Abstract
Background:
Growth hormone and insulin-like growth factor 1 are anabolic hormones that play a vital role in the growth of various physical organs. Exercise is one of the stimuli that affect GH and IGF-1 secretion.Objectives:
This study aimed to compare the effect of two types of high-intensity interval training (HIIT) on plasma levels of GH and IGF-l in overweight nurses.Methods:
In this study, 27 nurses were voluntarily selected and randomly assigned to three groups (9 participants for each group): 1. HIIT (type 1), including eight seconds of spring running and 12 seconds of active recovery, 2. HIIT (type 2), including a 40-meter shuttle run with maximum speed, 3. control. HIIT (type 1) was performed for four weeks, three sessions per week, each session 6 - 9 min with more than 90% HRmax. HIIT (type 2) was applied for four weeks, three sessions per week, with more than 90% HRmax. The control group did not participate in any training protocol. The serum value of GH and IGF-1 were compared in three groups. The data were analyzed by the dependent t-test and ANOVA. One-way analysis of variance (ANOVA) was used to analyze the intergroup data at P ⟨ 0.05.Results:
The results showed that HIIT (type 1) and (type 2) significantly increased plasma GH (P = 0.032 in group 2 and P = 0.010 in group 1) and IGF-l (P = 0.004 in group 2 and P = 10.013 in group 1) levels in nurses. The results showed a significant difference in the variables (GH and IGF-l) among HIIT (type 1), HIIT (type 2), and control groups.Conclusions:
It can be concluded that four weeks of adverse intermittent exercises are effective in increasing the concentration of GH and IGF-1 serum and decreased percentage body fat in young nurses with overweight, and proportional to the intensity of the exercise protocol response rate is different.Keywords
1. Background
Nurses are so active and working hard, and their physical health guarantees better service to patients. The physical health of nurses is at risk due to persistent stress and long-term insomnia. Exercise at different times of the morning or evening can improve the mood status and reduce depression (1). Stress and lack of sleep weaken the immune system and disrupted hormones that are associated with sleep. Hormones of GH and IGF-1 are increased at night when there is no psychological tension and during physical activity (2).
Insulin-like growth factor (IGF-1) plays a key role in regulating growth and muscle development after birth (1, 2). This factor also plays a role in certain diseases such as muscular dystrophy, delayed wound tissue, and impair in the tissue of internal organs in the body (3, 4). Hence reinforcing the growth factor can prevent and even help treat some growth-related disorders. Moreover, IGF-1 is the most important family member of somatomedin that plays an important role in physical growth, proliferation, cell differentiation, metabolism, and life (5).
This factor plays a crucial role in the development of embryonic and fetal, and postnatal growth and increases tissue repair and anabolism in the elderly (6). Full IGF-1 contains 70 amino acids and is connected to binding proteins in plasma circulating insulin-like growth factor-binding proteins (IGFBPs). The main source of IGF-1 is the liver. One of the main mechanisms for the production of IGF-1 is the effect of GH on the liver to produce IGF-1. Other tissues, such as muscle, brain, and bones, also produce IGF-1. Owing to its central role in metabolic processes, this study continues in connection with the growth factor (7).
Furthermore, IGF-1 plays important roles in the adaptation of muscle hypertrophy to resistance exercise (8, 9), the growth and bone health (10), short-term and long-term metabolic activity (11), and adaptation of the organism to exercise (1). Limited researches have shown the central role of GH/IGF-1 in response to the adverse intermittent exercise. Exercise stimulates the release of growth hormone, but there are conflicting data about the effect of acute exercise on IGF-1 and GH.
Some studies attributed the rising of IGF-1 after exercise to the insulin-like effects. Some studies have reported a relationship between IGF-1 in blood circulation and muscle mass (12). Hasani-Ranjbaret al. (7) showed that an adverse intermittent training session increases the concentration of IGF-1 and its affiliates like IGFBP3.
Stokes et al. (12) examined the effect of aerobic adverse exercise values on IGF-1, IGF-2 growth hormones. The training protocol was 30 seconds faster, bicycling on ergometer cycling with the strength of 7 or 9 percent of body weight. Stokes et al. (2010) examined the effect of aerobic adverse exercise values on IGF-1 and IGF2 growth hormone. The training protocol was 30 seconds faster bicycling on ergometer cycling with the strength of 7 or 9 percent of body weight. The results of this research showed that one session of adverse exercise stimulates GH release and has had no significant effect on IGF-1 and IGF2 concentration and IGF biological activity (12). Some other studies show a drastic incremental change increase (2, 8, 10), while some studies reported a decrease (13), and several studies reported no significant differences (11, 14). Some studies have reported the independence of these two hormones (15).
2. Objectives
According to the results of various studies and the release of IGF-1 from different tissues, it is not yet clear whether the increase in IGF-1 secretion occurs after exercise and follows an increase in GH or its increase is independent of the increase in GH. Also, because most research has been done on men and there is a difference between women’s and men’s hormonal responses to a different exercise; hence, the purpose of the study was to examine the effect of two types of intermittent exercise on GH and IGF-1 serum in severely overweight nurses.
3. Methods
The present research is quasi-experimental, and subjects were randomly divided into different training groups of two groups of adverse intermittent exercise (HIIT) and a control group (C).
3.1. Participants
The study population consisted of female nurses in Amir Al-Momenin Hospital in Gerash City. The study was conducted according to the Declaration of Helsinki. On a certain day, the volunteers were invited, and after presenting full comments on the process of the study and the benefits and potential harms of the study, written informed consent was obtained from volunteers. The participants were informed about the confidentiality of their information. They were also ensured that they could withdraw from the study whenever they desired. The inclusion criteria were the BMI index between 23 and 24.5, the ability to perform physical activity, and having no underlying disease. The exclusion criteria were unwillingness to continue the activity and a doctor’s diagnosis.
3.2. Study Design
Twenty-seven participants were selected to participate in the study using the convenience sampling method who were divided into three groups, including adverse intermittent exercise type 1 (eight seconds quick running and twelve seconds of active recovery), adverse intermittent exercise type 2 (sweep test with a maximum speed of 40 meters) and the control group (nine participants per group) (Figure 1). Before choosing the sample to the desired height, their weight, body fat percentage, BMI, and waist to hip ratio were measured to provide homogenous groups. The average age, height, and weight of groups were 25.81 ± 60 years old, 158.01 ± 67 cm, and 69.41 ± 25 kg. Also, the average BMI of the subjects was 29.11 ± 3.94 (kg/m2). Pre- and post-test were taken from all subjects.
Flow chart of the study
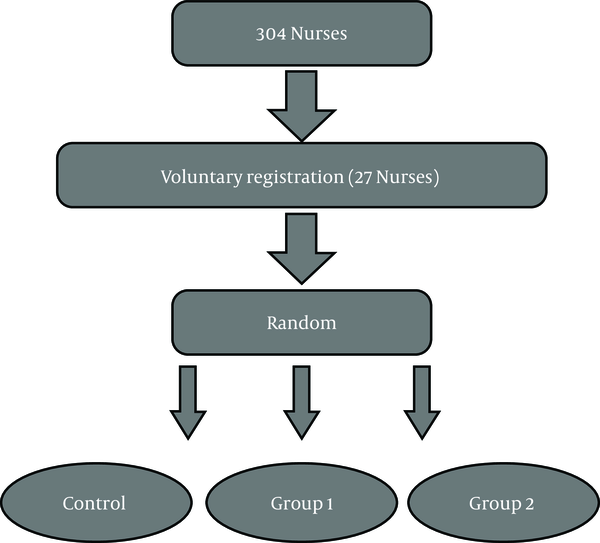
3.3. Training Protocol
To do the exercise, the subjects were randomly assigned to three groups (two groups of adverse intermittent exercise (HIIT) and control group (C). Experimental group 1 for four weeks and three sessions per week had exercises that include eight seconds of rapid running and twelve seconds of active recovery (walk) at the determined time in the evening (5 PM). This exercise protocol was piloted before the start of the study. At the same time, experimental group 2 conducted the exercise protocol of a maximum speed of 40 m derived from the sweep test of 40 meters with a maximum speed of three sessions per week for four weeks and were as follows (Figure 2).
HIIT exercise protocol of the study
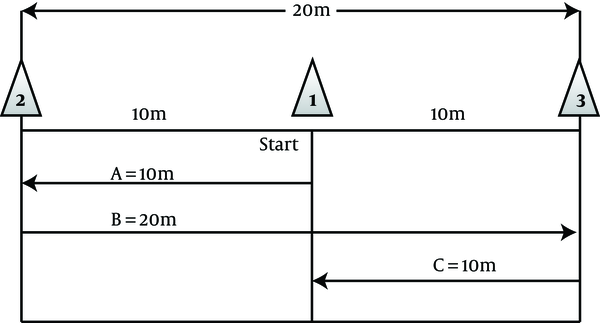
Starting with the exercise protocol, subjects ran with a maximum speed of start point (cone no.: 1) to the cone (2) (route A), then turn in the opposite direction with a maximum speed of 20 km to the cone (3) (route B) and then again returned to the starting point (cone (1)) with a maximum running speed (line C) to a distance of 40 meters (12).
3.4. Measurements
Twenty-four hours before the first practice session and 24 hours after the last section, 10 cc blood sample was taken from the brachial vein (antecubital) of all subjects in eight hours fasting status (8:30 AM). Blood samples were immediately poured into tubes containing EDTA (Ethylene Diamine Tetra Acetate) anticoagulant. Then were centrifuged at 3,000 rpm for 10 min at 4°C. Serum GH levels were used by the ELISA method and using specific ELISA kit (manufactured by Mediagnost Germany), and ELISA kit (manufactured by Koma Biotech) was used to measure IGF-1 according to the manufacturer’s instructions (11). We calculated BMI using the formula of weight (kg) that is divided by height square (m2). We calculated the percentage of fat using a body composition machine. Height and weight were calculated using SECA standard gauge and scale, respectively (12).
3.5. Statistical Analysis
The normality of the collected data was checked by the Kolmogorov-Smirnov test (KS). The value of P is considered to be at 0.05, and the calculations were performed using SPSS software version 16. An independent t-test was used to compare the mean values of groups studied, according to the study design.
4. Results
Twenty-seven nurses (mean age 25.81 ± 60 year, height 158.01 ± 67 cm, and weight 69.41 ± 25 kg) have voluntarily participated in this study. The results of general variables (BMI, height, weight, body fat percentage, and waist to hip ratio) and studied variables (GH and IGF-1) are presented in tables 1 and 2.
| Variables | Experimental Group 1 | Experimental Group 2 | Control Group | |||
|---|---|---|---|---|---|---|
| Pre-Test | Post-Test | Pre-Test | Post-Test | Pre-Test | Post-Test | |
| Age, y | 25.71 ± 63 | 25.91 ± 57 | 25.81 ± 60 | |||
| Height, cm | 162.70 ± 67 | 163.28 ± 67 | 162.99 ± 67 | |||
| Weight, kg | 61.40 ± 39 | 61.11 ± 39 | 61.31 ± 14 | 61.30 ± 39 | 61.52 ± 25 | 61.54 ± 39 |
| BMI, kg/m2 | 23.11 ± 3.9 | 23.14 ± 2.1 | 24.15 ± 3.6 | 24.11 ± 2.4 | 23.45 ± 1.8 | 23.44 ± 5.6 |
| Waist | 97.23 ± 1.4 | 94.80 ± 2.2 | 98.09 ± 3.3 | 96.42 ± 4.1 | 99.18 ± 2.6 | 98.70 ± 6.9 |
| WHR, ratio | 0.779 | 0.777 | 0.770 | 0.771 | 0.759 | 0.755 |
| Body fat, % | 22.98 | 18.97 | 23.08 | 20.08 | 23.33 | 23.71 |
Changes of Variables (GH and IGF-1) in the Experimental and Control Groups Before and After 4 Weeks of HIITa
| Variables | Groups | Pretest | Posttest | Difference | P Value |
|---|---|---|---|---|---|
| GH, μg/mL | Experimental 1 | 6.01 ± 14.02 | 16.73 ± 3.24 | 10.72 | 0.010b |
| Experimental 2 | 5.98 ± 5.32 | 10.04 ± 1.61 | 4.06 | 0.032b | |
| Control | 6.23 ± 9.21 | 6.24 ± 9.89 | 0.01 | 0.496 | |
| IGF-1, ng/mL | Experimental 1 | 278.64 ± 4.03 | 356.1 ± 7.27 | 77.46 | 0.013b |
| Experimental 2 | 275.63 ± 7.91 | 290.54 ± 3.12 | 14.91 | 0.004b | |
| Control | 280.60 ± 2.44 | 280.68 ± 1.2 | 0.08 | 0.076 |
Research findings showed that GH concentration increased significantly after performing HIIT for four weeks in 1 and 2 training group (P = 0.032 in group 2 and P = 0.010 in group 1) (Table 2), but no significant changes in the levels of GH were observed in the control group (Table 2). Also, the concentration of IGF-1 level increased significantly in the exercise group 1 and 2 (P = 0.004 in group 2 and P = 10.013 in group 1), while the control group showed no significant changes in IGF-1 levels (Table 2). Body fat percentage was reduced in both groups significantly, while none of the waist-to-hip ratio (WHR), BMI, and weight variables showed significant changes in the three groups.
5. Discussion
Growth hormone secreted from anterior pituitary cells stimulates IGF-1 secretion from liver cells and ultimately stimulates protein anabolic and fat breakdown. Moreover, HIIT exercises stimulate the breakdown of adipose tissue, resulting in weight loss and improved health indicators. Therefore, it is important to examine the effect of these exercises on these two growth factors. Weight training, BMI, and WHR had little effect, but both exercises reduced body fat by 3 to 4 percent. The reason for the slight decrease in the first three factors and the further decrease in body fat percentage is the replacement of muscle mass with fat mass. The results of the present research about the significant increase in GH and IGF-1 are in line with the results of Tanimotoet al. (11) and Maniniet al. (16) and is different from the results of Kim et al. (3). The reason for this difference could be the type of program and the duration of the exercise. In some studies, the GH levels increased after adverse intermittent exercise (5, 8, 17).
The intensity of the training was close in all of these studies, so the reason for the difference in results was probably the length of the training period. Based on the evidence available, the mechanism of IGF-1 response in circulating to adverse intermittent exercise in studies is different (4). The results of this study are associated with IGF-1 response to adverse intermittent exercise, which are consistent with the results of Fujita et al. (4), while is different from the results of Takano (18). The reason for this difference seems to be the type of subjects (18).
The sharp rise in GH could not be due to the increase IGF-1 immediately after exercise because the level of IGF-1 even 16 to 28 hours after the release of GH peak is not reached (19). In the present study, an increase in serum levels of IGF-1 was not observed even to 5.1 times compared to the pre-workout. Although there are significant differences between the groups in terms of IGF-1 levels, the inter-group analysis showed a significant difference in the group of moderate-intensity resistance training.
Never the less, compared to previous studies that showed a 10 to 30-fold increase in serum IGF-1 serum after exercise, the low increase in serum IGF-1 in the present study was due to the measurement of hormone levels before reaching its peak time. In general, based on the results of this study, although the pattern of change in serum levels of GH and IGF-1 was similar in both groups, adverse intermittent exercise protocols with less intensity can cause a further increase in anabolic hormones in young girls is high-intensity resistance training. Selection of girl groups is due to the homogeneity of the objects in terms of all aspects of physiological and structural variables, and the goal of the researchers was to compare the differences between the two types of exercise, not the difference in results between the two genders. Owing to the limitations of research in the women’s community and the higher prevalence of obesity and overweight in women than men, this study was conducted on women (20, 21). The limitations of the study were the lack of control over diet, sleep, and emotional states of the subjects during exercise. It is recommended that this study should be performed in a group of men to achieve better results. In addition to the blood factors taken, several functional factors should be measured to evaluate the functional effects of these activities. However, due to limited research on the impact of HIIT on GH/IGF-1 in young nurses, more research is needed in this area.
5.1. Conclusions
Based on the findings of the present study, it seems that applying four weeks of high-intensity interval training (HIIT) is a suitable training method to increase the concentration of serum GH and IGF-1 and reduced the percentage of body fat in overweight young nurses and the response rate varies according to the intensity of the training protocol. Also, the intensity of exercise as a crucial factor in increased serum concentrations of GH and IGF-1 in response to exercise can be noted.
References
-
1.
Irandoust K, Taheri M, Chtourou H, Nikolaidis PT, Rosemann T, Knechtle B. Effect of time-of-day-exercise in group settings on level of mood and depression of former elite male athletes. Int J Environ Res Public Health. 2019;16(19). [PubMed ID: 31546685]. [PubMed Central ID: PMC6801561]. https://doi.org/10.3390/ijerph16193541.
-
2.
Fujita T, Brechue WF, Kurita K, Sato Y, Abe T. Increased muscle volume and strength following six days of low-intensity resistance training with restricted muscle blood flow. Int J KAATSU Train Res. 2008;4(1):1-8. https://doi.org/10.3806/ijktr.4.1.
-
3.
Kim E, Gregg LD, Kim L, Sherk VD, Bemben MG, Bemben DA. Hormone responses to an acute bout of low intensity blood flow restricted resistance exercise in college-aged females. J Sports Sci Med. 2014;13(1):91.
-
4.
Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, et al. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol (1985). 2007;103(3):903-10. [PubMed ID: 17569770]. https://doi.org/10.1152/japplphysiol.00195.2007.
-
5.
Takarada Y, Tsuruta T, Ishii N. Cooperative effects of exercise and occlusive stimuli on muscular function in low-intensity resistance exercise with moderate vascular occlusion. Jpn J Physiol. 2004;54(6):585-92. [PubMed ID: 15760491]. https://doi.org/10.2170/jjphysiol.54.585.
-
6.
Abe T, Yasuda T, Midorikawa T, Sato Y, Kearns CF, Inoue K, et al. Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. Int J KAATSU Train Res. 2005;1(1):6-12. https://doi.org/10.3806/ijktr.1.6.
-
7.
Hasani-Ranjbar S, Soleymani Far E, Heshmat R, Rajabi H, Kosari H. Time course responses of serum GH, insulin, IGF-1, IGFBP1, and IGFBP3 concentrations after heavy resistance exercise in trained and untrained men. Endocrine. 2012;41(1):144-51. [PubMed ID: 21983797]. https://doi.org/10.1007/s12020-011-9537-3.
-
8.
Takarada Y, Nakamura Y, Aruga S, Onda T, Miyazaki S, Ishii N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol (1985). 2000;88(1):61-5. [PubMed ID: 10642363]. https://doi.org/10.1152/jappl.2000.88.1.61.
-
9.
Yasuda T, Brechue WF, Fujita T, Shirakawa J, Sato Y, Abe T. Muscle activation during low-intensity muscle contractions with restricted blood flow. J Sports Sci. 2009;27(5):479-89. [PubMed ID: 19253083]. https://doi.org/10.1080/02640410802626567.
-
10.
Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol (1985). 2008;104(5):1452-61. [PubMed ID: 18323467]. [PubMed Central ID: PMC2715298]. https://doi.org/10.1152/japplphysiol.00021.2008.
-
11.
Tanimoto M, Madarame H, Ishii N. Muscle oxygenation and plasma growth hormone concentration during and after resistance exercise: Comparison between “KAATSU” and other types of regimen. Int J KAATSU Train Res. 2005;1(2):51-6. https://doi.org/10.3806/ijktr.1.51.
-
12.
Stokes KA, Sykes D, Gilbert KL, Chen JW, Frystyk J. Brief, high intensity exercise alters serum ghrelin and growth hormone concentrations but not IGF-I, IGF-II or IGF-I bioactivity. Growth Horm IGF Res. 2010;20(4):289-94. [PubMed ID: 20472480]. https://doi.org/10.1016/j.ghir.2010.03.004.
-
13.
Pierce JR, Clark BC, Ploutz-Snyder LL, Kanaley JA. Growth hormone and muscle function responses to skeletal muscle ischemia. J Appl Physiol (1985). 2006;101(6):1588-95. [PubMed ID: 16888046]. https://doi.org/10.1152/japplphysiol.00585.2006.
-
14.
Yasuda T, Abe T, Sato Y, Midorikawa T, Kearns CF, Inoue K, et al. Muscle fiber cross-sectional area is increased after two weeks of twice daily KAATSU-resistance training. Int J KAATSU Train Res. 2005;1(2):65-70. https://doi.org/10.3806/ijktr.1.65.
-
15.
Hosseini KS, Sharifi MA, Hamedinia M, Azarnive M. A comparison of the effect of traditional resistance training with resistance training with vascular occlusion on muscular function and cardiovascular endurance in young females. Sport Biosciences (Harakat). 2011;(10):95-114.
-
16.
Manini TM, Clark BC. Blood flow restricted exercise and skeletal muscle health. Exerc Sport Sci Rev. 2009;37(2):78-85. [PubMed ID: 19305199]. https://doi.org/10.1097/JES.0b013e31819c2e5c.
-
17.
Reeves GV, Kraemer RR, Hollander DB, Clavier J, Thomas C, Francois M, et al. Comparison of hormone responses following light resistance exercise with partial vascular occlusion and moderately difficult resistance exercise without occlusion. J Appl Physiol (1985). 2006;101(6):1616-22. [PubMed ID: 16902061]. https://doi.org/10.1152/japplphysiol.00440.2006.
-
18.
Takano H, Morita T, Iida H, Asada K, Kato M, Uno K, et al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol. 2005;95(1):65-73. [PubMed ID: 15959798]. https://doi.org/10.1007/s00421-005-1389-1.
-
19.
Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35(4):339-61. [PubMed ID: 15831061]. https://doi.org/10.2165/00007256-200535040-00004.
-
20.
Avazpour S, Fazell Kalkhoran J, Mohseni F. Effect of 12 weeks of resistance training on serum, vaspin and chemerin in obese middle-aged women. Asian J Sports Med. 2020;11(1). https://doi.org/10.5812/asjsm.97363.
-
21.
Poorgholami F, Pouryousef S, Manesh EP, Mohseni F, Jahromi MK. A study of social support among non-depressed and depressed mothers after childbirth in Jahrom, Iran. Bangladesh J Med Sci. 2019;18(4):736-40. https://doi.org/10.3329/bjms.v18i4.42877.