response to vigorous activity results in increased platelets (35). In addition, the rise in platelet indices of PLT, PDW, MPW, and PCT after acute activity is attributed to increased blood concentration due to exercise (hemoconcentration) as well as platelet release from the spleen, liver, and lungs and the entry of fresh platelets into the bloodstream (36). Subjects in the present study were trained individuals with long, athletic experience. Therefore, part of the change in platelet indexes is related to the high level of readiness of the subjects so that a session of intense judo activity did not affect the platelet levels of the judoka subjects.
The results showed that one session of heavy judo activity had no impact on WBC, LYM, and NEU. Suzuki et al. (37) also showed that no changes in leukocytes (NEU and LYM) occurred after a judo tournament. In addition, Robson-Ansley et al. (38) also reported no change in leukocytes and neutrophils in response to an acute period of intensive periodic exercise training in endurance-trained men. Nevertheless, the results of the present study are inconsistent with the findings of Hulmi et al.’s study (39), who reported increased leukocyte and platelet counts in response to one bout of resistance exercise in young and old men. The invariability of WBC, LYM, and granulocyte variables indicate that a session of intensive judo activity in this study did not suppress the cellular immune system and open window phenomenon. There is strong evidence that hormones play an essential role in regulating exercise-induced changes in leukocyte levels and subgroups. It has been clearly demonstrated that hormones such as cortisol and epinephrine affect the distribution of leukocytes. Research has shown that epinephrine is responsible for increased leukocytosis during vigorous and short-term exercise. The leukocytosis level seems to be directly correlated with the intensity and duration of activity and inversely related to one’s readiness (40). The type of activity, gender, and age of the participants are determinants of changes in the immune system (41). Because the subjects in this study are trained individuals, a smaller increase might have occurred in these hormones with a slight effect on cellular immunity.
The focus of previous research has been directed mainly to identify changes in serum and salivary concentrations of immunoglobulins involved in the development of URTI after one bout of acute and intense exercise. The most crucial finding of the present study was concerned with a significant decrease in IgA and IgM levels after one session of intensive judo activity, although there was no significant change in IgE and IgG concentrations after the intensive judo activity. Overall, in exercise immunology, an increase in immunoglobulin concentration indicates the development of immunity, while the decrease is usually interpreted as immunosuppression (3). The present study is in line with the findings of Tsai et al. (14), where severe taekwondo training combined with rapid weight change resulted in reduced IgA levels in elite female taekwondo athletes. It has also been shown that intense, long-term swimming exercise reduces serum and saliva IgM, IgG, and IgA levels in elite swimmers, which in turn increases susceptibility to infection (42). In addition, McKune et al. (3) have reported a decrease in IgM concentration and an increase in total IgG concentration after an ultra-marathon in experienced runners.
In addition, Zakovska et al. (4) have reported a significant increase in IgG level and no significant change in IgA and IgM levels after 100 km of ultra-marathon under cold conditions in elite runners. In contrast, Karacabey et al. (11) reported that a 30-minute aerobic running on the treadmill and a 30-second Wingate anaerobic test had no significant effect on IgM, IgA, and IgG levels in elite volleyball players. Also, four sets of bench press and squat resistance exercise with 85% of one repetition maximum and rest intervals of 60, 90, and 120 seconds (12), as well as a kickboxing match (13), had an impact on serum IgA and total concentration and salivary IgG levels in resistance-trained men.
One mechanism to justify different IgM responses is that the isotopic switching of immunoglobulins varies during different exercise activities. This function is mediated by two groups of the cytokine family of tumor necrosis factors called B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL). Also, IgM can be converted to IgG by isotopic shift (3), which might be a reason for the decrease in IgM levels after exercise. Aldred et al. (43) reported that moderate and uniform exercise increased IgE levels in allergic subjects but did not affect IgE levels in healthy subjects. Therefore, part of the non-change in IgE levels of the subjects in this study was due to the lack of allergy in the subjects.
Based on the results, it can be concluded that intensive judo exercise causes transient anemia and suppresses humoral immunity in adolescent boys. However, it did not affect platelet and cellular immune parameters.
Acknowledgements
References
-
1.
Galazka-Franta A, Jura-Szoltys E, Smolka W, Gawlik R. Upper Respiratory Tract Diseases in Athletes in Different Sports Disciplines. J Hum Kinet. 2016;53:99-106. [PubMed ID: 28149415]. [PubMed Central ID: PMC5260580]. https://doi.org/10.1515/hukin-2016-0014.
-
2.
Spence L, Brown WJ, Pyne DB, Nissen MD, Sloots TP, McCormack JG, et al. Incidence, etiology, and symptomatology of upper respiratory illness in elite athletes. Med Sci Sports Exerc. 2007;39(4):577-86. [PubMed ID: 17414793]. https://doi.org/10.1249/mss.0b013e31802e851a.
-
3.
McKune AJ, Smith LL, Semple SJ, Wadee AA. Influence of ultra-endurance exercise on immunoglobulin isotypes and subclasses. Br J Sports Med. 2005;39(9):665-70. [PubMed ID: 16118307]. [PubMed Central ID: PMC1725321]. https://doi.org/10.1136/bjsm.2004.017194.
-
4.
Zakovska A, Knechtle B, Chlibkova D, Milickova M, Rosemann T, Nikolaidis PT. The Effect of a 100-km Ultra-Marathon under Freezing Conditions on Selected Immunological and Hematological Parameters. Front Physiol. 2017;8:638. [PubMed ID: 28955243]. [PubMed Central ID: PMC5600930]. https://doi.org/10.3389/fphys.2017.00638.
-
5.
Wu HJ, Chen KT, Shee BW, Chang HC, Huang YJ, Yang RS. Effects of 24 h ultra-marathon on biochemical and hematological parameters. World J Gastroenterol. 2004;10(18):2711-4. [PubMed ID: 15309724]. [PubMed Central ID: PMC4572198]. https://doi.org/10.3748/wjg.v10.i18.2711.
-
6.
Nieman DC, Wentz LM. The compelling link between physical activity and the body's defense system. J Sport Health Sci. 2019;8(3):201-17. [PubMed ID: 31193280]. [PubMed Central ID: PMC6523821]. https://doi.org/10.1016/j.jshs.2018.09.009.
-
7.
Schroeder HJ, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S41-52. [PubMed ID: 20176268]. [PubMed Central ID: PMC3670108]. https://doi.org/10.1016/j.jaci.2009.09.046.
-
8.
Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. [PubMed ID: 25368619]. [PubMed Central ID: PMC4202688]. https://doi.org/10.3389/fimmu.2014.00520.
-
9.
Peters EM, Shaik J, Kleinveldt N. Upper respiratory tract infection symptoms in ultramarathon runners not related to immunoglobulin status. Clin J Sport Med. 2010;20(1):39-46. [PubMed ID: 20051733]. https://doi.org/10.1097/JSM.0b013e3181cb4086.
-
10.
Hammouda O, Chtourou H, Chaouachi A, Chahed H, Ferchichi S, Kallel C, et al. Effect of short-term maximal exercise on biochemical markers of muscle damage, total antioxidant status, and homocysteine levels in football players. Asian J Sports Med. 2012;3(4):239-46. [PubMed ID: 23342222]. [PubMed Central ID: PMC3525820]. https://doi.org/10.5812/asjsm.34544.
-
11.
Karacabey K, Peker İ, Saygın Ö, Cıloglu F, Ozmerdivenli R, Bulut V. Effects of Acute Aerobic and Anaerobic Exercise on Humoral Immune Factors in Elite Athletes. Biotechnology & Biotechnological Equipment. 2014;19(1):175-80. https://doi.org/10.1080/13102818.2005.10817177.
-
12.
Rahimi R, Ghaderi M, Mirzaei B, Ghaeni S, Faraji H, Vatani DS, et al. Effects of very short rest periods on immunoglobulin A and cortisol responses to resistance exercise in men. Journal of Human Sport and Exercise. 2010;5(2):146-57. https://doi.org/10.4100/jhse.2010.52.05.
-
13.
Moreira A, Arsati F, Lima-Arsati YB, Franchini E, De Araujo VC. Effect of a kickboxing match on salivary cortisol and immunoglobulin A. Percept Mot Skills. 2010;111(1):158-66. [PubMed ID: 21058596]. https://doi.org/10.2466/05.06.16.25.PMS.111.4.158-166.
-
14.
Tsai ML, Ko MH, Chang CK, Chou KM, Fang SH. Impact of intense training and rapid weight changes on salivary parameters in elite female Taekwondo athletes. Scand J Med Sci Sports. 2011;21(6):758-64. [PubMed ID: 20456682]. https://doi.org/10.1111/j.1600-0838.2010.01099.x.
-
15.
Gleeson M. Immune function in sport and exercise. J Appl Physiol (1985). 2007;103(2):693-9. [PubMed ID: 17303714]. https://doi.org/10.1152/japplphysiol.00008.2007.
-
16.
Azarbayjani MA, Fathi R, Daloii AA, Abdi A, Fatolahi H. Acute Hematological Profile Response to One Session of Aerobic and Anaerobic Exercise among Young Male Kickboxers. Turkish Journal of Physical Medicine & Rehabilitation. 2014;60(2):92-7. https://doi.org/10.5152/tftrd.2014.24654.
-
17.
Tayebi SM, Hanachi P, Niaki AG, Ali PN, Ghaziani FG. Ramadan Fasting and Weight-Lifting Training on Vascular Volumes and Hematological Profiles in Young Male Weight-Lifters. Global Journal of Health Science. 2010;2(1). https://doi.org/10.5539/gjhs.v2n1p160.
-
18.
Ahmadizad S, El-Sayed MS. The acute effects of resistance exercise on the main determinants of blood rheology. J Sports Sci. 2005;23(3):243-9. [PubMed ID: 15966342]. https://doi.org/10.1080/02640410410001730151.
-
19.
Ghanbari Niaki A, Mohammadi S. Effect of 4 weeks of an aerobic (RAST) training on hematological changes in male kick-boxers. International Journal of Applied Exercise Physiology. 2010;5(10):75-87.
-
20.
Ozyener F, Gür H, Özlük K. Hematological changes following a brief exhaustive maximal exercise in sedentary males. Hacettepe J Sport Sci. 1994;6(2):27-37.
-
21.
Belviranli M, Okudan N, Kabak B. The Effects of Acute High-Intensity Interval Training on Hematological Parameters in Sedentary Subjects. Med Sci (Basel). 2017;5(3). [PubMed ID: 29099031]. [PubMed Central ID: PMC5635806]. https://doi.org/10.3390/medsci5030015.
-
22.
Kilic-Toprak E, Ardic F, Erken G, Unver-Kocak F, Kucukatay V, Bor-Kucukatay M. Hemorheological responses to progressive resistance exercise training in healthy young males. Med Sci Monit. 2012;18(6):CR351-60. [PubMed ID: 22648250]. [PubMed Central ID: PMC3560717]. https://doi.org/10.12659/msm.882878.
-
23.
Mairbaurl H. Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front Physiol. 2013;4:332. [PubMed ID: 24273518]. [PubMed Central ID: PMC3824146]. https://doi.org/10.3389/fphys.2013.00332.
-
24.
Alam T, Rahman SM, Alam T, Habib N, Umar BU, Banna QR, et al. Effect of Physical Exercise on some Hematological Parameters in Female Athletes in Bangladesh. JNMA J Nepal Med Assoc. 2014;52(195):892-6. [PubMed ID: 26982662].
-
25.
Cakir-Atabek H, Atsak P, Gunduz N, Bor-Kucukatay M. Effects of resistance training intensity on deformability and aggregation of red blood cells. Clin Hemorheol Microcirc. 2009;41(4):251-61. [PubMed ID: 19318718]. https://doi.org/10.3233/CH-2009-1176.
-
26.
Zoller H, Vogel W. Iron supplementation in athletes--first do no harm. Nutrition. 2004;20(7-8):615-9. [PubMed ID: 15212743]. https://doi.org/10.1016/j.nut.2004.04.006.
-
27.
Joborn H, Akerstrom G, Ljunghall S. Effects of exogenous catecholamines and exercise on plasma magnesium concentrations. Clin Endocrinol (Oxf). 1985;23(3):219-26. [PubMed ID: 4075536]. https://doi.org/10.1111/j.1365-2265.1985.tb00217.x.
-
28.
Lippi G, Schena F, Salvagno GL, Tarperi C, Montagnana M, Gelati M, et al. Acute variation of estimated glomerular filtration rate following a half-marathon run. Int J Sports Med. 2008;29(12):948-51. [PubMed ID: 18600608]. https://doi.org/10.1055/s-2008-1038745.
-
29.
Alis R, Sanchis-Gomar F, Risso-Ballester J, Blesa JR, Romagnoli M. Effect of training status on the changes in platelet parameters induced by short-duration exhaustive exercise. Platelets. 2016;27(2):117-22. [PubMed ID: 26023745]. https://doi.org/10.3109/09537104.2015.1047334.
-
30.
Kratz A, Wood MJ, Siegel AJ, Hiers JR, Van Cott EM. Effects of marathon running on platelet activation markers : direct evidence for in vivo platelet activation. Am J Clin Pathol. 2006;125(2):296-300. [PubMed ID: 16393676]. https://doi.org/10.1309/PRF5-N7P2-XM6E-243H.
-
31.
Aldemir H, Kilic N. The effect of time of day and exercise on platelet functions and platelet-neutrophil aggregates in healthy male subjects. Mol Cell Biochem. 2005;280(1-2):119-24. [PubMed ID: 16311912]. https://doi.org/10.1007/s11010-005-8238-8.
-
32.
Kırbaş Ş, Tetik S, Aykora E, Duran B. An examination of the impact of regular exercise participation on blood platelet parameters. World Journal of Medical Sciences. 2015;12:79-82.
-
33.
Whittaker JP, Linden MD, Coffey VG. Effect of aerobic interval training and caffeine on blood platelet function. Med Sci Sports Exerc. 2013;45(2):342-50. [PubMed ID: 22935739]. https://doi.org/10.1249/MSS.0b013e31827039db.
-
34.
Lippi G, Salvagno GL, Danese E, Tarperi C, Guidi GC, Schena F. Variation of red blood cell distribution width and mean platelet volume after moderate endurance exercise. Adv Hematol. 2014;2014:192173. [PubMed ID: 25197280]. [PubMed Central ID: PMC4147199]. https://doi.org/10.1155/2014/192173.
-
35.
Bakovic D, Pivac N, Eterovic D, Breskovic T, Zubin P, Obad A, et al. The effects of low-dose epinephrine infusion on spleen size, central and hepatic circulation and circulating platelets. Clin Physiol Funct Imaging. 2013;33(1):30-7. [PubMed ID: 23216763]. https://doi.org/10.1111/j.1475-097X.2012.01156.x.
-
36.
Heber S, Volf I. Effects of Physical (In)activity on Platelet Function. Biomed Res Int. 2015;2015:165078. [PubMed ID: 26557653]. [PubMed Central ID: PMC4628769]. https://doi.org/10.1155/2015/165078.
-
37.
Suzuki M, Nakaji S, Umeda T, Shimoyama T, Mochida N, Kojima A, et al. Effects of weight reduction on neutrophil phagocytic activity and oxidative burst activity in female judoists. Luminescence. 2003;18(4):214-7. [PubMed ID: 12950057]. https://doi.org/10.1002/bio.727.
-
38.
Robson-Ansley PJ, Blannin A, Gleeson M. Elevated plasma interleukin-6 levels in trained male triathletes following an acute period of intense interval training. Eur J Appl Physiol. 2007;99(4):353-60. [PubMed ID: 17165057]. https://doi.org/10.1007/s00421-006-0354-y.
-
39.
Hulmi JJ, Myllymaki T, Tenhumaki M, Mutanen N, Puurtinen R, Paulsen G, et al. Effects of resistance exercise and protein ingestion on blood leukocytes and platelets in young and older men. Eur J Appl Physiol. 2010;109(2):343-53. [PubMed ID: 20101405]. https://doi.org/10.1007/s00421-010-1360-7.
-
40.
Bobeuf F, Labonte M, Khalil A, Dionne IJ. Effect of resistance training on hematological blood markers in older men and women: a pilot study. Curr Gerontol Geriatr Res. 2009:156820. [PubMed ID: 19865492]. [PubMed Central ID: PMC2768009]. https://doi.org/10.1155/2009/156820.
-
41.
Nieman DC, Miller AR, Henson DA, Warren BJ, Gusewitch G, Johnson RL, et al. Effect of high- versus moderate-intensity exercise on lymphocyte subpopulations and proliferative response. Int J Sports Med. 1994;15(4):199-206. [PubMed ID: 8063469]. https://doi.org/10.1055/s-2007-1021047.
-
42.
Gleeson M, McDonald WA, Cripps AW, Pyne DB, Clancy RL, Fricker PA. The effect on immunity of long-term intensive training in elite swimmers. Clin Exp Immunol. 1995;102(1):210-6. [PubMed ID: 7554392]. [PubMed Central ID: PMC1553334]. https://doi.org/10.1111/j.1365-2249.1995.tb06658.x.
-
43.
Aldred S, Love JA, Tonks LA, Stephens E, Jones DS, Blannin AK. The effect of steady state exercise on circulating human IgE and IgG in young healthy volunteers with known allergy. J Sci Med Sport. 2010;13(1):16-9. [PubMed ID: 18977173]. https://doi.org/10.1016/j.jsams.2008.07.001.