Abstract
Background:
Cerebral ischemia and reperfusion (I/R) is a pathological condition that arises by reduction or cessation in cerebral blood flow and return of oxygen and metabolites to brain cells, which cause oxidative damage.Objectives:
The aim of this study was to investigate the neuroprotective effects of Withania coagulans (WC) extract on brain cortex in a rat model of I/R.Materials and Methods:
Thirty-two adult male Wistar rats weighing 280 - 300 g were used in this study. Animals were randomly divided to four groups (n = 8) as follow: sham operated group (I), I/R group (II), WCE500 + I/R (III) and WCE1000 + I/R groups (IV). Pretreatment with WC extract (500, 1000 mg/kg) was done by oral gavage for 30 days and global brain ischemia was induced by the common carotid occlusion for 30 minutes. After 72 hours, the animals were perfused transcardially and then the brains were prepared for histological study (H & E and TUNEL staining).Results:
The I/R group showed a significant increase in pycnotic (dying) neurons and pretreatment with WC at doses of 500 mg/kg and 1000 mg/kg significantly reduced pycnotic and TUNEL positive neurons, in a dose dependent manner in ischemic brain cortex.Conclusions:
Our findings indicated that WC has neuroprotective effects and is able to reduce histopathological alterations and apoptosis in brain cortex I/R in rats.Keywords
1. Background
Brain stroke is a pathological condition, which leads to disability or mortality in human beings (1). It is caused by cessation or reduction of blood flow in parts or the entire brain that cause oxygen and glucose deprivation of cerebral tissue (2). Initiation of perfusion due to free radical production, promotes tissue damage, inflammation and apoptosis (3). Nowadays, neuroprotective strategies to prevent, inhibit or diminish brain ischemia complications are concentrated in neuroscience investigations. Many natural product derivatives of plants possess antioxidant and anti-inflammatory effects and are used for treatment of neurological disorders like parkinson, alzheimer and brain ischemia (4-6). Withania coagulans (WC) belongs to the Solanaceae family; also known as “paneerbad’’, it has been used in traditional medicine in Iran (7). In vivo and in vitro studies have demonstrated that WC extract has anti-inflammatory, antioxidant and neuroprotective effects (8).
Withanolides are categories of alkaloids, which are the most bioactive constitutes of WC. The major withanolides in WC are withaferin A and withanolide A (8).
Withania and withanolides have been shown to act as potent inhibitors of pro-inflammatory transcription factors like nuclear factor-kappa B (NF-κB) (9).
Wube et al. showed the inhibitory effect of WC on cyclooxygenase-2 (COX-2) (10). Also it has been reported that withanolides from WC have neuroprotective effects in alzheimer’s disease and its associated problems (11, 12). Mohanty et al. demonstrated that withania has protective effects against myocardial ischemia-reperfusion injury (13). Jain et al. studied neuroprotective and antiapoptotic effects of withania in hippocampus of stressed rats (14).
2. Objectives
In the present study we investigated the protective role of extracts of WC in preventing apoptosis and histopathological alterations in rat’s brain cortex following ischemia and reperfusion (I/R).
3. Materials and Methods
Roots of WC plant were collected in May 2014 from the herbarium center of Sistan and Baluchestan university. The shade dried and ground roots of WC were extracted with a mixture of distilled water and ethanol (1:3, v/v) for 24 hours at room temperature. After filtration, the solvent was dried by rotary evaporation and the resulting crude extracts were stored at -20°C.
3.1. Animals
A total of 32 adult male Wistar rats (280 - 300 g) supplied by the laboratory animal research center of Zahedan University of Medical Sciences were used. The rats were kept in standard cages at a temperature of 22°C, humidity (40% - 60%), and light period-controlled (07.00 - 19.00 hours) environment with free access to food and water. Experimental procedures were confirmed by the ethics committee of Zahedan University of Medical Sciences and national institute of health (NIH) guide-lines for the care and use of laboratory animals (EC/93-8613). The animals were randomly divided to four groups (n = 8) and received vehicle or extract by oral gavage for 30 days:
Sham: received distilled water and surgical process without bilateral common carotid occlusion (BCCO).
Ischemia/reperfusion (I/R): received distilled water and BCCO.
I/R + WC500: received WC extract 500 mg/kg and BCCO.
I/R + WC1000: received WC extract 1000 mg/kg and BCCO.
Two hours after the last dose of the administration of the extract or vehicle, ischemia was induced by bilateral common carotid artery occlusion for 30 minutes (15) under anesthesia with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (8 mg/kg) mixture.
Seventy-two hours after ischemia, the animals were deeply anesthetized and transcardially perfused with paraformaldehyde (4%) in phosphate buffered saline (PBS). Next, brains were removed and post-fixed in the same fixator, overnight. After dehydration in graded concentration of ethanol, tissues were embedded in paraffin and then cut into 5-µm thick serial sections. A histopathological study was done on the sections stained with hematoxylin and eosin (H & E).
To detect apoptotic cell death, sections were processed for terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling (TUNEL) staining using an in situ cell death detection kit (Roche Molecular Biochemicals kit, Germany). Briefly, tissue sections were deparaffinised and treated with proteinase K solution. To block endogenous peroxidase activity, tissue sections were incubated in 3% H2O2 for ten minutes, re-incubated in TUNEL reaction buffer for ten minutes, then incubated in TUNEL reaction mixture for one hour at 37 - 40°C. Finally, sections were incubated with diaminobanzidine (DAB) substrate for one to two minutes to visualize apoptotic cells and counterstained with hematoxylin for 30 seconds. Apoptotic cells were observed under a light microscope with 400X magnification (Olympus, Hamburg, Germany). Four microscopic fields at 400X in five sections in each animal were captured and the numbers of pycnotic neurons in hematoxylin and eosin and apoptotic neurons in TUNEL staining were counted. Cell counts from the cortex on each of the five sections were averaged to provide the mean value (16).
3.2. Statistical Analysis
All Data were presented as means ± standard error of the mean (SEM). One way analysis of variance (ANOVA) and Tukey post hoc test was used for analysis of data between different groups. P values of < 0.05 were considered significant.
4. Results
4.1. Histological Assessment
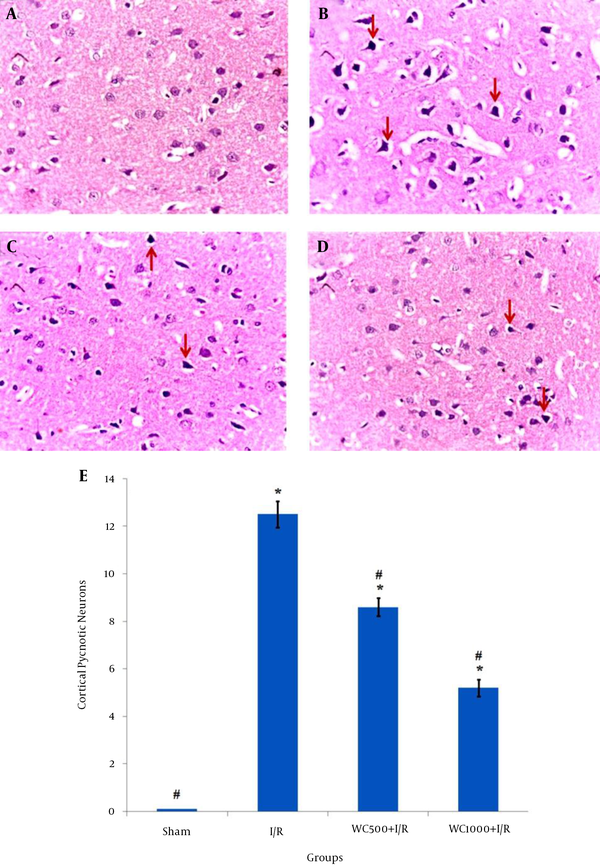
The histopathological results of the H & E staining are shown in Figure 1. As shown by Figure 1A, no histopathological abnormalities were observed in the sham group. In contrast, in the I/R group, perivascular edema was observed and most neurons were observed as shrunken with triangulated pycnotic nucleus, and wided perinuclear space (12.5 ± 0.56) (Figure 1B). In both pretreatment groups with WC, the pycnotic neurons in brain cortex markedly decreased (Figure 1C and Figure 1D) (P < 0.001). The number of these neurons in the pretreatment group with a dose of 1000 mg/kg was less (5.25 ± 0.36) than the 500 mg/kg dose (8.62 ± 0.36) (Figure 1E).
Effects of Withania coagulans Extract (WCE) on Ischemia/Reperfusion in the Brain Cortex of Different Groups
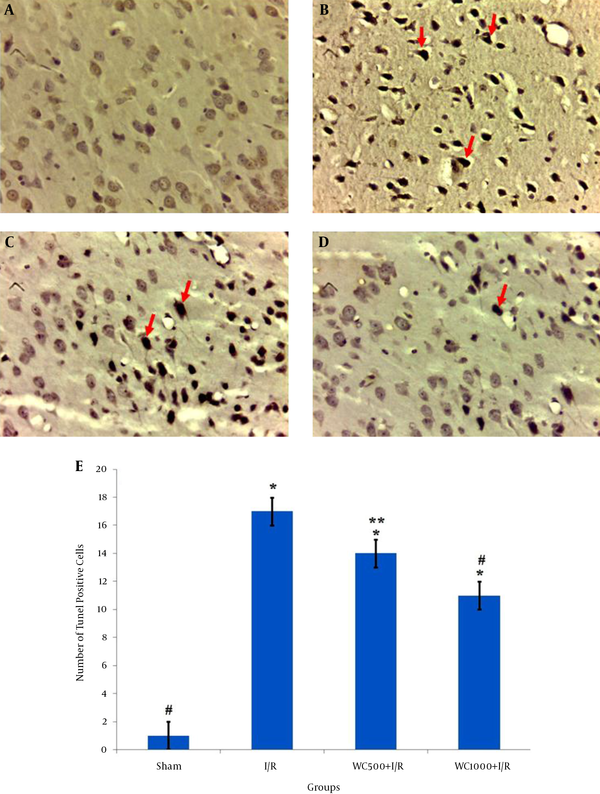
The TUNEL staining results are shown in Figure 2 ; TUNEL-positive neurons were very rarely detected in the sham group (Figure 2A). The number of apoptotic neurons remarkedly increased in the I/R group (17.8 ± 0.71) compared to the sham group (1 ± 0.16) (Figure 2B,2E ). Furthermore, TUNEL-positive cells in the WC 1000 mg/kg pretreated group was significantly lower (11 ± 1.3) than that of the WC 500 mg/kg (14.25 ± 0.95) compared to I/R group (P < 0.001) (2C-E).
Effects of Withania coagulans Extract (WCE) on Ischemia-Induced Apoptosis in the Brain Cortex of Different Groups
5. Discussion
The results of the present study show that the WC extract reduced histopathological changes in the brain cortex of the rat model of I/R. Furthermore, we found that pretreatment with WC extract can decrease apoptotic neurons after I/R. After reperfusion ischemia, production of free radicals in tissue increases, which leads to tissue damages and cell death (17).
Despite numerous defense mechanisms, the brain tissue is very sensitive to oxidative damages caused by I/R. Ischemia-induced histopathological alterations in the brain are prominent in the cortex, hippocampus and striatum regions (18). According to the report of Margaritescu et al. dark basophilic and shrunken nucleus with eosinophilic cytoplasm is the earliest signs of neuronal injury in the brain cortex (19).
In line with our results, Sarshoori et al. also showed that pycnotic neurons increased in the brain cortex after 24 hours of I/R (16). Some studies have shown that natural products of plant origin can prevent neuronal cell death (4, 20, 21). Withania coagulans has many properties, such as anti-inflammatory, antiapoptotic and neuroregeneration effects (8). Accumulating experimental evidence showed that withanolides of WC had antioxidant properties (22). Prasad et al. showed that WC extracts increased catalase and superoxide dismutase enzymes in diabetic rats (23). Another study showed that treatment with WC extract increases total antioxidant capacity (TAC) and decreases malondialdehyde (MDA) in benign prostatic hyperplasia in rats (24) According to previous studies, antioxidant therapy can attenuate side effects of ischemia reperfusion (25-27).
The results of our study showed that WC extract administration before ischemia was able to reduce the pycnotic nucleus and widened the perinuclear space in the parietal brain cortex, which is in agreement with the findings of Budhiraja et al. that showed cytoprotective effects of WC extract against CCL4-induced hepatotoxicity in rats (28). Another study showed that pretreatment with withania at a dose of 1000 mg/kg significantly improved the neurological deficit in rats (12).
The results of our study are in agreement with previous studies, which indicated that WC extract at a dose of 1000 mg/kg for 30 days leads to improved histopathological outcomes (12, 27).
Several studies indicated that, withaferin A purified from WC had an anti-inflammatory effect and could reduce tumor necrosis factor-alpha (TNF-α) and inhibit NF-κB activity (29, 30).
Evidences have shown that proinflammatory cytokines such as TNF-α have important roles in the pathogenesis of ischemic brain damage (31).
Experimental evidences suggest that attenuation of inflammation may reduce the progression of ischemia-induced brain damage (32, 33). In the present study, the beneficial effect of WC may be because of anti-inflammatory effects and the ameliorating of free radicals by improved anti-oxidant defense. We also showed that pretreatment with WC decreased TUNEL-positive cells in the brain cortex. Mohanty et al. also stated that WC extract pretreatment protected myocardial cells from apoptotic death in myocardial reperfusion. They proposed that this process could be exerted via upregulation of the expression of Bcl-2 and down-regulation of the expression of Bax (13).
In another study it was shown that WC extract with inhibition of excessive influx of Ca2+ in neuronal cells could protect them from apoptosis (34). We showed, in our previous study, that TUNEL-positive cells were markedly reduced in the hippocampal CA1 region of ischemic rats when pretreated with WC extract (27). These findings reveal the protective actions of WC extract against ischemia-induced apoptosis and cell injury.
The results of our study showed that the Withania coagulans extract significantly attenuates ischemia/reperfusion-induced brain cortex apoptosis and histopathological alterations.
Acknowledgements
References
-
1.
Chacon MR, Jensen MB, Sattin JA, Zivin JA. Neuroprotection in cerebral ischemia: emphasis on the SAINT trial. Curr Cardiol Rep. 2008;10(1):37-42. [PubMed ID: 18416999].
-
2.
Mahajan SK, Kashyap R, Sood BR, Jaret P, Mokta J, Kaushik NK, et al. Stroke at moderate altitude. J Assoc Physicians India. 2004;52:699-702. [PubMed ID: 15839445].
-
3.
Janardhan V, Qureshi AI. Mechanisms of ischemic brain injury. Curr Cardiol Rep. 2004;6(2):117-23. [PubMed ID: 14759356].
-
4.
Pasban-Aliabadi H, Esmaeili-Mahani S, Sheibani V, Abbasnejad M, Mehdizadeh A, Yaghoobi MM. Inhibition of 6-hydroxydopamine-induced PC12 cell apoptosis by olive (Olea europaea L.) leaf extract is performed by its main component oleuropein. Rejuvenation Res. 2013;16(2):134-42. [PubMed ID: 23394606]. https://doi.org/10.1089/rej.2012.1384.
-
5.
Durairajan SS, Liu LF, Lu JH, Chen LL, Yuan Q, Chung SK, et al. Berberine ameliorates beta-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer's disease transgenic mouse model. Neurobiol Aging. 2012;33(12):2903-19. [PubMed ID: 22459600]. https://doi.org/10.1016/j.neurobiolaging.2012.02.016.
-
6.
Zhao J, Yu S, Zheng W, Feng G, Luo G, Wang L, et al. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem Res. 2010;35(3):374-9. [PubMed ID: 19774461]. https://doi.org/10.1007/s11064-009-0065-y.
-
7.
Beigomi M, Mohammadifar MA, Hashemi M, rohani MG, Senthil K, Valizadeh M. Biochemical and rheological characterization of a protease from fruits of Withania coagulans with a milk-clotting activity. Food Sci Biotechnol. 2014;23(6):1805-13. https://doi.org/10.1007/s10068-014-0247-5.
-
8.
Khodaei M, Jafari M, Noori M. Remedial use of withanolides from Withania coagolans (Stocks) Dunal. Adv Life Sci. 2012;2(1):6-19. https://doi.org/10.5923/j.als.20120201.02.
-
9.
Kaileh M, Vanden Berghe W, Heyerick A, Horion J, Piette J, Libert C, et al. Withaferin a strongly elicits IkappaB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J Biol Chem. 2007;282(7):4253-64. [PubMed ID: 17150968]. https://doi.org/10.1074/jbc.M606728200.
-
10.
Wube AA, Wenzig EM, Gibbons S, Asres K, Bauer R, Bucar F. Constituents of the stem bark of Discopodium penninervium and their LTB4 and COX-1 and -2 inhibitory activities. Phytochemistry. 2008;69(4):982-7. [PubMed ID: 18086481]. https://doi.org/10.1016/j.phytochem.2007.11.001.
-
11.
Patil SP, Maki S, Khedkar SA, Rigby AC, Chan C. Withanolide A and asiatic acid modulate multiple targets associated with amyloid-beta precursor protein processing and amyloid-beta protein clearance. J Nat Prod. 2010;73(7):1196-202. [PubMed ID: 20553006]. https://doi.org/10.1021/np900633j.
-
12.
Choudhary MI, Nawaz SA, ul-Haq Z, Lodhi MA, Ghayur MN, Jalil S, et al. Withanolides, a new class of natural cholinesterase inhibitors with calcium antagonistic properties. Biochem Biophys Res Commun. 2005;334(1):276-87. [PubMed ID: 16108094].
-
13.
Mohanty I, Arya DS, Dinda A, Talwar KK, Joshi S, Gupta SK. Mechanisms of cardioprotective effect of Withania somnifera in experimentally induced myocardial infarction. Basic Clin Pharmacol Toxicol. 2004;94(4):184-90. [PubMed ID: 15078343]. https://doi.org/10.1111/j.1742-7843.2004.pto940405.x.
-
14.
Jain S, Shukla SD, Sharma K, Bhatnagar M. Neuroprotective effects of Withania somnifera Dunn. in hippocampal sub-regions of female albino rat. Phytother Res. 2001;15(6):544-8. [PubMed ID: 11536389].
-
15.
Yigitkanli K, Pekcec A, Karatas H, Pallast S, Mandeville E, Joshi N, et al. Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Ann Neurol. 2013;73(1):129-35. [PubMed ID: 23192915]. https://doi.org/10.1002/ana.23734.
-
16.
Sarshoori JR, Asadi MH, Mohammadi MT. Neuroprotective effects of crocin on the histopathological alterations following brain ischemia-reperfusion injury in rat. Iran J Basic Med Sci. 2014;17(11):895-902. [PubMed ID: 25691932].
-
17.
Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, et al. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14(8):1505-17. [PubMed ID: 20812869]. https://doi.org/10.1089/ars.2010.3576.
-
18.
Gaur V, Aggarwal A, Kumar A. Protective effect of naringin against ischemic reperfusion cerebral injury: possible neurobehavioral, biochemical and cellular alterations in rat brain. Eur J Pharmacol. 2009;616(1-3):147-54. [PubMed ID: 19577560]. https://doi.org/10.1016/j.ejphar.2009.06.056.
-
19.
Margaritescu O, Mogoanta L, Pirici I, Pirici D, Cernea D, Margaritescu C. Histopathological changes in acute ischemic stroke. Rom J Morphol Embryol. 2009;50(3):327-39. [PubMed ID: 19690757].
-
20.
Chandrashekhar VM, Ranpariya VL, Ganapaty S, Parashar A, Muchandi AA. Neuroprotective activity of Matricaria recutita Linn against global model of ischemia in rats. J Ethnopharmacol. 2010;127(3):645-51. [PubMed ID: 20025954]. https://doi.org/10.1016/j.jep.2009.12.009.
-
21.
Zhou XQ, Zeng XN, Kong H, Sun XL. Neuroprotective effects of berberine on stroke models in vitro and in vivo. Neurosci Lett. 2008;447(1):31-6. [PubMed ID: 18838103]. https://doi.org/10.1016/j.neulet.2008.09.064.
-
22.
Shrivastava V, Mathur D, Agrawal RC. Phytochemical Screening and Determination of Antioxidant Potential of Fruits Extracts of Withania coagulans. Recent Res Sci Technol. 2011;3(11).
-
23.
Prasad SK, Kumar R, Patel DK, Hemalatha S. Wound healing activity of Withania coagulans in streptozotocin-induced diabetic rats. Pharm Biol. 2010;48(12):1397-404. [PubMed ID: 20822338]. https://doi.org/10.3109/13880209.2010.486837.
-
24.
Sarbishegi M, Khani M, Salimi S, Valizadeh M, Sargolzaei Aval F. Antiproliferative and Antioxidant Effects of Withania coagulans Extract on Benign Prostatic Hyperplasia in Rats. Nephro Urol Mon. 2016;8(1). eee33180. https://doi.org/10.5812/numonthly.33180.
-
25.
Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295(2):H801-6. [PubMed ID: 18567706]. https://doi.org/10.1152/ajpheart.00377.2008.
-
26.
Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev. 2002;54(2):271-84. [PubMed ID: 12037143].
-
27.
Sarbishegi M, Heidari Z, Mahmoudzadeh-Sagheb H, Valizadeh M, Doostkami M. Neuroprotective effects of Withania coagulans root extract on CA1 hippocamus following cerebral ischemia in rats. Avicenna J Phytomed. 2016;Epub ahead of print:1-11.
-
28.
Budhiraja RD, Garg KN, Sudhir S, Arora B. Protective effect of 3-beta-hydroxy-2,3-dihydrowithanolide F against CCl4-induced hepatotoxicity. Planta Med. 1986;(1):28-9. [PubMed ID: 3754641]. https://doi.org/10.1055/s-2007-969059.
-
29.
Oh JH, Kwon TK. Withaferin A inhibits tumor necrosis factor-alpha-induced expression of cell adhesion molecules by inactivation of Akt and NF-kappaB in human pulmonary epithelial cells. Int Immunopharmacol. 2009;9(5):614-9. [PubMed ID: 19236958]. https://doi.org/10.1016/j.intimp.2009.02.002.
-
30.
Lalsare S, Chutervedi A. Anti-inflammatory and anthihyperlipidemic activity of various extract of fruits Withania coagulance. Pharmacologyonline. 2010;1:101-7.
-
31.
Bemeur C, Qu H, Desjardins P, Butterworth RF. IL-1 or TNF receptor gene deletion delays onset of encephalopathy and attenuates brain edema in experimental acute liver failure. Neurochem Int. 2010;56(2):213-5. [PubMed ID: 19931338]. https://doi.org/10.1016/j.neuint.2009.11.010.
-
32.
Webster CM, Kelly S, Koike MA, Chock VY, Giffard RG, Yenari MA. Inflammation and NFkappaB activation is decreased by hypothermia following global cerebral ischemia. Neurobiol Dis. 2009;33(2):301-12. [PubMed ID: 19063968]. https://doi.org/10.1016/j.nbd.2008.11.001.
-
33.
Capone C, Fabrizi C, Piovesan P, Principato MC, Marzorati P, Ghirardi O, et al. 2-Aminotetraline derivative protects from ischemia/reperfusion brain injury with a broad therapeutic window. Neuropsychopharmacology. 2007;32(6):1302-11. [PubMed ID: 17119539]. https://doi.org/10.1038/sj.npp.1301255.
-
34.
Bhatnagar M, Meena P, Barbar S, Joshi C. Neuroprotective response of the hippocampus region of the brain to Withania somnifera and Asparagus racemosus root extract: An in vitro study. Afr J Zool. 2013;2010:1.
