Abstract
Background:
Uterine leiomyoma (ULs) is the most common gynecological tumor and a significant health concern for many women .The interleukin-1 receptor antagonist (IL-1Ra) is a naturally occurring cytokine inhibiting interleukin- 1 (IL-1) activity by binding to the IL-1 receptors without signal transduction.Objectives:
The aim of this study was to investigate the association between interleukin-1 receptor antagonist gene variable number of tandem repeat (VNTR) polymorphism and ULs in women of the South- East of Iran.Patients and Methods:
A total number of 99 patients with leiomyoma and 102 controls were studied. Genotyping of IL-1Ra (VNTR) polymorphism was determined by gel electrophoresis after PCR amplification. Frequency of alleles and genotypes in patients and control group was statistically analyzed using χ2 test or fisher exact test.Results:
The frequency of alleles 1, 2 and 3 of IL-1Ra VNTR polymorphism were %71, %27 and %22 in control group and %74, %20 and %6 in the ULs patients, respectively and there were no significant differences between these two groups. No statistically significant differences were observed between the frequency of IL-1Ra genotypes in the study and control groups.Conclusions:
This study showed that 86bp VNTR polymorphism of IL-1Ra gene is not associated with leiomyoma in the studied population.Keywords
Leiomyoma Uterus Interleukin 1 Receptor Antagonist Protein Polymorphism Genetic
1. Background
Uterine leiomyomas (ULs), or Uterine fibroids (UF) as a common gynecological tumor, are diagnosed in 22% of women over 35 years of age. The ULs symptoms are observed in one-third of patients and make the most common indications for hysterectomy. ULs are benign smooth muscle tumors arising from uterine myometrium smooth-muscle cells containing large amounts of extracellular matrix components such as collagen, fibronectin and proteoglycans (1, 2). Most patients with these tumors are without symptoms and the tumors grow slowly (3). ULs can be usually diagnosed by gynecologic examination, ultrasonography or hysteroscopy and MRI (4). Regardless of different investigations, including genetic and molecular studies, the molecular pathology of this complication is still unknown. Leiomyoma is a multisystem disorder and several factors might be involved in its formation, including interactions between multiple genes, hormones, growth factors, cytokines, and environment (5, 6). Like ovarian and breast cancer, leiomyoma isaffected by autocrine and paracrine interactions of sex-steroid hormones. The development of leiomyoma was found to be estrogen dependent and elevated estrogen levels (e.g. in pregnancy) could affect the tumor size (7, 8).
Several evidences have indicated that immunological and inflammatory processes may play a role in tumor development. Moreover elevated levels of cytokines have been observed in uterine cavity containing leiomyoma compared to normal uterus (9, 10). The genetic factors contributing to the leiomyoma development have been investigated and the possible effects of cytokine gene polymorphisms in the leiomyoma pathogenesis has been suggested in several studies (11-13). Interleukin-1 (IL-1) as a pro-inflammatory cytokine has widespread biological activities and plays an essential role in inflammatory and immune diseases. This cytokine is regulated with interleukin-1 receptor antagonist (IL-1Ra) which binds to IL-1 receptors and blocks the biological activity of IL-1. The IL-1Ra (IL1RN) gene is located on chromosome 2, band q12–21 (14).
There are several polymorphisms in IL-1Ra gene but a variation in repeats of an 86bp variable number of tandem repeat (VNTR) polymorphism in intron 2, is the most studied one. five alleles have been reported for the IL-1Ra gene VNTR polymorphism: allele 1, four repeats; allele 2, two repeats; allele 3, five repeats; allele 4, three repeats and allele 5, six repeats (15). Moreover IL-1Ra alleles were divided into two groups: long genotypes (L: alleles 1, 3, 4, and 5) and short genotype (S: allele 2). The genotypes were classified as LL, 2L, and 22 (16).
2. Objectives
Since few reports are available about the association between IL-1Ra and leiomyoma a case-control study was conducted to investigate the relationship between IL-1Ra VNTR-polymorphism and leiomyoma in Iranian patients.
3. Patients and Methods
A total number of 99 women with clinically diagnosed uterine leiomyoma undergoing surgical intervention at the Department of Gynecology of the Zahedan University of Medical Sciences were included to the study between 2012 and 2013. Leiomyoma were diagnosed by detailed sonographic examination and confirmed by histological examination after hysterectomy or myomectomy. 102 age and BMI matched volunteers without leiomyoma recruited for the control group. Non-leiomyoma status was diagnosed after detailed sonographic examination. Blood samples were collected in 2 mL Na-EDTA tubes from patients and healthy controls. The genomic DNA was extracted from peripheral blood leukocytes according to salting out method. Quality and quantity of the extracted DNA were then determined using spectrophotometry (Biophotometer, Eppendrof, Germany). Two oligonucleotide primers forward: 5'- CTC AGC AAC ACT CCT AT -3' and reverse: 5-' TTC CAC CAC ATG GAA C -3' based on flanking region of the IL-1Ra gene were used to amplify the corresponding DNA fragments by polymerase chain reaction (PCR). PCR was performed in a 25 μL final volume containing 25 pmol of each primer, 0.1 mmol of dNTP (Fermentas, Lithuania), 0.5 μg of genomic DNA, 1.5 mmol/L of MgCl2, 2.5 μL of PCR buffer and 1.5 unit of Taq DNA polymerase (Fermentas, Lithuania) according to the following protocol: initial denaturation at 94ºC for 4 min; 30 cycles of denaturation at 94ºC for 45 s, annealing at 51ºC for 30 s, extension at 72ºC for 45 s; and final extension at 72ºC for 5 minutes. PCR products were separated by electrophoresis on a 2.5% agarose gel and visualized by ethidium bromide staining. The IL-1Ra alleles were described as follow: allele 1, four repeats; allele 2, two repeats; allele 3, five repeats; allele 4, three repeats and allele 5, six repeats (Figure 1).
1. Electrophoretic Pattern of PCR Amplified Products of IL-1 Ra Gene VNTR Polymorphism: M, 50 bp DNA Marker
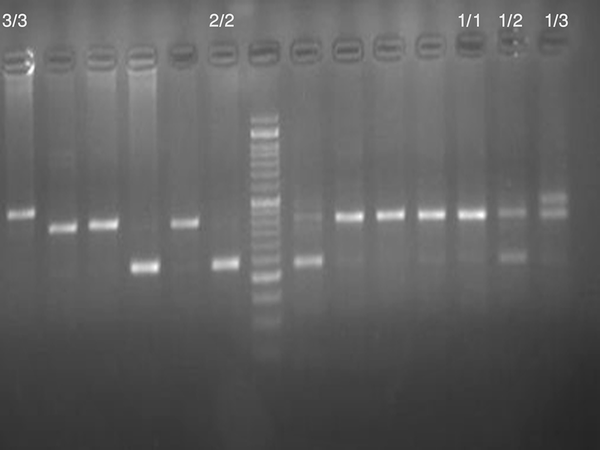
3.1. Statistical Analysis
Data were analyzed using the statistical software SPSS V. 18 (SPSS, Chicago, IL). Direct gene counting method was used to determine the frequency of genotypes and alleles. The χ2 test or Fisher's exact test was used to determine differences in allele and genotype frequencies of IL-1Ra polymorphism. The odds ratio (OR) and 95% confidence intervals (CI) were also estimated. Variations less than 0.05 were considered statistically significant.
4. Results
Demographic data of patients with leiomyoma and control group are shown in Table 1.
| Patients (n = 99) | Controls (n = 102) | P Value | |
|---|---|---|---|
| Age, y | 38.5 ± 9.8 | 36.7 ± 5.5 | NS |
| Ethnicity | |||
| Fars | 46 (46) | 50 (49) | NS |
| Balouch | 53 (54) | 52 (51) | NS |
| Marriage status | 98 (99) | 100 (98) | NS |
| BMI, kg/m2 | 25.3 ± 4.8 | 25.4 ± 4.7 | NS |
| Age of menarche, y | 13.1 ± 1.5 | 13.5 ± 1.1 | NS |
| Duration of menses, d | 6.6 ± 2.7 | 5.8 ± 1.5 | NS |
| Menstrual cycle, d | 28.6 ± 2.3 | 29.1 ± 3.4 | NS |
| Bleeding | 57 (58) | 6 (6) | 0.0001 |
| Pain | 28 (28) | 8 (8) | 0.0001 |
There were no significant differences in age, BMI and marriage status between two groups. No significant differences in menstrual histories, like age of menarche, duration of menses and menstrual cycle were found among the control and disease groups. However the frequency and intensity of bleeding and pain were significantly higher in patients (P < 0.0001). Genotype and allele frequency of IL1Ra VNTR polymorphism are shown in Table 2. Six genotypes (1/1, 1/2, 1/3, 2/2, 2/3 and 3/3) of IL1Ra VNTR polymorphism were observed in UF group, however five genotypes of them were also found in control subjects (except 3/3 genotype). Although the frequency of 2/2 genotype was higher in control group, which was not statistically significant (P = 0.08). The frequency of alleles 1(four repeats), 2(two repeats) and 3(five repeats) were 74, 20 and six percent in UF patients and 75, 22 and three percent in healthy controls respectively and not statistically different. Alleles 4 (three repeats) and 5 (six repeats) were not observed in the study population. There were no alleles and genotype differences detected, moreover no allele frequency differences were observed between two ethnic groups (P > 0.05)
Genotypes and Alleles Frequency of IL-1Ra Gene VNTR Polymorphism in Leiomyoma Patients and Control Group a
| Patients (n = 99) | Controls (n = 102) | P Value | Odds Ratio (95% CI) | |
|---|---|---|---|---|
| Genotype | ||||
| 1/1 | 58 (58.6) | 61 (60.4) | 1 | |
| 1/2 | 24 (24.2) | 18 (17.8) | 0.23 | 1.4 (0.7 - 2.9) |
| 1/3 | 6 (6.1) | 5 (5) | 0.5 | 1.3 (0.4 - 4.4 ) |
| 2/2 | 7 (7.1) | 16 (15.8) | 0.08 | 0.5 (0.2 - 1.2) |
| 2/3 | 2 (2) | 1 (1) | 0.5 | 2.1 (0.2 - 23.8) |
| 3/3 | 2 (2) | 0 (0) | 0.2 | 2 (1.7 - 2.5) |
| Allele | ||||
| 1 | 146 (74) | 145 (72) | 1 | |
| 2 | 40 (20) | 51 (25) | 0.2 | 0.8 (0.5 - 1.3) |
| 3 | 12 (6) | 6 (3) | 0.13 | 2 (0.7 - 5.4) |
5. Discussion
According to the findings of the present study, the frequency of the allele 2 of IL-1Ra VNTR polymorphism was lower in patients with leiomyoma than healthy controls (25% vs. 20%); however the difference was not significant. Cytokines are proteins playing key roles in the links between the immunological systems and compromised tissues. A number of cytokines and growth factors, including the ILs family, were investigated in the myometrium and leiomyoma and significantly elevated concentration of serum IL-1 was found in ULs patients (17). Inagaki et al. indicated that these cytokines may increase matrix metalloproteinase production, which may result in leiomyoma development stimulation (6). IL-1Ra has anti- inflammatory effects and prevents IL-1 signaling pathway by binding to IL-1 receptors and blocking its biological activity. Some evidences indicated that IL-1Ra may affect the host immune responses in the local and general environments of gynecological cancers (18). Moreover IL-1Ra could decrease tumor growth and angiogenesis (19). Altered IL1-Ra expression levels have been found to correlate with several types of tumors, like endometrial cancer (20) and gastric carcinoma (21). Since IL-1Ra belongs to the IL-1 family (IL-1α, IL-1β, and IL-1Ra) which consist of three linked genes, IL-1 family polymorphisms are located very close to each other. Therefore even mutation in only one of the three genes could alter their expression. Also, different IL-1Ra variants play major roles in IL-1 modulating (22).
There is a 86bp VNTR polymorphism in intron2 of IL-Ra gene, which contains three potential protein binding sites: an interferon α silencer A, an interferon β silencer B and an acute phase response element. It has been reported that the control of cell proliferation activity, was affected by IL-1Ra production through these three binding sites (23). Among the five alleles of IL-1Ra VNTR polymorphism, allele 2 seems to play an important role in the molecular basis of different diseases and autoimmune conditions. Furthermore, increased circulating IL-1Ra and even more elevated IL1β have been observed in individuals with IL-1Ra allele 2 (24). Recently an association between IL-1 family polymorphisms and leiomyoma has been investigated. There is only one published report about the association between IL-1Ra VNTR polymorphism and ULs by Hsieh et al. in 2007. In consistent to the present study, they observed no association between IL-1Ra VNTR polymorphism and ULs in Taiwan, however they reported a relation between G allele of IL-12Rbeta1 codon 378 and leiomyoma susceptibility (11) Pietrowski et al. reported an association between IL-1β-511C allele and leiomyomas in Austria (12). Taghizade-Mortezaee et al. demonstrated a significant relationship between IL-1ß-511C >T polymorphism and increased risk of uterine leiomyomas in Chaharmahal and Bakhtiari province of Iran (25). Other studies have examined the association between IL-1Ra VNTR polymorphism and endometriosis too. Chen et al. in Korea (26) and Hsieh in Taiwan (27) revealed no relation between IL-1Ra VNTR polymorphism and endometriosis. Nevertheless Wen et al. in china indicated that the allele2 of IL-1Ra gene may be a risk factor for endometriosis in the Chinese women (28). These different results are common in association studies are and may be due to different genetic backgrounds of various populations.
There were many limitations to this study, likelow sample size, environmental conditions and different ethnic groups (Balouch and Fars) existing in Southeast of Iran. Therefore due to the relatively small number of patients with leiomyoma and the racial differences, further investigations using larger sample sizes are necessary confirm the present findings. In conclusion, the present study showed no association between IL-1Ra gene polymorphism and leiomyoma in Southeast of Iran and consequently this polymorphism is not a valuable marker for the prediction of leiomyoma.
Acknowledgements
References
-
1.
Marino JL, Eskenazi B, Warner M, Samuels S, Vercellini P, Gavoni N, et al. Uterine leiomyoma and menstrual cycle characteristics in a population-based cohort study. Hum Reprod. 2004;19(10):2350-5. [PubMed ID: 15242998]. https://doi.org/10.1093/humrep/deh407.
-
2.
Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87(4):725-36. [PubMed ID: 17430732]. https://doi.org/10.1016/j.fertnstert.2007.01.093.
-
3.
Cook JD, Walker CL. Treatment strategies for uterine leiomyoma: the role of hormonal modulation. Semin Reprod Med. 2004;22(2):105-11. [PubMed ID: 15164305]. https://doi.org/10.1055/s-2004-828616.
-
4.
Evans P, Brunsell S. Uterine fibroid tumors: diagnosis and treatment. Am Fam Physician. 2007;75(10):1503-8. [PubMed ID: 17555142].
-
5.
Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111(8):1037-54. [PubMed ID: 12826476].
-
6.
Inagaki N, Ung L, Otani T, Wilkinson D, Lopata A. Uterine cavity matrix metalloproteinases and cytokines in patients with leiomyoma, adenomyosis or endometrial polyp. Eur J Obstet Gynecol Reprod Biol. 2003;111(2):197-203. [PubMed ID: 14597251].
-
7.
Sumitani H, Shozu M, Segawa T, Murakami K, Yang HJ, Shimada K, et al. In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology. 2000;141(10):3852-61. [PubMed ID: 11014242]. https://doi.org/10.1210/endo.141.10.7719.
-
8.
Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595-602. [PubMed ID: 11129693].
-
9.
Malyshkina AI, Posiseeva LV, Sotnikova NY, Antsiferova JS, Suvorkina EE, Arevadze IE. Local immunological markers of different rate of growth of uterine myoma. Russ J Immunol. 2002;7(1):58-62. [PubMed ID: 12687267].
-
10.
Yun AJ, Daniel SM. Sympathetic and T helper (Th)2 bias may ameliorate uterine fibroids, independent of sex steroids. Med Hypotheses. 2005;65(6):1172-5. [PubMed ID: 16213989]. https://doi.org/10.1016/j.mehy.2005.03.015.
-
11.
Hsieh YY, Chang CC, Tsai CH, Lin CC, Tsai FJ. Interleukin (IL)-12 receptor beta1 codon 378 G homozygote and allele, but not IL-1 (beta-511 promoter, 3953 exon 5, receptor antagonist), IL-2 114, IL-4-590 intron 3, IL-8 3'-UTR 2767, and IL-18 105, are associated with higher susceptibility to leiomyoma. Fertil Steril. 2007;87(4):886-95. [PubMed ID: 17222831]. https://doi.org/10.1016/j.fertnstert.2006.07.1541.
-
12.
Pietrowski D, Thewes R, Sator M, Denschlag D, Keck C, Tempfer C. Uterine leiomyoma is associated with a polymorphism in the interleukin 1-beta gene. Am J Reprod Immunol. 2009;62(2):112-7. [PubMed ID: 19614627]. https://doi.org/10.1111/j.1600-0897.2009.00718.x.
-
13.
Sosna O, Kolesar L, Slavcev A, Skibova J, Fait T, Mara M, et al. Th1/Th2 cytokine gene polymorphisms in patients with uterine fibroid. Folia Biol (Praha). 2010;56(5):206-10. [PubMed ID: 21138652].
-
14.
Patterson D, Jones C, Hart I, Bleskan J, Berger R, Geyer D, et al. The human interleukin-1 receptor antagonist (IL1RN) gene is located in the chromosome 2q14 region. Genomics. 1993;15(1):173-6. [PubMed ID: 8432529]. https://doi.org/10.1006/geno.1993.1025.
-
15.
Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27-55. [PubMed ID: 9597123]. https://doi.org/10.1146/annurev.immunol.16.1.27.
-
16.
Santtila S, Savinainen K, Hurme M. Presence of the IL-1RA allele 2 (IL1RN*2) is associated with enhanced IL-1beta production in vitro. Scand J Immunol. 1998;47(3):195-8. [PubMed ID: 9519856].
-
17.
Sikorski R, Kapec E, Zaleska W. [Serum levels of proinflammatory cytokines in women with uterine myomas]. Ginekol Pol. 2001;72(12A):1485-8. [PubMed ID: 11883301].
-
18.
Irvine KR, Rao JB, Rosenberg SA, Restifo NP. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J Immunol. 1996;156(1):238-45. [PubMed ID: 8598468].
-
19.
Corbacioglu S, Pietsch T, Koenig A, Welte K. Interleukin-1 receptor antagonist: a powerful inhibitor of tumor vascularization and tumor growth in vivo. Anderson Cancer Center. 1994;6:35.
-
20.
Van Le L, Haskill S, Jaffe GJ, Fowler WC, Jr. Expression of interleukin-1 and interleukin-1 receptor antagonists in endometrial cancer. Gynecol Oncol. 1991;42(2):161-4. [PubMed ID: 1832651].
-
21.
Iizuka N, Hazama S, Hirose K, Abe T, Tokuda N, Fukumoto T, et al. Interleukin-1 receptor antagonist mRNA expression and the progression of gastric carcinoma. Cancer Lett. 1999;142(2):179-84. [PubMed ID: 10463774].
-
22.
Vamvakopoulos J, Green C, Metcalfe S. Genetic control of IL-1beta bioactivity through differential regulation of the IL-1 receptor antagonist. Eur J Immunol. 2002;32(10):2988-96. [PubMed ID: 12355453]. https://doi.org/10.1002/1521-4141(2002010)32:10<2988::AID-IMMU2988>3.0.CO;2-9.
-
23.
Tarlow JK, Blakemore AI, Lennard A, Solari R, Hughes HN, Steinkasserer A, et al. Polymorphism in human IL-1 receptor antagonist gene intron 2 is caused by variable numbers of an 86-bp tandem repeat. Hum Genet. 1993;91(4):403-4. [PubMed ID: 8500797].
-
24.
Witkin SS, Gerber S, Ledger WJ. Influence of interleukin-1 receptor antagonist gene polymorphism on disease. Clin Infect Dis. 2002;34(2):204-9. [PubMed ID: 11740709]. https://doi.org/10.1086/338261.
-
25.
Taghizade-Mortezaee F, Hashemzadeh-Chaleshtori M, Kheiri S, Parvin N, Norbakhsh M, Etemadi S, et al. Association of Interleukin-1β (IL-1β) Gene Polymorphisms with Uterine Leiomyoma. Zahedan J Res Med Sci. 2012;14(7):53-6.
-
26.
Chun S, Kim H, Ku SY, Suh CS, Kim SH, Kim JG. The association between endometriosis and polymorphisms in the interleukin-1 family genes in Korean women. Am J Reprod Immunol. 2012;68(2):154-63. [PubMed ID: 22509941]. https://doi.org/10.1111/j.1600-0897.2012.01136.x.
-
27.
Hsieh YY, Chang CC, Tsai FJ, Wu JY, Shi YR, Tsai HD, et al. Polymorphisms for interleukin-1 beta (IL-1 beta)-511 promoter, IL-1 beta exon 5, and IL-1 receptor antagonist: nonassociation with endometriosis. J Assist Reprod Genet. 2001;18(9):506-11. [PubMed ID: 11665666].
-
28.
Wen J, Deng L, Zhang XM. [Research on relationship between gene polymorphisms of interleukin-1 family and endometriosis]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2006;35(6):653-7. [PubMed ID: 17177339].
