Abstract
Background:
Many studies examine the antibacterial effects of medicinal plants; however, little research is done to evaluate their effects on different cell types, especially dermal fibroblasts.Objectives:
The current study aimed to study the effect of different concentrations of Aloe Vera, henna, chamomile, myrtle, mint, licorice, cinnamon, ginger and cedar extracts and their synergistic effects on the viability of dermal fibroblasts.Methods:
To evaluate the performance of herbal extracts on dermal fibroblasts, in the first experiment different concentrations (6.25, 12.5, 25, 50, 100, 250, 500 and 1000 µg/mL) of the extracts were evaluated by the MTT cell proliferation assay. In the second experiment, the minimum effective concentrations of the plant extracts in triple combination were evaluated in the cells under study.Results:
The minimum effective concentrations of henna, chamomile, myrtle, mint, cinnamon, ginger and cedar were 12.5, 6.25, 6.25, 6.25, 6.25, 12.5 and 12.5µg/mL, respectively. Results showed that, by comparing the minimum effective concentration of herbal extracts, the viability of dermal fibroblasts significantly increased by cedar extract (P < 0.05). Combination of Aloe Vera, licorice and mint extracts significantly increased the viability of dermal fibroblasts (P < 0.05).Conclusions:
Based on the results of the current study, it was concluded that Aloe vera, licorice and mint extracts had synergistic effects on the viability of dermal fibroblasts; in addition, the combination of Aloe vera and licorice with either henna or myrtle, and Aloe vera and mint with either cedar or ginger resulted in synergistic effects on viability of dermal fibroblasts. The third category of triple combinations of herbal extracts with synergistic effects on the cells under study was the combination of Aloe Vera and mint with either chamomile or cinnamon and also Aloe vera and licorice with either myrtle or cedar.Keywords
1. Background
As a result of pharmacological properties and wide range of chemical and biological activities of herbs, consumption of natural bioactive compounds such as medical or health supplements is gaining popularity (1, 2), particularly for highly complex molecular structures such as polyphenols, since the synthesis of such organic chemicals would be laborious and even more costly (3). In addition, these components may reduce the level of oxidative stress in live cells; thus, much attention is recently focused on the activity of the herbal antioxidants (2). A wide variety of phytochemicals including polyphenolics, carotenoids, terpenoids, coumarins, saponins, phytosterols, curcuminoids, etc., are identified in numerous plants (4).
Aloe Vera is a member of the Liliaceae family, which is easily grown in tropical climates. The mucilaginous tissue, called Aloe Vera gel, is located in the center of the Aloe Vera leaf and over 75 active ingredients are identified in it (5, 6). Aloe Vera gel consists of 99.3% water and the remaining is made up of solids with glucose and mannose molecules, of which mannose is more concentrated than glucose (6, 7). Acemannan is the major polysaccharide in Aloe Vera gel and is known to induce immunological reaction (8). These components give the special properties as a skin care product (7). Polysaccharides from Aloe Vera promote both the proliferation of fibroblasts and the production of hyaluronic acid and hydroxyproline in fibroblasts, which play important roles in extracellular matrix remodeling during wound healing (6). Aloe Vera may also affect some intracellular processes important in wound healing, including enhancement of fibronectin production by fibroblasts (9). Henna is a plant which grows wild in abandoned areas (10), it is now widely cultivated throughout the tropics as an ornamental and dye plant (11). Its bark leaves and seeds are utilized in medicine, because of the high amount of phenolic compounds such as flavanol and phenolic acid. These phenolic compounds are well known antioxidants that help to reduce free radicals (3). The leaves are used as a prophylactic against skin diseases. The major phytochemical constituent of henna, lawsone, possesses significant anti-inflammatory, analgesic and antipyretic activities (10). One of the oldest and most widely used medicinal plants is chamomile suggested for a variety of healing applications (7). It contains around 120 secondary metabolites, including 28 terpenoids and 36 flavonoids (12). Myrtle is an aromatic and medicinal herb reported to have antibacterial, antifungal, antiviral, antioxidant and anti-mutagenicity properties. The dried leaves of this herb contain terpinolene, cineol, linalool, terpineol, linalyl acetate, tannins and flavonoids compounds (13). Mint is one of the most important medicinal plants with a wide use in pharmaceutical, food and hygienic industry (14). The phenolic compounds found naturally on it were suggested to be the major contributors to the antioxidant activities of the plant (1). Menthol is the major component of mint. It is known as a disinfectant with effective antimicrobial properties (14). Licorice originates from the dried roots of several Glycyrrhiza species. It is employed in demulcent and expectorant as well as a flavoring and sweetening agent, because of its antioxidative, neuroprotective, anti-allergic and anti-inflammatory activities (15). Previous phytochemical investigations of this plant identified several flavonoids, triterpenoids and polysaccharides (16). The roasted form of licorice is reported to possess anti-allergic, neuroprotective, anti-oxidative, and anti-inflammatory activities (15). The most favored chemical constituents of cinnamon are volatile oils such as cinnamaldehyde, eugenol, cinnamic acid and weiterhin, and also mucilage, diterpenes and proanthocyanidins. In addition, it is rich in essential oils and tannins, which inhibit microbial growth (17). Cinnamaldehyde, as the major active component in the cinnamon, induces type I collagen biosynthesis within dermal fibroblasts (18). Ginger is produced from the rhizome of Zingiber officinale. Gingerol is an active component of ginger and responsible for antibacterial, anti-angiogenic, anti-inflammatory and anti-tumor promoting activities (19, 20). Antioxidant molecules already reported from this plant are alflabene, cassumunene, cassumunaquinones I, II, cassumunins a, B, C and cassumunarins a, B, C (4). Ziziphus spina-christi L., commonly known by the Persian names “konar” or “sedr”, is widely grown in the South of Iran. The antibacterial, antiviral and antidiabetic effects of the extracts or fractions of the leaves of this plant are confirmed (20). Flavonoids, alkaloids, tannins, sterols, betulinic acid and triterpenoidal saponin glycosides are isolated from different species of Ziziphus (21).
Fibroblasts represent a major cell type in the dermis. They are maintaining the extracellular matrix homeostasis and responsible for collagen synthesis or fibrogenesis, flexibility, strength and vitality of the skin (8, 22). It is proven that fibroblasts are the key factor in wound healing [9]. Fibroblasts play an important role in generating collagen, elastin and hyaluronic acid (HA) as other ingredients of the dermis. Collagen forms the three-dimensional structure; elastin maintains the elasticity and HA is responsible for moisture retention in the skin (8).
2. Objectives
Much attention is recently paid on the activity of herbal extracts to prevent and treat skin diseases. Since the fibroblasts represent a major cell type in the dermis, the current study aimed to evaluate the effect of different concentrations of Aloe Vera, henna, chamomile, myrtle, mint, licorice, cinnamon, ginger and cedar extracts and their synergistic effects on the viability of dermal fibroblasts.
3. Methods
3.1. Chemicals
The culture media, the Dulbecco modified the Eagle medium (DMEM), and other chemicals such as phosphate-buffered saline (PBS), fetal bovine serum (FBS) and penicillin-streptomycin were obtained from Inoclon (Tehran, Iran) and the plastic ware was purchased from Falcon (Paignton, UK) unless stated otherwise.
3.2. Herbal Extracts
The stems and leaves of mint and the leaves of Aloe Vera, myrtle, henna and cedar and root of ginger and licorice and cinnamon bark and chamomile flowers were provided. Dirt was removed by early washing and in the second stage; plants were washed with distilled water. In the third stage, plants were immersed in 20% ethanol. Then the plants were placed in the oven for one day for drying. Dried plants were grounded and prepared for extraction. The aqueous extract was provided by Soxhlet apparatus; in a way that, 40 g of each plant powder was placed in bags. The diluted extracts obtained and put in the evaporator at 67°C, and finally 40 g dense extracts derived from each plant. Then samples were centrifuged (3000 rpm, five minutes) to separate impurities and suspended solids and purify the obtained extract. To identify the extracts, a part of grounded herbs were dried, weighted and the extracts were provided at the concentration of 20 mg/mL.
3.3. Establishment of Dermal Fibroblast Cultures
The ovine fibroblast cell line was provided from sheep fetus’s ear skin, as described by Shah et al. (23) and established in the stem cell and transgenic animal lab in Iranian research organization for science and technology (IROST). In summary, the tissue was held in sterile PBS and transported to the laboratory on ice. The tissues were then washed in culture medium (DMEM with 4,500 mg/L D-glucose, L-glutamine and sodium bicarbonate, and supplemented with 20% FBS, 100 IU/mL penicillin and 50 mg/mL streptomycin), and the skin and hair follicles were removed. The remaining tissues were minced into small pieces and washed again in culture medium, then seeded into 25 cm2 culture flasks and incubated at 37.5°C in a CO2 incubator (5% CO2). The monolayer migrated from cultured tissues and assumed as a primary cell culture of the respective cells. After cells grown at 70% confluency, the dermal fibroblasts were sub-cultured by partial trypsinization.
3.4. MTT Assay
The MTT assay involves the conversion of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide to formazan, an insoluble product, by mitochondrial reductase enzyme activity in living cells (24). In the current study after 24 hours of cell culturing with different concentrations of herbal extracts based on experimental design in 96-well microplates (5000 cells per well), the viability of cells was determined using a kit (Thermo Fisher Scientific, V-13154) as per the manufacturer’s protocol. Briefly, 12 mM MTT stock solution was prepared by adding 1 mL of sterile PBS to 5 mg of MTT. Then 10 µL of the 12 mM MTT stock solution was added to each well, including a negative control of 10 µL of the MTT stock solution added to 100 µL of medium alone, and incubated at 37°C and %5 CO2 for four hours. Then 100 µL of the sodium dodecyl sulfate -hydrochloric acid (SDS-HCl) solution (10 mL of 0.01 M HCl was added to 1 g SDS) was added to each well and the microplates were incubated at 37°C overnight in a humidified chamber. The formazan concentration was determined by optical density at 570 nm.
3.5. Experimental Design
To evaluate the performance of herbal extracts on dermal fibroblasts, in the first experiment different concentrations (6.25, 12.5, 25, 50, 100, 250, 500 and 1000 µg/mL) of the extracts were evaluated by the MTT cell proliferation assay. In the second experiment, the minimum effective concentrations of the plant extracts in triple combination were studied in the cells under study. Therefore, Aloe vera was used as the base plant and each of henna, chamomile, myrtle, mint and licorice were used as second and third plants and the other extracts added to the combination were cinnamon, ginger and cedar.
3.6. Statistical Analysis
Each experiment was repeated at least three times. Data were analyzed by SPSS ver. 16. Comparisons between means were done using one-way ANOVA followed by Duncan multiple-range test. Results are expressed as mean ± standard error of the mean (SEM), and P < 0.05 denoted a statistically significant difference.
4. Results
4.1. Experiment 1: The Effect of Different Herbal Extracts Concentrations on Viability of Dermal Fibroblasts
Among different herbal extracts under study, neither the concentration of Aloe vera nor licorice showed a negative effect on the viability of dermal fibroblasts. Accordingly, 50 µg/mL Aloe vera and 6.25 µg/mL licorice were the minimum effective concentrations that significantly increased the proliferation of dermal fibroblasts (P < 0.05). However, higher concentrations of henna, chamomile, myrtle, mint, cinnamon and ginger (50, 1000, 250, 50, 500 and 500 µg/mL, respectively) significantly decreased the viability compared with those of the control (P < 0.05). The minimum effective concentrations of henna, chamomile, myrtle, mint, cinnamon, ginger and cedar were 12.5, 6.25, 6.25, 6.25, 6.25, 12.5 and 12.5µg/mL, respectively (Figure 1).
Effect of Different Concentrations of Herbal Extracts on Viability of Dermal Fibroblasts

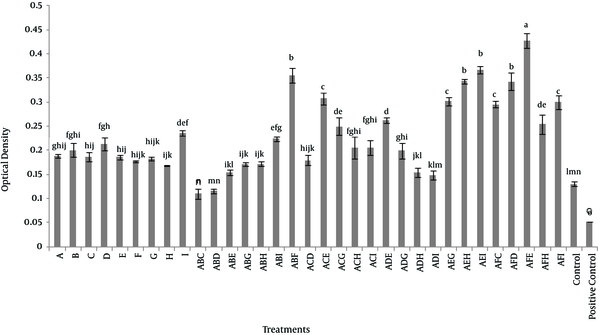
4.2. Experiment 2: Synergistic Effect of Nine Herbal Extracts
In the second experiment, the minimum effective concentration of the most effective extract on the viability of dermal fibroblasts in triple combination was used. In this experiment, Aloe vera was considered as the base plant and each of henna, chamomile, myrtle, mint and licorice were used as second combination and third plants and the other extracts added to the combination were cinnamon, ginger and cedar. Results indicated that a combination of Aloe vera, licorice and mint extracts significantly increased the viability of dermal fibroblasts (P < 0.05). When Aloe vera and licorice extracts were used in combination with either henna or myrtle, also extracts of Aloe vera and mint were used in combination with either cedar or ginger, the results showed a significant increase in the viability of dermal fibroblasts (P < 0.05) in comparison to other groups, except Aloe vera, licorice and mint. The third category of triple combination of herbal extracts were Aloe vera and mint with either chamomile or cinnamon and also Aloe vera and licorice with either myrtle and cedar, that showed a synergistic effect on viability of dermal fibroblasts, as compared to the time that plant extracts were used alone (Figure 2).
Synergistic Effect of Herbal Extracts
5. Discussion
Many studies examined the antibacterial effects of medicinal plants (17); however, little research is done on either minimum effective concentration or their effects on different cell types, especially dermal fibroblasts. The current study examined the effects of nine medicinal plant extracts on the viability of dermal fibroblasts. Besides identifying effective concentration, their synergistic effects on the viability of dermal fibroblasts were detected. Fibroblasts, the key cells involved in wound repair, perform a number of crucial functions including proliferation and remodeling of the new matrix at the wound site, and synthesis of growth factors and extracellular matrix components (e.g. collagen, fibronectin, glycosaminoglycans and proteoglycans) (9).
The results of the first experiment showed that cedar extract significantly increased the viability of dermal fibroblasts (P < 0.05). After that, myrtle and henna were more effective. Cedar leaves are a rich source of phenolic compounds, including simple and highly glycosylated flavonoids, proanthocyanidins and chlorogenic acids (21). Jamshidpoor et al. (25) (2015) showed that cedar (Z. spina-christi) extract had anticancer effects and induced significant inhibition of DNA synthesis. Moreover, the results indicated that treatment of the cells with cedar extract induces apoptosis. Zandi et al. (26), studied the effects of myrtle, mint, chamomile, henna and parsley on the viability of spermatogonial stem cells (SSCs) and Sertoli cells and concluded that myrtle, mint, chamomile and parsley significantly increased the number of dead cells in comparison to henna and control groups (P < 0.05). The results of this experiment provided evidence that henna with antibacterial activity had no detrimental effect on SSCs and Sertoli cells and was a good candidate for substitution of antibiotics.
Antioxidant activity in henna was higher when compared to vitamin E or tocopherol (11). This property was proportional with their phenolic components (27). Myrtle is an aromatic and medicinal herb reported to have antibacterial, antifungal, antiviral, antioxidant and anti-mutagenicity properties. In recent years, many of these researchers came to this understanding that the presence of phenolic compounds in the leaves of myrtle herb was due to the presence of flavenols (quercetin glycosides and myricetin) and derivatives of galloyl including galloyl glycosides, alajytanyn and the galvyl-quinic acid; the antibacterial activities of these compounds are attributed to the presence of polyphenols (13).
Among different herbal extracts under study, none of the concentrations of Aloe vera and licorice showed a negative effect on the viability of dermal fibroblasts; however, higher concentration of other plants resulted in cell death. In this regard, other studies revealed that plant polyphenols could be toxic (28).
The results of the second experiment revealed the synergistic effects of Aloe vera, licorice and mint extracts on the viability of dermal fibroblasts. Also the combination of Aloe vera and licorice with either henna or myrtle, and the Aloe vera and mint with either cedar or ginger, resulted in synergistic effects on viability of dermal fibroblasts. The third category of triple combination of herbal extracts with synergistic effects on the cells under study was the combination of Aloe vera and mint with either chamomile or cinnamon and also Aloe vera and licorice with either myrtle or cedar.
Aloe vera exhibits many activities including wound healing, immune boosting, hypoglycemic and hypolipidemic, and also antioxidant, antimicrobial, antitumor and antidiabetic properties. Many traditional uses are also reported such as burn injury, eczema, cosmetics, inflammation and fever (6). In addition, positive Aloe vera effects on wound healing are demonstrated in animal models (9). Aloe vera gel or its components directly affected proliferation, migration, cell-to-cell communication and other functions of fibroblasts (29). Tanaka et al. (8) reported that Aloe vera sterols stimulated collagen and HA production by human dermal fibroblasts.
Huo et al. (30) reported that licorice extract, by scavenging free radicals and stimulating activities of antioxidant enzymes, may play an important role in medicine formulation. They also revealed that the licorice components alone (triterpene, saponins and glycyrrhizic acid) or in combination with other components might be responsible for the reduction of hepatotoxicity. The favonoids possess anti-oxidative and anti-carcinogenic activities. From a structural point of view, the presence of phenolic OH groups contributes to their cytotoxicity (31).
Al-Juhaimi and Ghafoor (27) reported that mint leaves showed maximum total phenols and antioxidant activity in comparison to herbs such as coriander and parsley. But phenylpropanoids and quercetin components of ginger resulted in protection against oxidative stress in human skin cells (32). Recent studies suggest that chamomile caused complete wound healing faster than corticosteroids (12). Cinnamon bark extract and its polyphenol content are reported to have potent antioxidant activities (33). Farahpour and Habibi (34) reported that cinnamon extracts promote wound healing and are effective in wound contraction. In fact, antioxidant, anti-inflammatory, and antimicrobial effects are the main properties of remedies which speed up wound healing.
Acknowledgements
References
-
1.
Fatiha B. Optimisation of solvent extraction of antioxidants (phenolic compounds) from Algerian mint (Mentha spicata L.). Phcog Commn. 2012;2(4):72-86.
-
2.
Nidhal M, Salih AH, Hindi B, Hindi MJ. Antioxidant Activity of Dietary Plants: Peppermint. Pak J Nutr. 2013;12(6):571-4.
-
3.
Zohourian TH, Quitain AT, Sasaki M, Goto M. Polyphenolic contents and antioxidant activities of Lawsonia inermis leaf extracts obtained by microwave-assisted hydrothermal method. J Microw Power Electromagn Energy. 2011;45(4):193-204. [PubMed ID: 24428109].
-
4.
Sharma GJ, Chirangini P, Mishra KP. Evaluation of antioxidant and cytotoxic properties of tropical ginger, Zingiber montanum (J. Konig) A Dietr. Gardens' Bull Singapore. 2007;59(1):189-202.
-
5.
Maenthaisong R, Chaiyakunapruk N, Niruntraporn S, Kongkaew C. The efficacy of aloe vera used for burn wound healing: a systematic review. Burns. 2007;33(6):713-8. [PubMed ID: 17499928]. https://doi.org/10.1016/j.burns.2006.10.384.
-
6.
Radha MH, Laxmipriya NP. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J Tradit Complement Med. 2015;5(1):21-6. [PubMed ID: 26151005]. https://doi.org/10.1016/j.jtcme.2014.10.006.
-
7.
Irshad S, Butt M, Younus H. In-vitro antibacterial activity of Aloe barbadensis Miller (Aloe vera). Int Res J Pharmaceutic. 2011;1(2):59-64.
-
8.
Tanaka M, Misawa E, Yamauchi K, Abe F, Ishizaki C. Effects of plant sterols derived from Aloe vera gel on human dermal fibroblasts in vitro and on skin condition in Japanese women. Clin Cosmet Investig Dermatol. 2015;8:95-104. [PubMed ID: 25759593]. https://doi.org/10.2147/CCID.S75441.
-
9.
Abdullah KM, Abdullah A, Johnson ML, Bilski JJ, Petry K, Redmer DA, et al. Effects of Aloe vera on gap junctional intercellular communication and proliferation of human diabetic and nondiabetic skin fibroblasts. J Altern Complement Med. 2003;9(5):711-8. [PubMed ID: 14629848]. https://doi.org/10.1089/107555303322524553.
-
10.
Endrini S, Rahmat A, Ismail P, Yun Hin TY. Anticarcinogenic properties and antioxidant activity of henna (lawsonia inermis). J Med Sci. 2002;2(4):194-7.
-
11.
Chaudhary G, Goyal S, Poonia P. Lawsonia inermis Linnaeus: a phytopharmacological review. Int J Pharm Sci Drug Res. 2010;2(2):91-8.
-
12.
Srivastava JK, Shankar E, Gupta S. Chamomile: A herbal medicine of the past with bright future. Mol Med Rep. 2010;3(6):895-901. [PubMed ID: 21132119]. https://doi.org/10.3892/mmr.2010.377.
-
13.
Taheri A, Seyfan A, Jalalinezhad S, Nasery F. Antibacterial effect of Myrtus communis hydro-alcoholic extract on pathogenic bacteria. Zahedan J Res Med Sci. 2013;15(6):19-24.
-
14.
Tabari MA, RezaYoussefi M, Ghasemi F, Tabari RG, Esmaili RH, Behzadi MY. Comparison of antibacterial effects of Eucalyptus essence, mint essence and combination of them on Staphylococcus aureus and Escherichia coli isolates. Middle East J Sci Res. 2012;11(4):536-40.
-
15.
Ahn S. Determination of optimal concentration of deglycyrrhizinated licorice root extract for preventing dental caries using a bacterial model system. J Dent Sci. 2014;9:214-20.
-
16.
Jayaprakasam B, Doddaga S, Wang R, Holmes D, Goldfarb J, Li XM. Licorice flavonoids inhibit eotaxin-1 secretion by human fetal lung fibroblasts in vitro. J Agric Food Chem. 2009;57(3):820-5. [PubMed ID: 19132888]. https://doi.org/10.1021/jf802601j.
-
17.
Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med. 2006;6:39. [PubMed ID: 17134518]. https://doi.org/10.1186/1472-6882-6-39.
-
18.
Rosales Castro M, Gonzalez Laredo RF, Bae YS, Kim JK, Morre J, Karchesy JJ. Characterization and antioxidant properties of the condensed tannins from Alaska Cedar inner bark. Rec Nat Prod. 2014;8(3):217-27.
-
19.
Topman G, Lin FH, Gefen A. The natural medications for wound healing - Curcumin, Aloe-Vera and Ginger - do not induce a significant effect on the migration kinematics of cultured fibroblasts. J Biomech. 2013;46(1):170-4. [PubMed ID: 23084784]. https://doi.org/10.1016/j.jbiomech.2012.09.015.
-
20.
Kim EC, Min JK, Kim TY, Lee SJ, Yang HO, Han S, et al. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2005;335(2):300-8. [PubMed ID: 16081047]. https://doi.org/10.1016/j.bbrc.2005.07.076.
-
21.
Karar MGE, Quiet L, Rezk A, Jaiswal R, Rehders M, Ullrich MS, et al. Phenolic Profile and In Vitro Assessment of Cytotoxicity and Antibacterial Activity of Ziziphus spina-christi Leaf Extracts. Med Chem. 2016;6(3):143-56.
-
22.
Sajjad A, Subhani Sajjad S. Aloe vera: An Ancient Herb for Modern Dentistry-A Literature Review. J Dental Surg. 2014;2014:1-6.
-
23.
Shah RA, George A, Singh MK, Kumar D, Anand T, Chauhan MS, et al. Pregnancies established from handmade cloned blastocysts reconstructed using skin fibroblasts in buffalo (Bubalus bubalis). Theriogenology. 2009;71(8):1215-9. [PubMed ID: 19168209]. https://doi.org/10.1016/j.theriogenology.2008.10.004.
-
24.
Ebrahimi M, Rezaei Tavirani M, Heidari Keshel S, Raeisossadati R, Hoseinzade Salavati B, Daneshimehr F. Appraisal of fibroblast viability in different concentration of glucose as mimicry diabetic condition. J Paramed Sci. 2011;2(4):36-41.
-
25.
Jamshidpoor A, Ahmadi R, Mazloomifar H, Mahdavi E. The Effects of Konar Extract on Kidney Cell Line in Cell Culture. J Adv Chem Engg Biol Sci. 2015;2(1):57-8.
-
26.
Zandi M, Jasour F, Shariatinia A, Sanjabi MR. Henna, An Antimicrobial Herbal Medicine With Less Negative Effect on Spermatogonial Stem Cell Culture. Gene Cell Tissue. 2016;3(2):1-5.
-
27.
Al Juhaimi F, Ghafoor K. Total phenols and antioxidant activities of leaf and stem extracts from coriander, mint and parsley grown in Saudi Arabia. Pak J Bot. 2011;43(4):2235-7.
-
28.
Gramza Michałowska A, Abramowski Z, Jovel E, Hes M. Antioxidant potential of herbs extracts and impact on HepG2 cells viability. Acta Sci Pol Technol Aliment. 2008;7(4):61-72.
-
29.
Grazul-Bilska A, Johnson ML, Bilski LJ, Abdullah KM, Abdullah A. Effect of aloe vera on expression of fibroblast growth factor receptor 2 IIIc mRNA in human diabetic and non-diabetic skin fibroblast. J Alter Med Res. 2010;2(2):215-22.
-
30.
Huo HZ, Wang B, Liang YK, Bao YY, Gu Y. Hepatoprotective and antioxidant effects of licorice extract against CCl(4)-induced oxidative damage in rats. Int J Mol Sci. 2011;12(10):6529-43. [PubMed ID: 22072903]. https://doi.org/10.3390/ijms12106529.
-
31.
Ohno H, Araho D, Uesawa Y, Kagaya H, Ishihara M, Sakagami H, et al. Evaluation of cytotoxiciy and tumor-specificity of licorice flavonoids based on chemical structure. Anticancer Res. 2013;33(8):3061-8. [PubMed ID: 23898061].
-
32.
Schadich E, Hlavac J, Volna T, Varanasi L, Hajduch M, Dzubak P. Effects of Ginger Phenylpropanoids and Quercetin on Nrf2-ARE Pathway in Human BJ Fibroblasts and HaCaT Keratinocytes. Biomed Res Int. 2016;2016:2173275. [PubMed ID: 26942188]. https://doi.org/10.1155/2016/2173275.
-
33.
Balekar N, Bodhankar S, Mohan V, Thakurdesai PA. Modulatory activity of a polyphenolic fraction of Cinnamomum zeylanicum L. bark on multiple arms of immunity in normal and immunocompromised mice. J App Pharm Sci. 2014;4(7):114-22.
-
34.
Farahpour MR, Habibi M. Evaluation of the wound healing activity of an ethanolic extract of Ceylon cinnamon in mice. Vet Med. 2012;57(1):53-7.