Abstract
Background:
Industrial dyestuffs are the main sources of the discharge of dye pollutants into the environment, which are hardly degradable in the conventional biological treatment plants. Therefore, finding an effective method of treatment is urgent for color removal from dye-contaminated effluents.Objectives:
In the present study, the photocatalytic degradation of C.I. Sulphur Red 14 was studied by using UV/ZnO process.Materials and Methods:
In this work, ZnO catalyst and mercury lamp (UV-C, 254 nm) have been applied for removal of the C.I. Sulphur Red 14 dye from aqueous environments. The influence of various parameters such as the amount of ZnO, initial dye concentration, pH level, and H2O2 presence were also examined.Results:
The results showed that the optimum catalyst dose was found to be 50 mg/L. The maximum rate of decolorization was obtained in acidic condition at pH 5 and the initial dye concentration of 10 mg/L. Photodegradation of C.I. Sulphur Red 14 increased by addition of proper dose of hydrogen peroxide. Mineralization of the dye was also investigated as Chemical Oxygen Demand (COD) measurements showed increase in the COD removal with increase in decolorization rate.Conclusions:
The UV/ZnO system has been found an effective process with 90.2% COD removal and 95.6% dye removal after 50 minutes treatment.Keywords
1. Background
Various industries such as paper, plastics, food, cosmetics, and textile use considerable volume of water, chemicals, and dyes in order to color their products. Consequently, they generate a significant amount of dye-contaminated effluents (1-3). Dye removal from wastewater has been seriously targeted in the last years, not only because of its potential toxicity to some organisms, including aquatic animals but also due to their aesthetical problems in receiver waters (4). Each of the methods for treatment of dye-containing wastewater has specific advantages and disadvantages. Physical processes are generally non-destructive, and further treatment is necessary. Chemical treatment by chlorine or ozone is effective for color removal, but they are not cost-effective (as a result of their high dosages). Biological treatment methods are usually inefficient due to non-biodegradable organic compounds and other hazardous chemicals into the effluents.
The heterogeneous photocatalysis in the presence of UV radiation has been found an attractive advanced oxidation process for degradation of azo reactive dyes (5, 6). Recently, various advanced oxidation processes (AOP) such as ozonation, peroxone, Fenton, photo-Fenton, UV/O3, UV/H2O2, UV/TiO2, and UV/ZnO have been extensively used for mineralization of many pollutants in aqueous solution (7-10). Among them, TiO2 or ZnO nanoparticles are used as effective, inexpensive, and nontoxic heterogeneous photocatalysis for the degradation of a wide range of toxic and bio-resistant compounds and synthetic dyes into H2O and CO2, without generating any harmful by-products (8). Also, owning to similar photodegradation mechanism, ZnO nanopowder is a suitable alternative to TiO2 (11). Also, ZnO nanopowder is used in various wastewater treatment such as pulp mill bleaching wastewater treatment (12, 13) and used for degradation of 2-phenyphenol and phenol in the photocatalytic degradation system (14).
2. Objectives
The principal objective of the present study is to evaluate photocatalytic degradation of C.I. Sulphur Red 14, in the presence of ZnO nanopowder by UV-C light under various conditions. In this regard, the effect of several operating parameters such as pH level, the amount of nanocatalyst, and the initial dye concentration were examined. The influence of H2O2 addition for enhancing the decolorization of synthetic dye-containing wastewater was studied too.
3. Materials and Methods
3.1. Materials
C.I. Sulphur Red 14 (purchased from Alvan Sabet Co.) was used without further purification. Its structure and characteristics are given in Table 1. Diameter, specific surface area, and band-gap energy of ZnO (purchased from FLUKA) were 14 nm, 10 m2/g, and 2.92 eV, respectively. The pH of the solution was adjusted with NaOH or HCl solution from Merck. Deionized and double distilled water was used throughout this study. Reagents used for COD measurement such as potassium dichromate, silver sulfate, mercuric sulfate, ferrous ammonium sulfate, sulfuric acid, and Ferroin indicator were obtained from Merck. H2O2 (30%, Merck) was used for UV/ZnO/H2O2/ system.
Structure and Characteristics of C.I. Sulphur Red 14
| Variable | Value |
|---|---|
| Structure | |
| Molecular Formula | C38H16N4O4S2 |
| Molecular Weight, g/mol | 656.69 |
| ʎ max, nm | 565 |
3.2. Experimental Apparatus and Procedure
Dye removal experiments with UV/ZnO system were carried out in a batch water jacketed photoreactor with the total capacity of 800 mL. This reactor is double-walled made of a quartz, in which the temperature was maintained constant throughout the reaction by circulating water in its jacketed wall. To carry out the photodegradation of C.I. Sulphur Red 14, a solution containing desired concentration of dye and an appropriate amount of ZnO was prepared and transferred into 800 mL reactor. The radiation source was a 15 W mercury lamp, which was placed above the reactor. We measured COD by the standard method of potassium dichromate oxidation. The pH of the solution was adjusted using dilute sulphuric acid and sodium hydroxide solutions and measured by pH meter (Philips PW 9422). After that, the lamp was switched on to initiate the reaction. During the reaction time, the system was constantly agitated by a magnetic stirrer to keep the photocatalysts suspended. For separating of ZnO nanoparticles from suspension, the samples were centrifuged at 5500 rpm, for 10 minutes. The concentration of the dye in each sample was evaluated by UV-visible spectrophotometer (Lambda 25, USA) at λmax = 565 nm. Then, the concentrations of dye in the samples were determined by calibration curves. The definition of dye degradation percentage (DDP) is as follows:
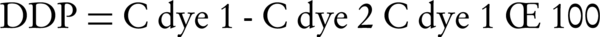
Where, DDP (100) is the degradation percentage of the reactor, C1 is the initial concentration of dye (mg/L), C2 is the concentration of dye (mg/L) after reaction for (t) time.
In this study, for determination of optimum pH and ZnO dose, 30 and 36 samples were used, respectively. Also, the number of samples for the evaluation of dye concentration effect, H2O2 addition, and evaluation of COD removal in UV/ZnO process under optimized conditions were 24, 20, and 7 samples, respectively, when each test is replicated three times.
4. Results
4.1. Effect of Initial pH
Solution pH is an important parameter that influences photodegradation efficiency of dye. Therefore, the effect of pH on the dye degradation was assessed at different ranges from 3 to 11 for constant dye concentration (10 mg/L) and catalyst dose (75 mg/L). Figure 1 shows the effect of initial pH on decolorization efficiency of C.I. Sulphur Red 14. The results showed that the decolorization efficiency increased with decrease in pH. As shown in Figure 1, pH 5 is found to be more favorable for decolorization. The obtained result from our study was in agreement with other studies for decolorization of azo dyes (12, 15-17). The interpretation of pH effects on the efficiency of the photocatalytic degradation process is a very difficult task since it has multiple roles. Because of the amphoteric nature of the most metal oxide surface, pH could easily affect the surface-charge properties of the semiconductor particles. On the other hand, pH could influence dye dissociation and OH radical formation (18). The experimental results revealed decolorization rate for the C.I. Sulphur Red 14 increased with the decrease in reaction pH and highest efficiency was observed at pH 5. These findings may be related to the interactions between the dye anions and negative charged in the catalyst surface leading to strong adsorption on the catalyst surface.
Effect of pH on Degradation of C.I. Sulphur Red 14
![Effect of pH on Degradation of C.I. Sulphur Red 14 [C.I. Sulphur Red 14] 0 = 10 mg/L; [ZnO] = 75 mg /L.](http://services.brieflands.com/cdn/serve/313ea/bb5c1001c2fed2d5843ce6f3de616484b11b99b5/healthscope-4-2-22248-i001-preview.png)
4.2. Effects of ZnO Dosage
Figure 2 shows the decolorization efficiency at different concentrations of ZnO (5, 10, 25, 50, 75, and 100 mg/L) at pH 5. Experiment results indicate a trend of reduced removal with increasing ZnO concentration up to 50 mg/L. Decolorization efficiency decreased with Increasing of ZnO nanopowder from 50 to 100 mg/L. This observation can be explained by the increase in accessibility of active sites on the ZnO surface through increasing in the amount of catalyst. On the other hand, at higher catalyst loadings (above the limiting value), and accumulation of ZnO particles the number of surface active sites decreases and the turbidity of the suspension and light scattering of ZnO particles increase as a result of decrease in the passage of light through the suspension (11, 19). Therefore, the ZnO dose of 50 mg/L was performed for decolorization of C.I. Sulphur Red 14, for further studies.
Effect of ZnO Amount on Decolorization Efficiency of C.I. Sulphur Red 14
![Effect of ZnO Amount on Decolorization Efficiency of C.I. Sulphur Red 14 [C.I. Sulphur Red 14] C0 = concentration of dye at initial time = 10 mg/L; pH 5.](http://services.brieflands.com/cdn/serve/313ea/bd9f37f35582779ccfcda6fba2bbf677520387d3/healthscope-4-2-22248-i002-preview.png)
4.3. Effect of Initial Dye Concentration
Although industrial wastewaters often contain different concentrations of dye, the majority of the studies on photocatalytic decolonization have been performed at specific range of initial concentration. Therefore, it is worthwhile to study the influence of initial concentration of dye on decolorization efficiency. The influence of various initial C.I. Sulphur Red 14 dye concentrations on photocatalytic decolorization by UV/ZnO process at optimal conditions obtained from pervious stages, (pH 5, [ZnO] = 50 mg/L) carried out by changing the initial concentrations of dye from 10 - 40 mg/L (Figure 3). The results showed that an increase in initial dye concentration from 10 to 100 mg/L decreases the C.I. Sulphur Red 14 decolorization from 96% to 62.2% after 50 minutes treatment. Likely explanations are given by other studies (4, 20). The presumed reason is that when initial dye concentration increases, more and more dye concentration is adsorbed on the ZnO surface. Therefore, the generation of hydroxyl radicals will be reduced, which consequently lowers the catalytic activity. In addition, When the initial dye concentration increases, (due to the photons get intercepted before they can receive the ZnO surface) the absorption of photons by the ZnO decreases, and the decolorization efficiency decreases too (21-23).
Effect of Initial Dye Concentration on Decolorization Efficiency of C.I. Sulphur Red 14
![Effect of Initial Dye Concentration on Decolorization Efficiency of C.I. Sulphur Red 14 [ZnO] = 50 mg l/L; pH 5.](http://services.brieflands.com/cdn/serve/313ea/36eb8898954fb4844f181dbd5071ab01e56bb748/healthscope-4-2-22248-i003-preview.png)
4.4. Effect of H2O2 Concentration
In photocatalytic degradation studies, it was indicated that addition of hydrogen peroxide was significantly improved the oxidation of the organic compounds. In order to determine the effluence of H2O2 concentration on the decolorization rate of C.I. Sulphur Red 14 using fluid suspension of 50 mg/L ZnO, different doses of H2O2 in the range of 0 to 200 mg/L were added (Figure 4). The results showed that the decolorization of the dye increases as the hydrogen peroxide concentration increases until an optimal H2O2 concentration reaches, when above that level, the decolonization rate decreases. An explanation for this behavior is that at higher concentrations of H2O2, it can also act as a scavenger of valence band holes and hydroxyl radicals (24). Therefore, H2O2 could improve the reaction by producing hydroxyl radicals, but it can act as a radical or a hole scavenger at excess concentrations. As shown in Figure 4, the decolonization rate increases when H2O2 concentration increases from 0 to optimal concentration (100 mg/L). At low concentration of H2O2, a relatively low concentration of hydroxyl radicals was formed for dye oxidation, which resulted in a low decolorization rate. However, the improvement of decolonization by using hydrogen peroxide is due to the production of OH, as shown by Equations 2 and 3
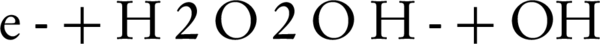
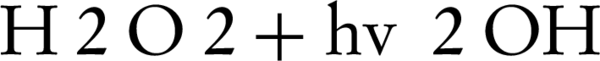
Also, H2O2 can produce OH by reacting with O2 or by direct photolysis:
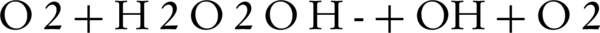
Above 100 mg/L H2O2, the decolorization efficiency decreases due to inhibition of H2O2 via reaction of excess H2O2 with OH (Equations 5 and 6).
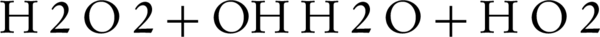
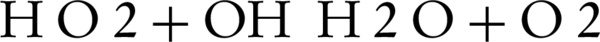
Effect of H2O2 Addition on Decolorization Efficiency of C.I. Sulphur Red 14
![Effect of H2O2 Addition on Decolorization Efficiency of C.I. Sulphur Red 14 [C.I. Sulphur Red 14] 0 = 10mg/L; [ZnO] = 50 mg/L; pH 5.](http://services.brieflands.com/cdn/serve/313ea/ad8ca0d894342886942403fe4580626721cb47da/healthscope-4-2-22248-i004-preview.png)
4.5. Chemical Oxygen Demand (COD) Removal
Since reduction of COD reveals the amount of degradation rate of an organic materials, its reduction was investigated under optimized conditions obtained from pervious stages (pH 5, [ZnO] = 50 mg/L, dye = 10 mg/L). Figure 5 presents the color and COD removal rate as a function of UV/ZnO process at an initial C.I. Sulphur Red 14 concentration of 10 mg/L, representing increase in COD reduction, accompany with increase in the color removal. As shown in Figure 5, the decolorization efficiency in UV/ZnO process for time reactions of 5, 10, 20, 30, 40, and 50 minutes in the optimized conditions were 55.3%, 65.2%, 74.3%, 84.2%, 92.5%, and 95.6%, respectively, representing a trend of increased decolorization efficiency by increasing the time reaction. The COD reduction efficiency for the corresponding time reaction of UV/ZnO process were 24.4%, 36.7%, 46.3%, 60.6%, 88.5%, and 90.2%, respectively, indicating a similar reduction trend. However, the rate of change in COD was significantly higher than that for dye. These findings show that decolorization efficiency in the initial time of reaction had no significant influence on COD removal efficiency. A possible reason for this result would be the greater ability of UV/ZnO process for degradation of color than for their organic intermediates; thus, in the initial time of reaction, more organic intermediates were generated from degradation of dye and accumulated in the reactor that contributed to the COD measurement.
Color and COD Removal in UV/ZnO Process Under Optimized Conditions
![Color and COD Removal in UV/ZnO Process Under Optimized Conditions [C.I. Sulphur Red 14] 0 = 10mg/L; [ZnO] = 50 mg/L; pH 5.](http://services.brieflands.com/cdn/serve/313ea/05e2370ede54f82e38aae7d40623224d428b5b2e/healthscope-4-2-22248-i005-preview.png)
5. Discussion
In the present study, the photocatalyst decolorization of C.I. Sulphur Red 14 by UV irradiation in the presence of ZnO nanopowder was studied. The results confirm that simultaneous presence of the catalyst and light is essential for the photodegradation of organic compounds in water. It was found that the rate of dye degradation is significantly affected by the pH, the initial dye concentration, and catalyst concentration. The results indicated that optimal loading of ZnO was 50 mg/L, with the dye concentration of 10 mg/L and pH 5. The results show that the rate of decolorization increases as the catalyst dose increases up to an optimum loading. But above that, any increase in catalyst dose had no effect. Also, H2O2 was proved to be useful for improvement in the catalytic degradation of dye-containing solutions in the UV/ZnO/H2O2 process as it significantly accelerates dye degradation and decreases the required time of reaction compared to UV/ZnO process alone.
Acknowledgements
References
-
1.
Ali M, Sreekrishnan TR. Aquatic toxicity from pulp and paper mill effluents: a review. Adv in Enviro Res. 2001;5(2):175-96. https://doi.org/10.1016/s1093-0191(00)00055-1.
-
2.
Pokhrel D, Viraraghavan T. Treatment of pulp and paper mill wastewater--a review. Sci Total Environ. 2004;333(1-3):37-58. [PubMed ID: 15364518]. https://doi.org/10.1016/j.scitotenv.2004.05.017.
-
3.
Dehghani M, Jaafari J, Alghasi A, Porkar G. Using medium pressure ultraviolet reactor for removing azo dyes in textile wastewater treatment plant. World Appl Sci J. 2011;12:797-802.
-
4.
Peternel IT, Koprivanac N, Bozic AM, Kusic HM. Comparative study of UV/TiO2, UV/ZnO and photo-Fenton processes for the organic reactive dye degradation in aqueous solution. J Hazard Mater. 2007;148(1-2):477-84. [PubMed ID: 17400374]. https://doi.org/10.1016/j.jhazmat.2007.02.072.
-
5.
Tariq MA, Faisal M, Muneer M. Semiconductor-mediated photocatalysed degradation of two selected azo dye derivatives, amaranth and bismarck brown in aqueous suspension. J Hazard Mater. 2005;127(1-3):172-9. [PubMed ID: 16095814]. https://doi.org/10.1016/j.jhazmat.2005.07.001.
-
6.
Bizani E, Fytianos K, Poulios I, Tsiridis V. Photocatalytic decolorization and degradation of dye solutions and wastewaters in the presence of titanium dioxide. J Hazard Mater. 2006;136(1):85-94. [PubMed ID: 16406296]. https://doi.org/10.1016/j.jhazmat.2005.11.017.
-
7.
Koprivanac N, Kusic H, Vujevic D, Peternel I, Locke BR. Influence of iron on degradation of organic dyes in corona. J Hazard Mater. 2005;117(2-3):113-9. [PubMed ID: 15629569]. https://doi.org/10.1016/j.jhazmat.2004.03.023.
-
8.
Daneshvar N, Rasoulifard MH, Khataee AR, Hosseinzadeh F. Removal of C.I. Acid Orange 7 from aqueous solution by UV irradiation in the presence of ZnO nanopowder. J Hazard Mater. 2007;143(1-2):95-101. [PubMed ID: 17030415]. https://doi.org/10.1016/j.jhazmat.2006.08.072.
-
9.
Zarei M, Khataee AR, Ordikhani-Seyedlar R, Fathinia M. Photoelectro-Fenton combined with photocatalytic process for degradation of an azo dye using supported TiO2 nanoparticles and carbon nanotube cathode: Neural network modeling. Electrochimica Acta. 2010;55(24):7259-65. https://doi.org/10.1016/j.electacta.2010.07.050.
-
10.
Moussavi G, Mahmoudi M. Degradation and biodegradability improvement of the reactive red 198 azo dye using catalytic ozonation with MgO nanocrystals. Chem Eng J. 2009;152(1):1-7. https://doi.org/10.1016/j.cej.2009.03.014.
-
11.
Daneshvar N, Salari D, Khataee AR. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. Photochem and Photobio A: Chemistry. 2004;162(2-3):317-22. https://doi.org/10.1016/s1010-6030(03)00378-2.
-
12.
Lizama C, Freer J, Baeza J, Mansilla HD. Optimized photodegradation of Reactive Blue 19 on TiO2 and ZnO suspensions. Catal Today. 2002;76(2-4):235-46. https://doi.org/10.1016/s0920-5861(02)00222-5.
-
13.
Yeber MC, Rodriguez J, Freer J, Baeza J, Duran N, Mansilla HD. Advanced oxidation of a pulp mill bleaching wastewater. Chemosphere. 1999;39(10):1679-88. [PubMed ID: 10520485].
-
14.
Khodja AA, Sehili T, Pilichowski JF, Boule P. Photocatalytic degradation of 2-phenylphenol on TiO2 and ZnO in aqueous suspensions. Photochem and Photobiol A: Chemistry. 2001;141(2-3):231-9. https://doi.org/10.1016/s1010-6030(01)00423-3.
-
15.
Akyol A, Yatmaz HC, Bayramoglu M. Photocatalytic decolorization of Remazol Red RR in aqueous ZnO suspensions. APPL CATAL B-ENVIRON. 2004;54(1):19-24. https://doi.org/10.1016/j.apcatb.2004.05.021.
-
16.
Kansal S, Kaur N, Singh S. Photocatalytic degradation of two commercial reactive dyes in aqueous phase using nanophotocatalysts. Nanoscale Res Lett. 2009;4(7):709-16. [PubMed ID: 20596421]. https://doi.org/10.1007/s11671-009-9300-3.
-
17.
Sakthivel S, Neppolian B, Shankar MV, Arabindoo B, Palanichamy M, Murugesan V. Solar photocatalytic degradation of azo dye: comparison of photocatalytic efficiency of ZnO and TiO2. SOL ENERG MAT SOL C. 2003;77(1):65-82. https://doi.org/10.1016/s0927-0248(02)00255-6.
-
18.
Stumm W, Morgan JJ. Aquatic chemistry: chemical equilibria and rates in natural waters. 126. John Wiley and Sons; 2012.
-
19.
Behnajady MA, Modirshahla N, Hamzavi R. Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst. J Hazard Mater. 2006;133(1-3):226-32. [PubMed ID: 16310945]. https://doi.org/10.1016/j.jhazmat.2005.10.022.
-
20.
Muruganandham M, Swaminathan M. TiO2-UV photocatalytic oxidation of Reactive Yellow 14: effect of operational parameters. J Hazard Mater. 2006;135(1-3):78-86. [PubMed ID: 16386844]. https://doi.org/10.1016/j.jhazmat.2005.11.022.
-
21.
Wu CH. Comparison of azo dye degradation efficiency using UV/single semiconductor and UV/coupled semiconductor systems. Chemosphere. 2004;57(7):601-8. [PubMed ID: 15488922]. https://doi.org/10.1016/j.chemosphere.2004.07.008.
-
22.
Qamar M, Saquib M, Muneer M. Photocatalytic degradation of two selected dye derivatives, chromotrope 2B and amido black 10B, in aqueous suspensions of titanium dioxide. DYES PIGMENTS. 2005;65(1):1-9. https://doi.org/10.1016/j.dyepig.2004.06.006.
-
23.
Daneshvar N, Rabbani M, Modirshahla N, Behnajady MA. Kinetic modeling of photocatalytic degradation of Acid Red 27 in UV/TiO2 process. PHOTOCHEM PHOTOBIOL A: Chemistry. 2004;168(1-2):39-45. https://doi.org/10.1016/j.jphotochem.2004.05.011.
-
24.
Hoseini M, Safari GH, Kamani H, Jaafari J, Ghanbarainm M, Mahvi AH. Sonocatalytic degradation of tetracycline antibiotic in aqueous solution by sonocatalysis. Toxicol Environ Chem. 2014;24(10):1680-9.