Abstract
Background:
Pyrocatechol or 1, 2-dihydroxybenzene, or 2-hydroxyphenol is considered as apriority pollutant since it is harmful to organisms at low concentrations, and has been classified as hazardous pollutants.Objectives:
The objective of this study was to examine whether Azolla filiculoides, is able to remove Pyrocatechol from aqueous solutions.Materials and Methods:
This study is an experimental research. In this study, the aquatic fern Azolla with different biomass (0.3, 0.6, 0.9, 1.2 g) has been cultured in solutionwhichwas contained 5, 10, 25, and 50 ppm Pyrocatechol. Each exam repeated twice. Samples were collected every 2 days from all of containers. The analytical determination of Pyrocatechol was performed by using DR4000 spectrophotometer by analyzing the color resulting with the wavelength of 600nm.Results:
The results showed that Azolla has high ability to remove Pyrocatechol from aqueous solutions. The Pyrocatechol removal was 60-90%. The removal efficiency wasincreasing with decreasing Pyrocatechol concentration, and increasing biomass amount, and vice versa. The removal efficiency was more than 90% when Pyrocatechol concentration was 5 ppm, and amount of biomass was 0.9 gr.Conclusions:
It is concluded that Azolla is able to accumulate and remove Pyrocatechol from the aqueous solutions. Since conventional methods of Pyrocatechol removal need high cost and energy, Phytoremediation by Azolla as a natural treatment system can decrease those issues, and it can be a useful and beneficial method for theremoval of Pyrocatechol.Keywords
1. Background
Nowadays, controlling of pollution is the prime concerns. Discharge of minicipal and industrial wastewater to water resources may pose a serious problem to the environment (1-4). Phenolic compounds are the most common forms of organic pollutants among the organic chemical pollutants in industrial wastewater (5, 6). High concentrations of phenol and phenolic compounds are typically found in aqueous effluents of oil refineries, petrochemical, ceramic, and steel plants, coal conversion processes, phenolic resin, and pharmaceutical industries (7). Catechol, also known as pyrocatechol or 1,2-dihydroxybenzene, is an organic compound with the molecular formula of C6H4(OH)2 (8).
Catechol is used as a topical antiseptic, and in photography, fur dying, leather tanning, antifungal preservation of seed potato pieces, and in polymerization inhibitors as well as a chemical intermediate, and an antioxidant in many industries. It is also used in chemical laboratories for the detection and determination of many ions. Catechol, therefore, frequently contaminates wastewaters generated by several industries including rubbers, chemical, dye, photographic, pharmaceutical, cosmetics, and oil industry (9-12).
Catechol, as a phenolic compound has adverse effects on the quality of water resource, and it is strongly irritant to eyes, skin, and respiratory tract, and can cause DNA damaging, diminishing of liver function, vascular collapse, coma, and death (13). The metabolites of catechol may start many cancers, and neurodegenerative diseases. Thereforethe United States Environmental Protection Agency (EPA) has ranked phenols the 11th in the list of 126 toxic chemicals which have been designated as priority pollutants, and stringent restrictions have been imposed by local authorities on levels of phenol contaminants in water and wastewater (10).
Small amounts of catechol occur naturally in fruits and vegetables, along with the enzyme polyphenol oxidase (6, 14). Thus, catechol removal from water and wastewater is an important issue to protect public health and environment. Many methods have been proposed for the removal of phenol and phenolic compounds including the adsorption, chemical oxidation, precipitation, distillation, solvent extraction, ion exchange, membrane processes, and reverse osmosis, chemical oxidation, and electrochemical processes (15-18). Aforementioned methods have some problems such as high cost, low effectiveness and generation of toxic by-products (19). Recently, Biological processes such as phytoremidation have been considered (20). In the process of phytoremediation pollutants are collected by the roots of plant, and either decomposed to less harmful forms or accumulated in the plant tissues. Therefore, this process is environmentally friendly and inexpensive. Azolla is a small aquatic fern. In fact, it is a symbiotic pair of Azolla filiculoides and a heterocystous blue-green alga Anabaena azolla. Azolla has been used as a fertilizer in botanical gardens because of its nitrogen-fixing capability, therefore has been used for several decades as green manure in rice fields. Studies showed that this plant could decrease nitrogen and phosphorus from well water and surface water effectively and has the ability to remove acid dye from water (1, 21-23).
2. Objectives
The objective of this study was to examine whether Azolla is able to remove pyrocatechol from aqueous solution.
3. Materials and Methods
3.1. Cultivation of Azolla
This plant can be found in watery zones, especially in stagnant water or in low velocity water (24). In this study, living Azolla was collected from the surface of rice-field near the south shores of Caspian Sea in Sari County (north of Iran), and was cultivated and kept in aquarium.
3.2. Lab Studies
Alga growth may happen in aquarium which is used to azolla cultivation. The alga growth may be reduced pyrocatechol concentration because alga degraded the pyrocatechoal. Therefore, the alga growth, Annoying and interferer organisms must be inhibited from aquarium. There are two main methods to prevent algae growth: A) Use of copper sulfate, B) Covering aquarium from besides that light can enter only from upside of aquarium. To dissolve the azollascurfing problem, temperature and light regulation was required, and it was performed in all stages (25).
The Pyrocatechol was purchased from Merck .CO. For this examination, 32 plastic containers were used with the height and width of 15 and 10 cm, respectively. The containers were used for the examination only, and 2 aquariums were used to keep azolla. The capacity of the container was 200 ml. stock solutions (1000 ppm) of pyrocatechol were prepared, and desired concentrations were made from the stock solution. In this study, desired concentrations of pyrocatechol solution were 5, 10, 25, 50 ppm. In next stage, azolla wasused in known weight of biomass including 0.3, 0.6, 0.9, and 1.2 gr along with the pyrocatechol concentrations. Primary culture medium and solution were poured in plastic containers, and after that azolla with known weight was added to the containers, and then allowed to growth of azolla in container to acclimatize with pyrocatechol in different times. All of 32 containers must be placed inside a large container which there is water and a heater inside of the container to fixthe optimum temperature of azolla growth. 4 containers of azolla were used for each concentration of pyrocatechol. To ensure the validity and accuracy of experiments, all steps were performed 2 times.
Pyrocatechol concentrations in each experiment were determined by DR4000 spectrophotometer by analyzing color with wavelength 600nm (26, 27). Concentration of pyrocatechol wasmeasured after 2, 4, 6, 8, 10, 12, and 14 days ofexposure. Standard curve wasused to determine the pyrocatechol concentration. The standard curve was prepared by concentrations of Pyrocatechol of 0.1- 1ppm.
Measuring pyrocatechol by spectrophotometric method requires3 solutions including buffer solution, 4-aminoantipyrine, and potassium ferrocyanide. 100 ml of sample was taken, and 2ml of buffer solution was poured and shaken to mixing, then 2 ml of 4-aminoantipyrine was shed and shaken, and finally 2 ml of potassium ferrocyanide was poured and shaken to mixing. After 15 minute, colorimetery was performed (26).
4. Results
4.1. Temperature Effect on Azolla Growth Rate
The results indicated that azolla grows at 25-35˚C, and the optimum temperature is 32˚C. The effect of temperature on growing of azolla is shown in Figure 1. This study was performed in 5 steps and by using 5 heaters. Initial concentration (0.3 gr) of azolla was added to whole containers, and temperature was regulated by heater. Biomass of azolla was weighed every 4 days.
Effect of Temperature onAzolla Growth

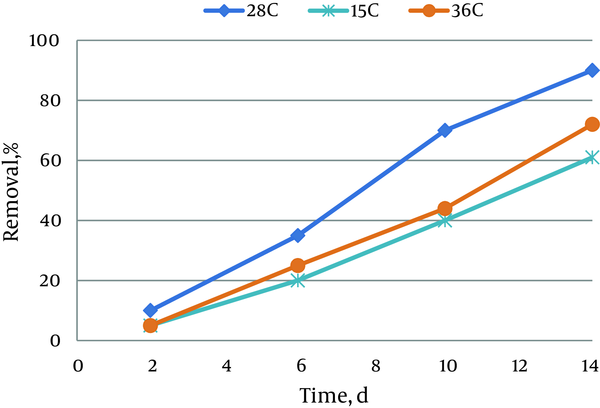
4.2. Effect of Temperature on Pyrocatechol Removal
The effect of temperature on removal efficiency of pyrocatechol is shown in Figure 2. According to the results, the maximum removal percent of pyrocatechol can be observed in 25-30˚C, which is the optimum temperature. Removal rate reduced below and above the optimum temperature, significantly. The amount of azolla growth in natural conditions, and at temperaturesabove 50˚C is low. The growth rate over time and by keeping constant the temperature above 50˚C is stopped. In this experiment, 6 containers were chosen with the same condition.The purpose of same conditions was existing 0.6 g biomass, and 5 ppm of pyrocatechol in each container. Then temperature was regulated by using heater to desired temperature. 3 containers were to ensure the accuracy of experiments and reducing of errors.
Effect of Temperature on Pyrocatechol Removal
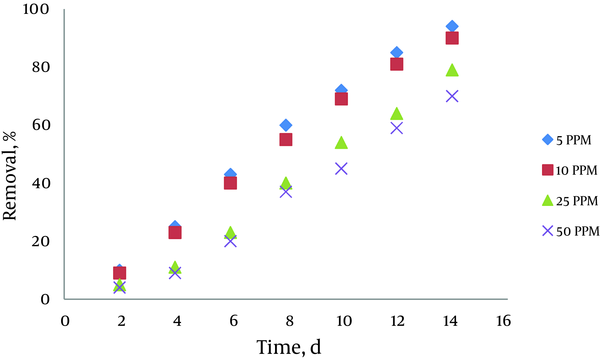
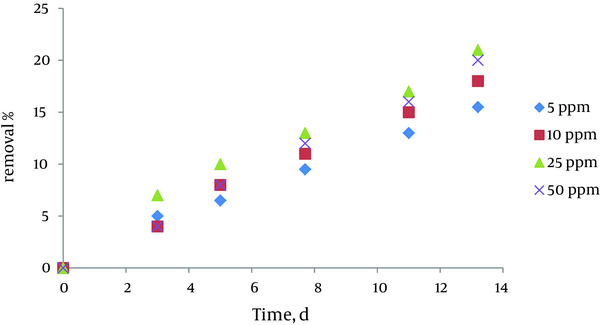
4.3. Effect of Initial Concentration of Pyrocatechol
The results indicated that azolla has high ability to remove pyrocatechol in aqueous solution, and the removal efficiency is between 65% and 85% in various concentrations. Removal efficiency was increased by biomass increasing and decreasing initial pyrocatechol concentration. Removal efficiency was 85% in biomass amount of 1.2 gr, and initial concentration of pyrocatechol of 5 ppm. Also, there was no significant difference in removal efficiency in 5 and 10 ppm, and the removal rate was equal approximately. The result is shown in Figure 3. In this experiment, 4 concentrations of pyrocatechol including 5, 10, 25, and 50 ppm were chosen. 2 containers were used to each concentration, which one of the containers was due to the accuracy of experiments. Specific biomass amount of 0.9gr was added to containers. The measurement of pyrocatechol was performed in known days. Removal rate was higher in this biomass concentration.
Effect of InitialPyrocatechol Concentration on Removal Efficiency
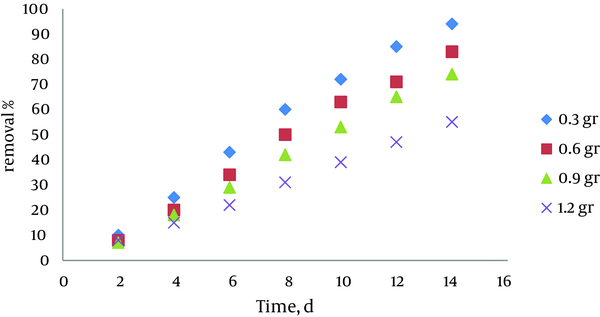
4.4. Effect of Initial Biomass Concentration
The effect of initial biomass concentration on pyrocatechol removal efficiency is shown in Figure 4. The results show that when half of the surface of each container was covered by azolla, the best conditions can be achieved to remove pyrocatechol. Since all of containers surface was covered by 1.2gr biomass, thus removal efficiency decreased more than lower weights. In this experiment, pyrocatechol concentration of 5ppm was chosen, and 4 biomass weights were contacted with specific concentration, and the removal rate was determined once a 2 days.
Effect of Initial Biomass Concentration on Efficiency ofPyrocatechol Removal
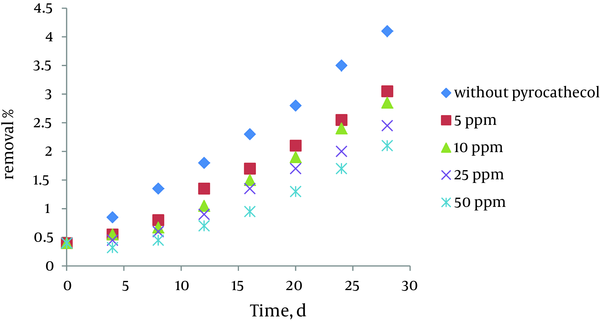
4.5. Effect of Initial Concentrations of Pyrocatechol on Inhibition Growth of Azolla
The effect of initial pyrocatechol concentration on inhibition growth of azolla is shown in Figure 5. According to the results when initial concentrations of pyrocatechol, increased the growth rate and the growth stopped at concentrations above 50 ppm. Temperature and initial concentration of biomass werekept constant at 28 ° C, and 0.9 gr, respectively.
Effect of Initial Concentrations of Pyrocatechol on Inhibition Growth of Azolla
4.6. Determination of Pyrocatechol Evaporation
The results of the experiments showed that about 15% to 25% of the pyrocatechol is removed by evaporation. At this stage, some samples that contain pyrocatechol and are without biomass were prepared to achieve pyrocatechol removal rates through evaporation. Finally, the removal rate by biomass (actual removal rate by azolla) was achieved from the difference between total removal, and removal through evaporation (Figure 6).
Evaporation of Pyrocatechol to Obtain Actual Removal Rates by Azolla
5. Conclusions
Contact time: the removal rate increased by increasing the contact time. The removal rate waslow at first days, due to plant adjusting with the new condition. The plant was adjusted from 4th day, and removal rate of pyrocatechol was increased. The result of this study is correspondent tothe result of study that was conducted by Dianati. R and also, other survey performed by Li (28, 29). Effect of temperature: azolla is found in temperate regions and stagnant waters. Best growth temperature is 25- 35˚C, and optimum temperature is 32˚C. The result is consistent with Nezamabadi. M survey result (30).
The pyrocatechol concentration: pyrocatechol removal rate decreased by increasing the Pyrocatechol rate, and maximum removal occurred in low concentration of Pyrocatechol. The removal rate was decreased with pyrocatechol concentration more than 10 ppm whichis consistent with other researches (28, 31). Increasing pyrocatechol concentration can damage the azolla, and can decrease azolla growth. This plant cannot grow in pyrocatechol concentration above 50 ppm. Increasing the pyrocatechol initial concentration can increase damagesto plant, and decrease azolla growth. Azolla cannot adapt itself with condition at concentrationsabove 50 ppm, and major amount of biomass is lost. Maximum growth of azolla occurs in pyrocatechol concentration of less than 10 ppm. The removal is 90% in this concentration which this result is exactly corresponding to the result of other study (29). The growth rate of azolla in a concentration less than 10 ppm was threefold compared to azolla growth rate in concentration of 50 ppm. Since azolla is found plentiful in our country, especially in Anzali,fen, and it is searched for finding some methods to remove this plant, so there is no worry about quantity of this plant in the the natural ecosystems (32).
Mechanism of uptake of pollutants by plants through the mechanism of phytodegradation (also known as phytotransformation) is the breakdown of contaminants taken up by plants through metabolic processes within the plant, or the breakdown of contaminants external to the plant through the effect of compounds (such as enzymes) produced by the plants. Also the Rhizodegradation Mechanism is the breakdown of an organic contaminant in soil through microbial activity whichis enhanced by the presence of the root zone (33).
Finally, the results of this study indicated that azolla isable to remove organic compound from wastewater, and since conventional methods such as AOP, adsorption and other methods, are costly, and need high energy methods, and since all countries are faced with energy shortage problem today, thus natural systems, for instance the use of azolla, can be good alternatives to conventional systems to remove this compounds from wastewater.
Acknowledgements
References
-
1.
Padmesh TV, Vijayaraghavan K, Sekaran G, Velan M. Batch and column studies on biosorption of acid dyes on fresh water macro alga Azolla filiculoides. J Hazard Mater. 2005;125(1-3):121-9. [PubMed ID: 15955624]. https://doi.org/10.1016/j.jhazmat.2005.05.014.
-
2.
Padmish T. Application Of Azolla Rongpong On Biosorption Of Acid Red 88,Acid Green 3 From Synthetic Solution. Hazard Mat. 2006;122(24):55-63.
-
3.
Zhao M. Removal And Recovery Of Nickel From Aqueous Solution And Electroplating Rinse Effulent Using Azolla. Process Biochem. 1998;33(3):249-55.
-
4.
Bazrafshan E, Mostafapour FK, Zazouli MA. Methylene blue (cationic dye) adsorption into Salvadora persica stems ash. African J Biotech. 2012;11(101):16661-8.
-
5.
Kuleyin A. Removal of phenol and 4-chlorophenol by surfactant-modified natural zeolite. J Hazard Mater. 2007;144(1-2):307-15. [PubMed ID: 17112660]. https://doi.org/10.1016/j.jhazmat.2006.10.036.
-
6.
Bazrafshan E, Mostafapour FK, Faridi H, Zazouli MA. Application of Moringa peregrina seed extract as a natural coagulant for phenol removal from aqueous solutions. African J Biotech. 2012;11(103):16758-66.
-
7.
Zazouli MA, Taghavi M. Phenol Removal from Aqueous Solutions by Electrocoagulation Technology Using Iron Electrodes: Effect of Some Variables. J Water Res Protect. 2012;(4):980-3.
-
8.
Rijnkels J. Health council of the Nether lands; 2011.Evaluation of the Carcinogenicity And Genotoxicity(1,2-Catechol (pyrocatechol).
-
9.
Monte MRR. Catechol Biodegradation kinetics Using Candida Parapsilopsis. Bio Tech.
-
10.
Shakir K, Ghoneimy HF, Elkafrawy AF, Beheir Sh G, Refaat M. Removal of catechol from aqueous solutions by adsorption onto organophilic-bentonite. J Hazard Mater. 2008;150(3):765-73. [PubMed ID: 17587494]. https://doi.org/10.1016/j.jhazmat.2007.05.037.
-
11.
Ugiyanti M. Spectrophometric Determination Of Pyrocathecol And Pyrogallol Based On Their Redox Reaction With Iron Phenanthroline System Chemistry. 2002;2(3):161-6.
-
12.
Nematollahia D, Rafieeb M. Mechanistic Study of Homogeneous Reactions Coupled With Electrochemical Oxidation Of Catechols. Chemic Soc. 2009;6(3):448-76.
-
13.
Yue S. Adsorption Of Catechol From Aqueous Solution By Aminated Hypercrosslinked Polymer. Env Sci. 2005;17(4):584-8.
-
14.
Cerda A. Determination Of Iodide In Table Salt By Flow Injection Analysis Using Pyrocatechol Violet. Food Chem. 1993;46(1):95-9.
-
15.
Bodalo A, Gomez Jl, Gomez M, Leon G, Hidalgo A, Ruiz M. Removal From Water By Hybrid Processes: Study Of The Membrane Process Step, Desalination. 2008;223:323.
-
16.
Srihari V, Das A. Comparative studies on adsorptive removal of phenol by three agro-based carbons: equilibrium and isotherm studies. Ecotoxicol Environ Saf. 2008;71(1):274-83. [PubMed ID: 17915320]. https://doi.org/10.1016/j.ecoenv.2007.08.008.
-
17.
Kujawski W, Warszawski A, Ratajczak W, Porebski T, Capala W, Ostrowska I. Removal Of From Wastewater By Different Separation Techniques. Desalination. 2004;163:287-96.
-
18.
Yun-Hwei S. Removal Of From Water By Adsorption-Flocculation Using Organobentonite. Water Res. 2002;36:1107-15.
-
19.
Abdelwahab O, Amin NK, El-Ashtoukhy E-S. Electrochemical removal of phenol from oil refinery wastewater Hazardous Materials. 2009;163:711-6.
-
20.
Bennicelli R, Stepniewska Z, Banach A, Szajnocha K, Ostrowski J. The ability of Azolla caroliniana to remove heavy metals (Hg(II), Cr(III), Cr(VI)) from municipal waste water. Chemosphere. 2004;55(1):141-6. [PubMed ID: 14720557]. https://doi.org/10.1016/j.chemosphere.2003.11.015.
-
21.
Forni C, Chen J. Evaluation Of The Fern Azolla For Growth Nitrogen And Phosphorus From Wastewater. Water Res. 2001;35(6):1592-8.
-
22.
Khosravi M, Rakhshaee R, Ganji MT. Pre-treatment processes of Azolla filiculoides to remove Pb(II), Cd(II), Ni(II) and Zn(II) from aqueous solution in the batch and fixed-bed reactors. J Hazard Mater. 2005;127(1-3):228-37. [PubMed ID: 16111810]. https://doi.org/10.1016/j.jhazmat.2005.07.023.
-
23.
Mashkani S, Tajer P. Biotechnological Potiential Of Azolla Filiculoides For Biosorption Of Cs And Sr Application Of Micro Pixe Forbmeasurement Of Biosortion. Biores Tech. 2008;2:1-7.
-
24.
Velan M. Batch And Column Studies On Biosorption Of Acid Dyes On Fresh Water Macro Alga Azolla Filiculoides. J Hazardous Materials B125. 2005:121-9.
-
25.
Duncan J. Removal And Recovery Of Zinc From Solution And Electroplating Effluent Using Azolla Filiculoides. Water Res. 1999;33(6):1516-22.
-
26.
Forni C. Evaluation Of The Fern Azolla For Growth Nitrogen And Phosphorus From Wastewater. Water Res. 2001;35(6):1592-8.
-
27.
Rigo M. Catechol Biodegradation Kinetics Using Candida Parapsiopsis. Brazilian Arch BioTech. 2010;53(2):481-6.
-
28.
Standard Methods for the Examination of Water and Wastewater. 17th ed. Washington, DC: Washington, DC; 1989.
-
29.
Dianati Tilaki R. Affect Of Glucose And Lactose On Uptake Of By Lemna Minor. Hazardous Materials. 2010;7(2):123-8.
-
30.
Li L, Zhu W, Zhang P, Chen Z, Han W. Photocatalytic oxidation and ozonation of catechol over carbon-black-modified nano-TiO2 thin films supported on Al sheet. Water Res. 2003;37(15):3646-51. [PubMed ID: 12867330]. https://doi.org/10.1016/S0043-1354(03)00269-0.
-
31.
Nezamabadi S. Analysis Research On Technology Management and Water Plant Azolla in Northern Iran Anzali lagoon and wetlands. Agri Sci. 2008;13(2):549-62.
-
32.
Taghavi M, Zazouli M, Bazrafshan E. Influences of Solution Chemistry on Phenol Removal From Aqueous Environments by Electrocoagulation Process Using Aluminum Electrodes. Health Scope. 2012;1(2):66-70.
-
33.
Gholizade Y. Ecological Study In Excessive Growth Of Azolla In Anzali And Quality Control Iran Natural Rresources. 2002;55(1):65-82.
-
34.
2000. Introduction to Phytoremediation. Environmental Protection Agency Office of Research and Development. Available from: www.InfoClearinghouse.com.