Abstract
Background:
To increase the therapeutic effect of drugs to combat diseases, combination therapy with current chemical drugs and new medicines derived from medicinal plants is necessary.Objectives:
The present work aimed to investigate the effect of hydroalcoholic extract of two medicinal plants, Ephedra major and Momordica cacharantia (Carla), and resveratrol drug on cell viability and expression levels of caspase-3 gene in MCF-7 cell line.Methods:
In this experimental study, the hydroalcoholic extraction of tested plants was done with a Soxhlet extractor. The MTT assay and real-time PCR were used to determine cell toxicity and caspase-3 gene expression levels, respectively.Results:
The highest and lowest cytotoxic effects of plant extracts and resveratrol were observed at concentrations of 500 and 150 µg/mL, respectively. The highest level of the caspase-3 gene expression was observed after 72 h of incubation by different concentrations of plant extracts and resveratrol.Conclusions:
It can be concluded that both plant extracts could influence cell viability in MCF-7 cells via the increase of cell toxicity and expression of caspase-3 gene. Thus, these species could be used in the pharmaceutical industry.Keywords
1. Background
Breast cancer is one of the most common cancers in women, with a high level of mortality over the past decades (1). Surgical side effects and adverse effects after breast cancer surgery and radiotherapy, such as persistent postsurgical and musculoskeletal pain, are major clinical problems after breast cancer surgery and radiotherapy (2). Therefore, numerous researchers are trying to discover and develop plant-based alternatives, particularly for the rational management of cancer control (3). The potential of dietary natural products to influence health has been investigated by scientific research, and it is believed that these compounds could affect the progression of different diseases in various ways (4).
The efficiency and effectiveness of medicinal plants were determined based on traditional experiences and ethnomedical practices (5, 6). Ephedra major species belong to the Ephedraceae family and are known to comprise approximately 35 to 45 species (7). The biological activity of Ephedra species has been studied and reported in the literature, and antioxidant, antibacterial, and antifungal effects of different species have been reported (8). The chemical compounds isolated from the Ephedra species include alkaloids, flavonoids, tannins, and polysaccharides, by which anticancer activity of flavonoid compounds of Ephedra species has been demonstrated (8, 9).
Momordica charantia L. or bitter melon (Cucurbitaceae) is widely distributed in tropical regions of the world. In various systems of traditional medicine, Momordica charantia has been used for several diseases such as diabetes, abdominal pain, rheumatism, antifertility, and malady hepatotoxicity (10). The potential chemopreventive activity of phytoalexin resveratrol has been reported. This compound is found at high levels in many plant species, including those often consumed by humans, such as grapes, peanuts, and berries (11). Because resveratrol is a naturally occurring compound, it has been highly studied for the prevention and treatment of many diseases, including cancer disease. The multiple anticancer effects of resveratrol application against both tumor initiation and cancer progression pathways have been extensively reviewed in numerous in-vitro studies (12, 13).
2. Objectives
In this research, we attempted to investigate the effect of ephedra and Carla plant extracts and resveratrol drug on cell viability and expression levels of Caspas-3 gene in a breast cancer cell line (MCF-7) by the MTT assay and real-time PCR method, respectively.
3. Methods
3.1. Plant Materials
Ephedra major species were collected at the coordinates 30° 03′ 24″ N - 54° 35′ 26″ E in May 2018. A dry sample of the plant was kept in the Herbarium of Botanic Laboratory of Yazd University, Yazd, Iran (EFYZ-2018). The green fruits of M. charantia were harvested from the northern regions of Iran and subsequently were cleaned and cut into small pieces and dried at room temperature.
3.2. Plant Material and Hydroalcoholic Extraction
Shade-dried plant tissues were crushed into powders using a blender. The powdered plant material (20 g) was subjected to hydroalcoholic extraction (80%) at 37°C for 16 h. The mixture was filtered, and the extraction procedure was repeated twice. A rotator evaporator under reduced pressure at 40°C was used to remove the solvent, and it was protected from light (14).
3.3. Cell Culture and Treatment
In this study, 105 MCF-7 cell lines were cultured in a cell culture plate containing RPMI-1640 culture medium (Gibco, USA) with 10% Fetal Bovine Serum (FBS, Life Technologies, South Korea), streptomycin (500 μg/mL), and penicillin. The culture plates were transferred to a humidified incubator (Memert, Germany) at 37°C with an atmosphere of 5% CO2 and 95% air. Cells were passaged when they covered the plate (70 - 80% confluence).
Cells incubated with the vehicle (DMSO) at a final concentration of 0.1% were used as the control. Different incubation times (24, 48, and 72 h) with different plant extracts (100, 200, 300, 400, and 500 µg/mL) and resveratrol (25, 50, 75, 100, and 150 µg/mL) were used for the MCF-7 cell viability assay.
3.4. Cell Viability Assay
The MTT assay was performed for the determination of the effects of plant extracts and resveratrol drug (15, 16) on cell viability. The development of purple color due to the formation of formazan crystals in the presence of viable cells was visualized by the spectrophotometric method. Absorbance was measured at the 570 nm wavelength using an ELISA plate reader (SpectroStar® Nano, Labtech, Germany). All the measurements were done in triplicate and the data were reported as mean ± SD. Cell viability was calculated using the equation:
The IC50 values (µg/mL) were calculated using the graph of cell viability versus plant extract and resveratrol drug concentration for the MCF-7 cell line (17).
3.5. Real-time RT-PCR
Treated MCF-7 cells with various concentrations of plant extracts and resveratrol were incubated for different times (24, 48, and 72 h) and subjected to RNA extraction using a Cinnagen RNA purification kit (Sina Clon, Iran). The quality and quantity of the extracted RNA were assayed by using agarose gel electrophoresis and NanoDropTM 1000 (Thermo Scientific, Whatham, MA, USA), respectively. The cDNA synthesis was done with a two-step RT-PCR kit (Sina Clon Bioscience, Iran). The cDNA samples were subjected to quantitative PCR using Eva Green qPCR Mix, ROX (Pishgam, Iran) in a light cycler apparatus (Rotor-Gene 3000, Corbett Robotics, Australia) with 40 cycles consisting of 94°C for 15 s, 58°C for 30 s, and 72°C for 30 s. Melting curve analysis was performed from 60 to 95°C to assess the specificity of PCR products. The caspase-3 gene primers (forward AAGCGAATCAATGGACTCTGG and reverse CTGTACCAGACCGAGATGTC) (18) and GAPDH primers as the reference gene (forward CAGCCTCAAGATCATCAGCA and reverse TGTGGTCATGAGTCCTTCCA) were used in this study.
3.6. Statistical Analysis
All in vitro experiments were performed at least in triplicate to confirm reproducibility. The differences between means were determined using Student’s t test or one-way ANOVA with Dennett’s multiple comparison test. Statistical significance was set at *P < 0.05 or **P < 0.01. The 2-∆∆Ct method was used to calculate the data from relative changes in gene expression specified from RT-PCR.
4. Results
4.1. Cell Viability
4.1.1. Ephedra Plant Extract
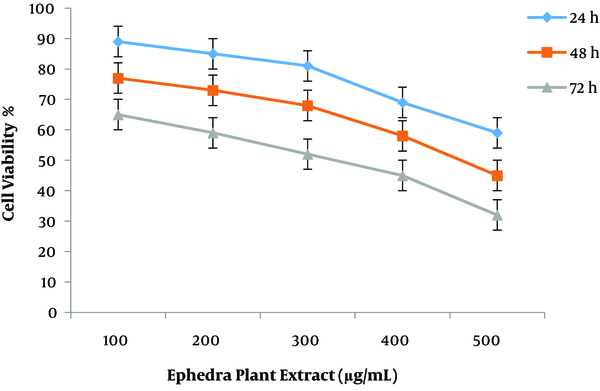
Cytotoxic properties of the two plant extracts and resveratrol drug against MCF7 cells were assessed using a standard MTT assay. The cell inhibition ability of all plant concentrations is shown in Figure 1. The highest cell death occurred in MCF-7 cells incubated for 72 h, and the lowest effect of plant extract was observed at 24 h treatment. The IC50 measured at 72 h time period was found to be better concerning anti-breast cancer activity. The MCF-7 cell line treated with ephedra extract for 24 and 48 h after incubation in the dose-response curve showed modest growth inhibition (Figure 1).
Effect of ephedra extract on the growth of MCF-7 breast cancer cell line.
4.1.2. Bitter Melon (Carla) Plant Extract
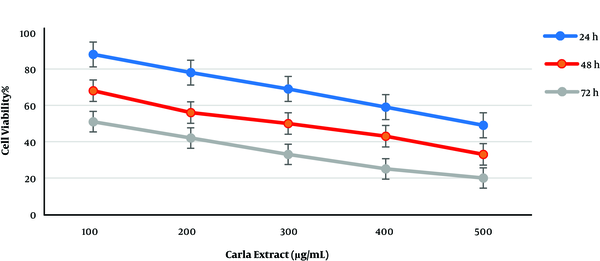
Like ephedra extract, cell viability was significantly affected when cultured MCF-7 cells were treated with various concentrations (100, 200, 300, 400, and 500 µg/mL). As shown in Figure 2, cell viability decreased at all concentrations. The increased incubation time and Carla plant extract concentration caused a reduction in cell viability when compared to other incubation times. The comparison of plant extract effects showed that Carla extract was more effective than ephedra extract with IC50 less than 100 µg/mL after 72 h of incubation. Variable IC50 values were observed for different incubation times, from 100 to 500 µg/mL of Carla extract (Figure 2).
Effect of Carla extracts on the growth of MCF-7 breast cancer cell line.
4.1.3. Resveratrol Drug
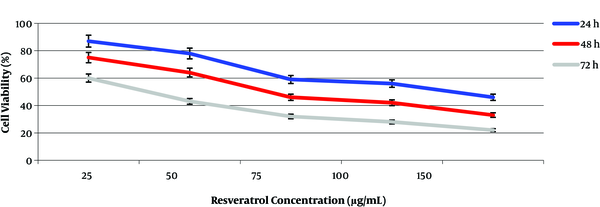
The results of cell viability performed by the MTT assay showed that the resveratrol drug had a high efficiency for cell viability at low concentrations when compared to the plant extract (Figure 3). The highest cell inhibitory effect was observed after 72 h of incubation. For this time period, the maximum and minimum growth inhibition were observed at 60 µg/mL and 20 µg/mL concentrations of resveratrol, respectively. The IC50 values for resveratrol drug were determined at 50 and 75 µg/mL of resveratrol concentrations during 72 and 48 h of incubation, respectively (Figure 3). A time-dependent relationship in cells exposed to the extract and drug occurred in our experiments.
Effect of resveratrol drug on the growth of MCF-7 breast cancer cell line.
4.1.4. Caspase-3 in Breast Cancer Cell Line Treated with Plant Extracts
The expressions of the caspase-3 gene as the target gene and the GAPDH gene as the reference gene were investigated in three groups of 24, 48, and 72 h incubation times with real-time PCR. Gene expression analysis was done on treated and non-treated MCF-7 cells in three time periods. Data obtained for non-treated cells were used as the negative control. The expression level of the caspase-3 gene increased in the range of 1.85 - 2.55 fold change at a concentration of 300 of ephedra extract. The highest increase in gene expression at this concentration was observed at 72 h time period (Figure 4). At a high concentration (400 - 500 µg/mL) of the plant extract, the expression level of the caspase-3 gene gradually decreased compared to lower concentrations.
The relative expression level of caspase-3 gene in MCF-7 breast cancer cell line treated with ephedra extract.
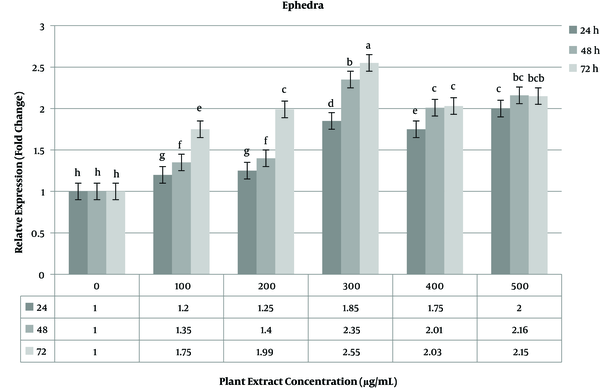
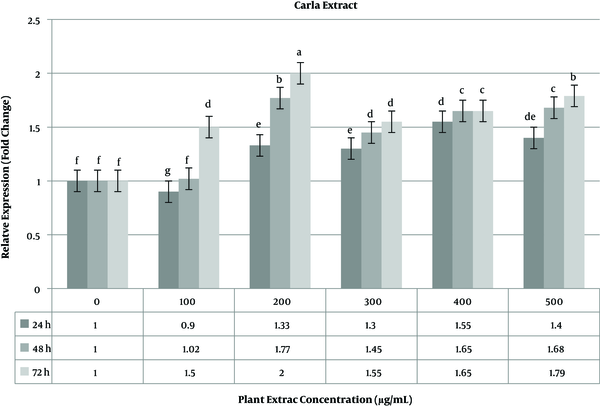
The level of caspase-3 gene expression was constantly stable (> 2 fold change) in 48 h time period. The highest expression level of the caspase-3 gene occurred in 72 h time period (Figure 4). Unlike the ephedra extract, in the Carla experiment, the highest expression level was calculated at 200 µg/mL of the plant extract. The increase in the plant extract concentration did not lead to an increase in the caspase-3 gene. As shown in Figure 5, a time-dependent relationship was observed for gene expression.
The relative expression level of caspase-3 gene in MCF-7 breast cancer cell line treated with Carla extract.
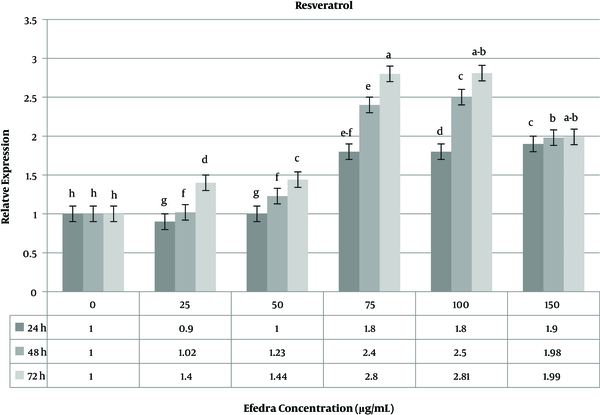
The effect of resveratrol as a drug was investigated on the MCF-7 cell line. The use of resveratrol at various concentrations showed a significant change in the expression level of the caspase-3 gene compared to untreated cells. The highest (2.81 fold change) and lowest (0.09 fold change) caspase-3 gene expressions were observed at doses of 25 µg/mL and 100 µg/mL of resveratrol, respectively. At a low resveratrol concentration, the variability of the expression level was not significant in 24 and 48 h time periods, but 72 h after treatment, the expression level of the caspase-3 gene significantly increased (Figure 6). The melting curve analysis for the caspase-3 gene used in the real-time gene expression analysis showed the high efficiency of specific primers to produce expected products (data not shown).
The relative expression level of caspase-3 gene in MCF-7 breast cancer cell line treated with resveratrol drug.
5. Discussion
Medicinal plants are traditionally used to combat diseases because they contain extensive and diverse chemical compositions, some of which have potent activity for cancer treatment. The use of drugs derived either from chemical materials or from natural products is not efficient after cancer development and progression. The use of natural products in the food chain and diet has been recommended in traditional medicine to prevent breast cancer. In this research, based on traditional experiments reviewed in the literature, we selected two plant species to test their efficiency in the MCF-7 cancer cell line.
Ephedra species have been demonstrated to be effective for the treatment of allergies, nasal congestion, and bronchial asthma, but there are a few reports available on the clinical use of ephedra in cancer patients. Carla (bitter melon) is known as the best plant for diabetes. There are some studies on testing the effectiveness of Carla extract in different cancers, but for native Iranian species, there are few studies performed on breast cancer. One-way analysis of variance (ANOVA) used for the IC50 assay indicated that the highest and lowest effects on cell viability (death) occurred 72 h after treatment. The inhibitory effect of the saffron extract on cell viability of gastric adenocarcinoma cell line (AGS) showed time and dose-dependent cell toxicity (19), which are in concordance with our results. The limits of crude extracts activity for the 50% inhibition of tumor cells have been defined to be less than 30 mg/mL (20). Anticancer activity of the methanolic extract of micro-algae showed IC50 values lower than 20 mg/mL (21). Our data showed a low IC50 (200 - 300 µg/mL), which was less than the accepted dose (30 mg/mL). This different IC50 level can be due to plant species and plant extraction methods. The high toxicity of ephedra extract could have led to these data. A reduction in cell toxicity with increased concentration and incubation time could be resulted from detoxificaion of plant extract by cell line. The plant extraction method and cancer cell types can affect cell toxicity.
The biological efficiency of plant extract on cell viability depends on multiple factors such as selected plant tissue, solvent application and concentration, the solvent to solid ratio, and extraction time period (22). The MTT assay of six plant extracts at the concentrations of 10 - 1000 μg/mL on Vero cells showed that the mode of plant extraction could influence the cell toxicity of the extract (23). Anti-proliferative effects of hydroalcoholic extract of Levisticum (stems and leaves) on MCF-7 and MDA-MB-468 cell lines showed IC50 values of 150 and 200 μg/mL for MDA-MB-468 and MCF-7 cells, respectively (24), which are approximately in agreement with our results. The chemopreventive activity of resveratrol as a natural phytoalexin against cancer has been well studied. The effect of resveratrol on cell proliferation is not similar in all breast cancer cell lines (25). The efficiency of resveratrol for 50% growth inhibition of H446 cancer cells was determined at 40 µg/mL resveratrol concentration (26), while in this work, the MCF-7 cell growth inhibition was observed at 75 µg/mL of resveratrol.
The expression levels of the caspase-3 gene have been measured in different cancer cell lines. The treatment of Hone1, AGS, HCT-116, and CL1-0 cell lines with Carla extract led to increased in caspase-3 activity (two-fold changes) (27). An increase in the caspase-3 gene (three-fold changes) was reported in treated cancer stem cells from the human colon with 125 µg/mL of methanolic extract of M. charantia (Carla) when compared to control cells (28), but in this work, the highest expression was recorded at 200 µg/mL of Carla extract after 72 h of treatment, which is in concordance with similar studies.
The evaluation of some medicinal plants for anticancer activity with three time periods, including 24, 48, and 72 h with 1.5 mg/mL plant concentrations, showed the time-dependent effect of tested plants, which is in agreement with our results (29). In HT29 cancer cells, the activity of Caspase group genes was measured by real-time PCR under treatment of Brucea javanica extract (fruit). The highest expression of these genes was observed at 100 μg/mL concentration after 24 h treatment (30) that is near to that of Carla (200 μg/mL) and resveratrol (75 - 100 μg/mL) but is not in agreement with ephedra extract (300 μg/mL). This difference can be due to the plant type (area, tissue, and species) and the method of extraction. Thus, based on these results, the aqueous extract powders of Carla and ephedra as edible vegetables appear to be a safe alternative of treatment for patients with breast cancer.
5.1. Conclusion
These results indicate that all the three tested drugs could influence cell viability, but resveratrol was more cytotoxic than the plant extracts. This high effect can be due to drug purity. In this study, we further investigated the characterization of caspase-3 gene expression response in incubated cells during three time periods. The increase in inducers did not show any increase in gene expression, and a moderate concentration was observed to induce gene expression; so, the increase in the drug could have adverse effects.
References
-
1.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29. [PubMed ID: 25559415]. https://doi.org/10.3322/caac.21254.
-
2.
Andersen KG, Christensen KB, Kehlet H, Bidstup PE. The effect of pain on physical functioning after breast cancer treatment: Development and validation of an assessment tool. Clin J Pain. 2015;31(9):794-802. [PubMed ID: 25679946]. https://doi.org/10.1097/AJP.0000000000000156.
-
3.
Shah SM, Sadiq A, Shah SM, Khan S. Extraction of saponins and toxicological profile of Teucrium stocksianum boiss extracts collected from District Swat, Pakistan. Biol Res. 2014;47:65. [PubMed ID: 25730474]. [PubMed Central ID: PMC4271446]. https://doi.org/10.1186/0717-6287-47-65.
-
4.
George P. Concerns regarding the safety and toxicity of medicinal plants-An overview. J Appl Pharm Sci. 2011;1(6):40-4.
-
5.
Wang S, Li Z, Yang G, Ho CT, Li S. Momordica charantia: A popular health-promoting vegetable with multifunctionality. Food Funct. 2017;8(5):1749-62. [PubMed ID: 28474032]. https://doi.org/10.1039/c6fo01812b.
-
6.
Dans AM, Villarruz MV, Jimeno CA, Javelosa MA, Chua J, Bautista R, et al. The effect of Momordica charantia capsule preparation on glycemic control in type 2 diabetes mellitus needs further studies. J Clin Epidemiol. 2007;60(6):554-9. [PubMed ID: 17493509]. https://doi.org/10.1016/j.jclinepi.2006.07.009.
-
7.
Rydin C, Pedersen KR, Crane PR, Friis EM. Former diversity of Ephedra (Gnetales): Evidence from Early Cretaceous seeds from Portugal and North America. Ann Bot. 2006;98(1):123-40. [PubMed ID: 16675607]. [PubMed Central ID: PMC2803531]. https://doi.org/10.1093/aob/mcl078.
-
8.
Khan A, Jan G, Khan A, Gul Jan F, Bahadur A, Danish M. In vitro antioxidant and antimicrobial activities of Ephedra gerardiana (root and stem) crude extract and fractions. Evid Based Complement Alternat Med. 2017;2017:4040254. [PubMed ID: 28491106]. [PubMed Central ID: PMC5405573]. https://doi.org/10.1155/2017/4040254.
-
9.
Zhang BM, Wang ZB, Xin P, Wang QH, Bu H, Kuang HX. Phytochemistry and pharmacology of genus Ephedra. Chin J Nat Med. 2018;16(11):811-28. [PubMed ID: 30502763]. https://doi.org/10.1016/S1875-5364(18)30123-7.
-
10.
Raza H, Ahmed I, John A, Sharma AK. Modulation of xenobiotic metabolism and oxidative stress in chronic streptozotocin-induced diabetic rats fed with Momordica charantia fruit extract. J Biochem Mol Toxicol. 2000;14(3):131-9. [PubMed ID: 10711628]. https://doi.org/10.1002/(sici)1099-0461(2000)14:3<131::aid-jbt2>3.0.co;2-q.
-
11.
Carter LG, D'Orazio JA, Pearson KJ. Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer. 2014;21(3):R209-25. [PubMed ID: 24500760]. [PubMed Central ID: PMC4013237]. https://doi.org/10.1530/ERC-13-0171.
-
12.
Nakagawa H, Kiyozuka Y, Uemura Y, Senzaki H, Shikata N, Hioki K, et al. Resveratrol inhibits human breast cancer cell growth and may mitigate the effect of linoleic acid, a potent breast cancer cell stimulator. J Cancer Res Clin Oncol. 2001;127(4):258-64. [PubMed ID: 11315261]. https://doi.org/10.1007/s004320000190.
-
13.
Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci. 2011;1215:1-8. [PubMed ID: 21261635]. https://doi.org/10.1111/j.1749-6632.2010.05870.x.
-
14.
Pitchakarn P, Ogawa K, Suzuki S, Takahashi S, Asamoto M, Chewonarin T, et al. Momordica charantia leaf extract suppresses rat prostate cancer progression in vitro and in vivo. Cancer Sci. 2010;101(10):2234-40. [PubMed ID: 20731662]. https://doi.org/10.1111/j.1349-7006.2010.01669.x.
-
15.
van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: The MTT assay. Methods Mol Biol. 2011;731:237-45. [PubMed ID: 21516412]. https://doi.org/10.1007/978-1-61779-080-5_20.
-
16.
Bahuguna A, Khan I, Bajpai VK, Kang SC. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J Pharmacol. 2017;12(2).
-
17.
Lara E, Mai A, Calvanese V, Altucci L, Lopez-Nieva P, Martinez-Chantar ML, et al. Salermide, a Sirtuin inhibitor with a strong cancer-specific proapoptotic effect. Oncogene. 2011;28(8):1168. [PubMed ID: 19060927]. https://doi.org/10.1038/onc.2008.436.
-
18.
O’Donovan N, Crown J, Stunell H, Hill ADK, McDermott E, O’Higgins N, et al. Caspase 3 in breast cancer. Clin Cancer Res. 2003;9(2):738-42.
-
19.
Bathaie SZ, Miri H, Mohagheghi MA, Mokhtari-Dizaji M, Shahbazfar AA, Hasanzadeh H. Saffron aqueous extract inhibits the chemically-induced gastric cancer progression in the wistar albino rat. Iran J Basic Med Sci. 2013;16(1):27-38. [PubMed ID: 23638290]. [PubMed Central ID: PMC3637902].
-
20.
Suffness M, Pezzuto JM. Methods in plant biochemistry: Assays for bioactivity. London, UK: Academic Press; 1990.
-
21.
Tavares-Carreon F, De la Torre-Zavala S, Arocha-Garza HF, Souza V, Galan-Wong LJ, Aviles-Arnaut H. In vitro anticancer activity of methanolic extract of Granulocystopsis sp., a microalgae from an oligotrophic oasis in the Chihuahuan desert. PeerJ. 2020;8. e8686. [PubMed ID: 32201642]. [PubMed Central ID: PMC7073244]. https://doi.org/10.7717/peerj.8686.
-
22.
Kwatra D, Subramaniam D, Ramamoorthy P, Standing D, Moran E, Velayutham R, et al. Methanolic extracts of bitter melon inhibit colon cancer stem cells by affecting energy homeostasis and autophagy. Evid Based Complement Alternat Med. 2013;2013:702869. [PubMed ID: 23533514]. [PubMed Central ID: PMC3606719]. https://doi.org/10.1155/2013/702869.
-
23.
Chan SM, Khoo KS, Sit NW. Interactions between plant extracts and cell viability indicators during cytotoxicity testing: Implications for ethnopharmacological studies. Trop J Pharm Res. 2015;14(11):1991-8.
-
24.
Lotfian Sargazi M, Saravani R, Shahraki A. Hydroalcoholic extract of Levisticum officinale increases cGMP signaling pathway by down-regulating PDE5 expression and induction of apoptosis in MCF-7 and MDA-MB-468 breast cancer cell lines. Iran Biomed J. 2019;23(4):280-6. [PubMed ID: 30388886]. [PubMed Central ID: PMC6462291].
-
25.
Pozo-Guisado E, Alvarez-Barrientos A, Mulero-Navarro S, Santiago-Josefat B, Fernandez-Salguero PM. The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: cell-specific alteration of the cell cycle. Biochem Pharmacol. 2002;64(9):1375-86. [PubMed ID: 12392819]. https://doi.org/10.1016/s0006-2952(02)01296-0.
-
26.
Li W, Li C, Ma L, Jin F. Resveratrol inhibits viability and induces apoptosis in the smallcell lung cancer H446 cell line via the PI3K/Akt/cMyc pathway. Oncol Rep. 2020;44(5):1821-30. [PubMed ID: 32901891]. [PubMed Central ID: PMC7550979]. https://doi.org/10.3892/or.2020.7747.
-
27.
Li CJ, Tsang SF, Tsai CH, Tsai HY, Chyuan JH, Hsu HY. Momordica charantia extract induces apoptosis in human cancer cells through caspase- and mitochondria-dependent pathways. Evid Based Complement Alternat Med. 2012;2012:261971. [PubMed ID: 23091557]. [PubMed Central ID: PMC3471438]. https://doi.org/10.1155/2012/261971.
-
28.
Dia VP, Krishnan HB. BG-4, a novel anticancer peptide from bitter gourd (Momordica charantia), promotes apoptosis in human colon cancer cells. Sci Rep. 2016;6:33532. [PubMed ID: 27628414]. [PubMed Central ID: PMC5024301]. https://doi.org/10.1038/srep33532.
-
29.
Solowey E, Lichtenstein M, Sallon S, Paavilainen H, Solowey E, Lorberboum-Galski H. Evaluating medicinal plants for anticancer activity. ScientificWorldJournal. 2014;2014:721402. [PubMed ID: 25478599]. [PubMed Central ID: PMC4248331]. https://doi.org/10.1155/2014/721402.
-
30.
Lou GG, Yao HP, Xie LP. Brucea javanica oil induces apoptosis in T24 bladder cancer cells via upregulation of caspase-3, caspase-9, and inhibition of NF-kappaB and COX-2 expressions. Am J Chin Med. 2010;38(3):613-24. [PubMed ID: 20503476]. https://doi.org/10.1142/S0192415X10008093.