Abstract
Background:
The threat of heavy metals such as Cr and Pb is already known; they have serious negative effects on animal and human health and also persist for a long time in the environment, which is the reason they are frequently detected in soil and water and efforts are made to convert them to nontoxic forms and stabilize them.Objectives:
The current study aimed at finding an efficient organism with high transforming capability of Cr and Pb and well surviving ability to get back into the soil further, and adding biochar to stabilize the transformed metals.Methods:
The current study first dealt with Cr and Pb transformation by Aspergillus sp. in different concentrations and further applied to contaminated soil to treat contaminant, next untransformed metal from the soil was stabilized by bio-charcol amendment provided from Pistia stratiotes leaves.Results:
The obtained results demonstrated that Aspergillus sp. could transform 96% of Cr and Pb at 40 mg/L. At 80 and 100 mg/L, transformation was up to 87.22% and 74.87% for Cr and 76.52% and 68.12% for Pb by forming well biomass in malt extract medium. Further application to soil containing 20 and 40 mg/kg of Cr and Pb respectively, Aspergillus sp. minimized significant quantities of both metals in the exchangeable fraction; at 20 mg/kg there was almost no available form of metals, but at 40 mg/kg there was about 5 mg/kg of Cr and about 3 mg/kg of Pb, but in the control, exchangeable form contained almost the same concentration about 17 mg/kg and 38.6 mg/kg of Cr and 18.6 mg/kg and 38.3 mg/kg of Pb were found. Further biochar was applied to Aspergillus sp. treated soil and great impact was observed on the stabilization of metals and it also reduced the exchangeable fraction of metals.Conclusions:
Based on the obtained results, it can be concluded that the strains used in the current study efficiently transformed the metals and biochar successfully stabilized metal at greater extent in the soil. Therefore, it is suggested as a considerably important factor to treat metal contaminated sites in the environment.Keywords
Environment Metals Biochar Soil Aspergillus sp. Contaminated Sites
1. Background
Human activities such as mining and industries are the major sources of heavy metals discharge into the environment. Up to certain instances, these metal concentrations increase gradually and accumulate into the living system and cause toxic effects to the ecosystem. Heavy metals are currently of much environmental concern; especially Cr and Pb cause serious negative effects to environment, animal, and human health and concurrently persist for a long time. Normally Cr exists in the environments in two forms of Cr III and VI. Cr VI is very mobile and hazardous to humans and seems to be highly toxic and cancerogenic, even at low concentrations, whereas Cr III is comparatively less mobile, but still toxic (1). U.S. Environmental Protection Agency (EPA) determines the regulation of Cr, in total all the existing forms of Cr in the environment should be less than 2 mg/L. Different industries such as leather, textile, mining, wood preservation, anticorrosion, power plant, nuclear plant, jet aircrafts etc. are important sources of Cr (2). Lead (Pb) is also recognized as an emerging hazardous heavy metal; in nature, Pb exists in two oxidation states of 0 or +2, however, Pb2+ is the most reactive and common form of lead. Hundreds and thousands of tons of lead are produced from lead smelting, electric battery manufacturing, and mining activities; internal combustion engines (3) also release large amounts of contaminating leads that pollute soil and water. As per EPA regulation, Pb concentration in water and soil should be less than 15 and 50 µg/L (or µg/kg), respectively (4). Pb and Cr are non-essential elements and toxic at lower concentrations and cause cell and tissue damage even cell death, dysfunction of different organs, decrease fertility, respiratory diseases, etc. (5). Pb is considered as a serious pollutant and included in the list of European Union for hazardous metals (6). Whereas Cr is listed in the United States Food and Drug Administration (USFDA) (7). Considering their toxicity, they should be removed or reduced in a considerable amount of the waste before their disposal into the environment. However, the industries employ several methodologies based on the electrochemical and physiochemical methods to treat waste, but these methods are not suitable for the remediation and heavy metals remain in biological forms. Therefore, it is essential to search and develop a method that can transform the contaminants into less toxic form and not available in biological form. Biological or biochemically mediated processes are more attractive and cost-effective with several advantages over the other methods (3, 6). Therefore, the current study selected an efficient fungus, Aspergillus scleotiorum, to transform the metals and further used biochar as a potential stabilizer for them. Biochar amendments potentially reduce the bioavailability of heavy metals to enter the food chain, possibly by means of physicochemical reactions and adsorption (8). Biochar is an alkaline available in nature and is produced by means of pyrolysis with the ability to increase soil pH and stabilize the heavy metals. Numerous studies previously confirmed that the biochar had enormous potential to adsorb and fix the metals in the soil (8, 9). The addition of biochar to stabilize the metals in contaminated soils may provide a new route for the soil remediation. Therefore, the current study used a biological approach (Aspergillus sp.) to transform metals and further stabilize them in the soil using biochar (prepared from Pistia stratiotes) to make them biologically unavailable.
2. Methods
2.1. Collection of Strain
Aspergillus scleotiorum slants were collected from the Department of Biotechnology, National Institute of Technology, Raipur (CG) India, and further subcultured repeatedly for five times on potato dextrose agar. Further, the strain was used for the experimental purposes.
2.2. Growth, Resistance, and Metal Transformation at Shake Flask Level
The responses were observed in 500-mL Erlenmeyer flasks, closed tightly using cotton plug, incorporated with 100 mL of malt extract agar (MEA), and inoculated with Aspergillus scleotiorum and were screened for their tolerance to Cr and Pb. One week old mycelia discs (2 x 5 mm inoculum) cut from the actively growing mycelia were inoculated into different concentrations (10, 20, 40, 80, 100 mg/L) of metals (Cr and Pb) and further kept on a shaker at 120 rpm and 30°C. For the control non-inoculated samples, MEA was used by the same concentrations and conditions. Aspergillus scleotiorum inhibited more than 25% at 40 mg/L, about 40% at 60 mg/L, and about 75% at 80 mg/L of both Cr and Pb. The biomass were harvested after 10 days of incubation, washed with distilled water, and the dry biomass was measured.
The specific concentration of MEA was described (g/L): malt extracts 20; dextrose 20; peptone 6, final pH 6. Metal salts were filtered (Cr, Pb) and supplemented to the medium after sterilization/autoclave. The required MEA plates were prepared using 15 g/L agar. For inoculum preparation, well-grown Aspergillus scleotiorum culture was taken, then centrifuged, and biomass was obtained by rinsing two times by the fresh saline solution. Cr and Pb transformation was monitored for 168 hours of incubation by collecting 1 mL of the sample. Growth was observed by means of biomass formation that was harvested daily for seven days of incubation and their dry weight was measured. Further sample was acidified using 1% HCl and kept at 4°C and within seven days the atomic absorption spectroscopy (AAS) was analyzed.
2.3. Inoculation/Remediation of Soil by Aspergillus scleotiorum
Farmland soil samples were collected from Raipur (CG), India. Samples were air-dried, sieved, and sterilized before use. The Cr and Pb remediation experiment was performed in a beaker containing 200 g of soil supplemented with Cr and Pb at two different concentrations of 40 and 60 mg/kg separately to observe Aspergillus sp. potential in the soil. Cr VI and Pb II were mixed thoroughly into the soil. The well-grown Aspergillus sp. was inoculated with soil samples and the biomass was mixed carefully. Control remediation studies were also conducted in a similar way without adding biomass. The experiment was performed at room temperature (30°C) and terminated in 10 days. Further, Cr and Pb were extracted from different fractions (exchangeable, carbonate bound, Fe-Mn bound, oxide bound) methods described by Tessier et al. (10), and the concentration was measured using an atomic absorption spectrometer according to the above explained parameters and conditions.
2.4. Preparation, Characterization, and Application of Biochar in Batch Experiment
Biochar was prepared by means of pyrolysis at 350˚C. For biochar, 200 g of the sample was placed in a ceramic cup; the biochars used in the current study were obtained from Pistia stratiotes leaves, by means of slow pyrolysis methods, dried Pistia stratiotes leaves were kept in a ceramic cup, and further incubated in a Muffle Furnace for five to six hours at 350˚C, the method was described in details by Cao and Harris (11). The solid burnt material was obtained in black color as a biochar. The biochar was separately applied to Aspergillus-treated soil in order to stabilize Cr and Pb. About 2 mm biochar was applied to the soil. For this purpose, 10% biochar was added to the soil sample and mixed properly and incubated for seven days. Further, total Cr and Pb concentrations of the soil were evaluated using the five-stage sequential extractions described by Tessier et al. (10), which included the following fractions: soluble/exchangeable, Fe-Mn bound, carbonate bound, organic bound, and residual fraction.
2.5. Differential Extraction of Metals and Atomic Absorption spectroscopy Investigation
Differential extraction of Cr VI and Pb was obtained from the soil in five different soluble fractions, Fe-Mn bound, carbonate bound, organic bound, and residual fraction mentioned by Tessier et al. (10). Diphenylcarbazide method was used to estimate the Cr VI concentration. About 0.25 mL diphenylcarbazide solution was added to 5 mL of the sample (the solution was prepared by dissolving 0.5 g diphenylcarbazide in 10 mL acetone), and further acidified by H2SO4 (0.05 M), and the absorbance was measured spectrophotometrically at 540 nm (12).
2.6. Characterization of Soil and Biochar
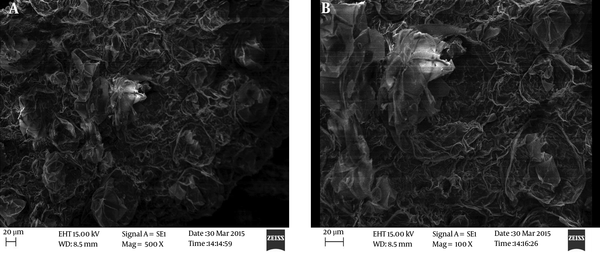
The soil and biochar were physiochemically characterized for pH, carbon, nitrogen, hydrogen, and sulfur. For the analysis, about 2 g of oven dried (105°C for 24 hours) sample was taken as 0.1 mm. The dried sample was grinded using a mortar pestle to get the desired particle size. The sample was analyzed using an elemental analyzer (Thermo Scientific, USA). Metals were analyzed after acidic digestion using AAS technique. The morphology of biochar was analyzed using scanning electron microscope (SEM) (Zeiss EVO 18 Special Editions, USA).
2.7. Data Analysis
Data were statistically analyzed by the analysis of variance (ANOVA) and the mean differences were compared by the Tukey-Kramer multiple comparison test at P < 0.05. All experiments were performed in three replicates and analyses were performed with GraphPad Prism version 4.03 (CA, USA).
3. Results and Discussion
3.1. Characterization, Growth, and Transformation at Shake Flask Level
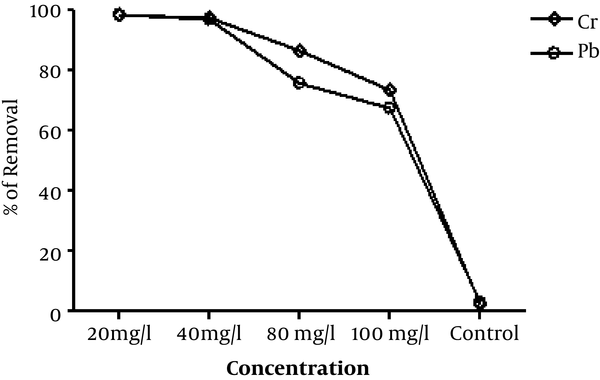
First of all, the collected soil sample was physiochemically characterized for pH, carbon, hydrogen, nitrogen, and sulfur as described in Table 1. Then, metals such as Fe, Zn, Cu, Mn, Pb, and Cr were also evaluated both in soil and biochar sample. It was found that Pb and Cr concentrations were less than the desired concentration; therefore, Cr and Pb were supplemented into the soil for the experiment. SEM picture of biochar sample was also taken and presented in Figure 1. Next, Aspergillus scleotiorum well-grown in MEA was supplemented with Cr and Pb at 10, 20, 40, 80, 100 mg/L. Aspergillus sp. grown luxuriously at 10, 20, and 40 mg/L showed approximately similar growth patterns without inhibition and reached the stationary phase after five days, but at 80 and 100 mg/L the growth was affected significantly and reduced compared with the previous concentrations (Tables 2 and 3), which may be due to the higher concentration of Cr and Pb. Aspergillus sp. transformation was approximately 96% at 20 and 40 mg/L of Cr and 0.6 to 0.8 mg/L of Pb, which proved the potential of the organism. At 80 and 100 mg/L Aspergillus sp. successfully transformed up to 87.22% (10.34) and 74.87% (26.22) for Cr and 76.52% (11.12) and 68.12% (33.12) for Pb; shown in Figure 2 in the bracket after the percentage of the left concentration is mentioned.
Scanning electron microscopy (SEM) of biochar sample used for biremediation experiment
Transformation observed for Cr and Pb at different concentrations with Aspergillus sp. at shake flask level
Physiochemical Characterization of Soil and Biochar Sample
| Serial No. | Metals | Soil | Biochar |
|---|---|---|---|
| 1 | pH | 6.4 ± 0.5 | 7.3 ± 0.3 |
| 2 | Carbon (%) | 21.24 ± 2 | 72.25 ± 3.2 |
| 3 | Hydrogen (%) | 2.1 ± 0.7 | 3.5 ± 0.9 |
| 4 | Nitrogen (%) | 2.9 ± 0.5 | 0.7 ± 0.02 |
| 5 | Sulfur (%) | 0.5 ± 0.01 | 0.1 ± 0.03 |
| 6 | Phosphorus (%) | 1.7 ± 0.4 | 0.4 ± 0.05 |
| 7 | Fe (mg/kg) | 14.51 ± 3 | 0.71 ± 0.02 |
| 8 | Zn (mg/kg) | 8.33 ± 2.5 | 5.99 ± 1.2 |
| 9 | Cu (mg/kg) | 4.99 ± 0.45 | 2.30 ± 0.6 |
| 10 | Mn (mg/kg) | 3.88 ± 0.9 | 1.80 ± 0.5 |
| 11 | Pb (mg/kg) | 7.2 ± 1.5 | 0.0 ± 0 |
| 12 | Cr (mg/kg) | 9.16 ± 1.7 | 0.7 ± 0.01 |
Growth of Aspergillus sp. at Different Concentrations of Cr in Terms of the Observed Dry Weight
| Time, d | Different Concentrations of Cr and Their Observed Dry Weight, g/L | |||||
|---|---|---|---|---|---|---|
| 0 | 0.00 | 10 | 20 | 40 | 80 | 100 |
| 1 | 0.00 | 0.29 ± 0.01 | 0.25 ± 0.01 | 0.13 ± 0.03 | 0.12 ± 0.02 | 0.11 ± 0.01 |
| 2 | 0.00 | 1.02 ± 0.01 | 0.89 ± 0.04 | 0.30 ± 0.02 | 0.28 ± 0.03 | 0.21 ± 0.02 |
| 3 | 0.00 | 1.06 ± 0.04 | 1.02 ± 0.03 | 0.49 ± 0.01 | 0.47 ± 0.05 | 0.28 ± 0.02 |
| 4 | 0.00 | 1.09 ± 0.06 | 1.07 ± 0.06 | 0.73 ± 0.05 | 0.61 ± 0.07 | 0.31 ± 0.06 |
| 5 | 0.00 | 1.07 ± 0.02 | 1.06 ± 0.06 | 0.73 ± 0.06 | 0.59 ± 0.03 | 0.29 ± 0.05 |
| 6 | 0.00 | 1.06 ± 0.01 | 1.05 ± 0.05 | 0.75 ± 0.07 | 0.64 ± 0.05 | 0.25 ± 0.03 |
| 7 | 0.00 | 1.06 ± 0.03 | 1.05 ± 0.03 | 0.73 ± 0.03 | 0.64 ± 0.03 | 0.23 ± 0.02 |
Growth of Aspergillus sp. at Different Concentrations of Pb in Terms of the Observed Dry Weight (g/L)
| Time, d | Different Concentrations of Pb and Their Observed Dry Wight, g/L | |||||
|---|---|---|---|---|---|---|
| 0 | 0.00 | 10 | 20 | 40 | 80 | 100 |
| 1 | 0.00 | 0.21 ± 0.01 | 0.22 ± 0.02 | 0.19 ± 0.02 | 0.16 ± 0.02 | 0.12 ± 0.04 |
| 2 | 0.00 | 1.12 ± 0.06 | 1.19 ± 0.09 | 1.30 ± 0.04 | 0.62 ± 0.01 | 0.18 ± 0.03 |
| 3 | 0.00 | 1.26 ± 0.04 | 1.22 ± 0.06 | 1.29 ± 0.01 | 0.67 ± 0.03 | 0.28 ± 0.04 |
| 4 | 0.00 | 1.22 ± 0.05 | 1.27 ± 0.04 | 1.13 ± 0.06 | 0.51 ± 0.01 | 0.21 ± 0.06 |
| 5 | 0.00 | 1.22 ± 0.06 | 1.26 ± 0.07 | 1.11 ± 0.03 | 0.59 ± 0.02 | 0.23 ± 0.02 |
| 6 | 0.00 | 1.25 ± 0.02 | 1.25 ± 0.03 | 1.05 ± 0.07 | 0.61 ± 0.02 | 0.26 ± 0.05 |
| 7 | 0.00 | 1.23 ± 0.01 | 1.25 ± 0.05 | 1.03 ± 0.4 | 0.57 ± 0.02 | 0.23 ± 0.02 |
3.2. Cr and Pb Transformation in Soil by Aspergillus scleotiorum
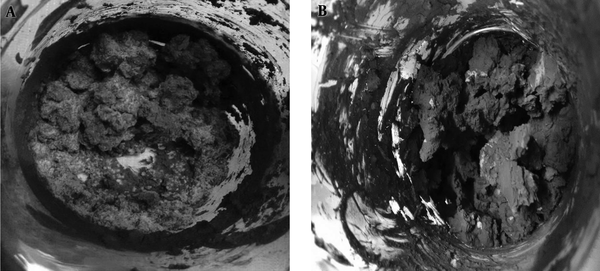
The composition of soil component, especially the metal content, is very important, since soil is used as a sink to eliminate contaminants from industrial waste material; due to lack of proper practice and management, soil is progressively observed as a major source of heavy metal. The heavy metals reduce the fertility of soil; plantation grown in the contaminated soil can transfer and increase metal content into the foodstuff and cause harmful effects on human and animal health (13). Due to its environmental importance, since many studies reported the risks caused by the metals, it is important to pay attention to the metal transformation and remediation (14). The current study applied Aspergillus sp., which significantly reduced Cr and Pb in soil. Aspergillus sp. was inoculated into contaminated soil at two different concentrations of 40 and 60 mg/kg containing Cr and Pb, separately. Figure 3 clearly showed that Aspergillus sp. grew luxuriously and formed hyphae through the soil, which was the 1st step for successful bioremediation by means of transformation and adsorptions. After extraction of Cr and Pb by fraction distribution, a significant reduction was found in the available form of both metals compared with that of the control. It was observed that the addition of Aspergillis sp. brought significant redistribution in the soil. Aspergillis sp. minimized a significant quantity of both metals in the exchangeable fraction; at 20 mg/kg there was almost no available form of metals. But at 40 mg/kg there was about 5 mg/kg (for Cr) and about 3 mg/kg (for Pb) (Figure 4), but the control exchangeable form contained almost the same concentration about 17 mg/ kg and 38.6 mg/kg for Cr and 18.6 mg/kg and 38.3 mg/kg for Pb. Further, in the redistribution, maximum concentration was observed in Fe-Mn bound, carbonate-bound, organic bound, and residual bound in Cr contaminated soil, but in the Pb-contaminated soil, maximum concentration was observed in Fe-Mn bound, organic bound, carbonate-bound, and residual bound and the minimum concentration in soluble-exchangeable fraction was observed in bioremediated soil, which showed the lowest mobility and bioavailability of Pb. In bioremediated soil samples, the soluble-exchangeable form reduced significantly, whereas Fe-Mn bound and Pb bound increased extensively, which suggested that the available Pb was converted into unavailable Pb, indicating the improvement of Pb contaminated soil after bioremediation (15). Rama Krishna and Philip (16) observed 70% reduction in Cr VI in the soil by the application of Ganoderm lucidum. Bennett et al., (17) applied six indigenous microorganisms obtained from contaminated soil and water; Klebsiella pneumoniae, Bacillus firmus, and Mycobacterium sp. were capable of absorbing Cr VI efficiently (by biomass), whereas the fungal isolates including Aspergillus flavus, Aspergillus sp., and A. niger were capable of transforming Cr VI to Cr III relative to cell-wall-binding properties and decreased the contamination. Kumar et al., (18) used different fungi (Penicillium chrysogenum, Aspegillus nidulans, Aspergillus flavus, Rhizopus arrhizus, and Trichoderma viride). Aspergillus nidulans, Rhizopus arrhizus, and Trichoderma viride showed the maximum uptake capacity of 25.67 mg/g for Pb, 13.15 mg/g for Cd, and 2.55 mg/g of Cr, respectively, which indicated the potential of these fungi and bacteria as biosorbent for the removal of high concentration metals from industrial effluents. The mechanisms involved in the transformation of metal ions in the soil are sorption, precipitation, complexation reactions, and leaching. Further, it was also affected by soil environment and soil properties and environmental factors. Environmental microbiologists understand well about the solubility of metals, and their contamination and solubilized fraction, which is readily available to the living organisms and express their toxic effect (8). By observing and the comparing the result of previous studies, it was found that Aspergillus sp. were efficient and degraded the two metals, Cr and Pb, efficiently at a shake flask level as well as in soil. Therefore, this strain can be applied to the contaminated site in the environment.
Picture showing the growth of Aspergillus sp. in Cr- (A) and Pb- (B) contaminated soil used for remediation, supplemented with 40 mg/kg of each metal
Picture showing differential extraction and observation of Cr and Pb in soil treated with Aspergillus sp.
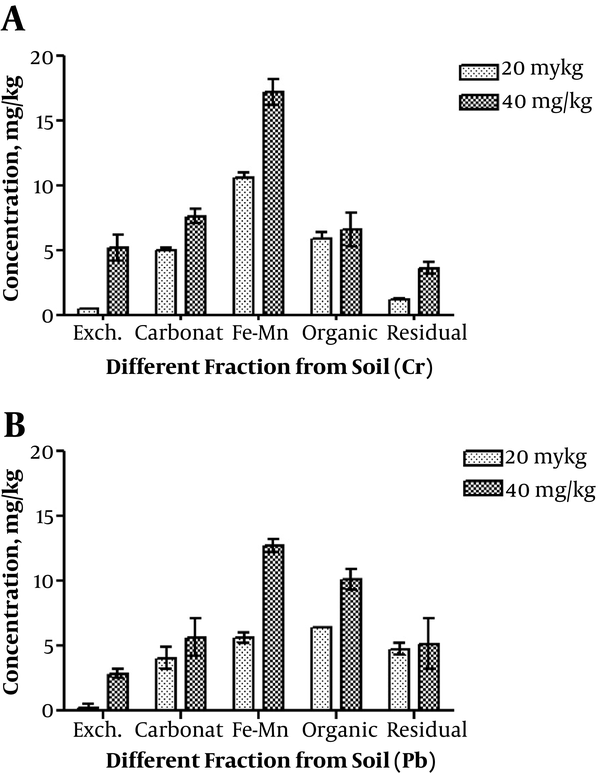
3.3. Application of Biochar to the Aspergillus sp. Treated (Contaminated) Soil
After application of Aspergillus sp., biochar was added to stabilize the left Cr and Pb in the soil to prevent it becoming easy bioavailable due to its variable environmental nature. The current study results indicated that biochar stabilized the metals and also reduced the left exchangeable fraction of the metals (Figure 5). Biochar is an environmentally acceptable alternative for sustainable management to minimize the soil contamination (19).
Distribution of Cr and Pb in soil in different fractions after biochar addition to the soil treated with Aspergillus sp. (“a” for Cr and “b” for Pb)
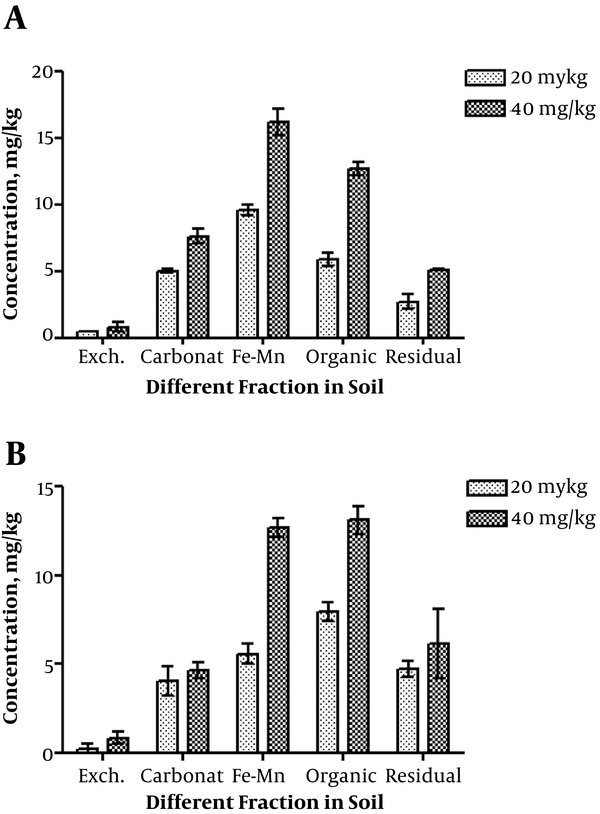
Adding biochar to soil has many positive effects on soil such as increasing the organic content, increasing heavy metals absorption capacity, preservation of moisture and nutrient, increasing soil structure, and increasing soil pH (19). Previously, Cui et al. (20) reported that addition of biochar to soil significantly reduced the concentration or level of metals from the plant parts. Lu et al., (21) observed Pb2+ stabilization in soil by using biochar, probably by different mechanisms such as surface precipitation, co-precipitation, inner-sphere complexation with humus substances, sorption, complex formation biochar mineral oxide and hydroxide, complex formation with different functional groups, the physical adsorption, and surface precipitation that contribute to the stabilization of Pb2+. Cao and Harris (22) demonstrated that the precipitation of insoluble Pb phosphate may be the mechanism to retain Pb using biochar from dairy manure. Trakal (23) also evaluated Pb sorption behavior after biochar application into a metal-contaminated soil.
Choppala et al., (24) applied biochar to a Cr-contaminated soil sample and found that biochar improved the reduction of mobile Cr VI to less mobile Cr III and declining the leaching of Cr. The decreased leaching of Cr III is probably recognized by means of adsorption of Cr III onto cation exchange sites and precipitation as Cr(OH)3 due to discharge of OH− ions during the Cr VI reduction process (25). Biochar was broadly applied to stabilize metals and also reduce the leaching of metals by means of redox reactions of metals. Recently Rinklebe et al., (26) used 10 g/kg biochar to remediate soils contaminated with potential toxic elements under dynamic redox conditions such as arsenic (As), cadmium (Cd), copper (Cu), aluminum (Al), zinc (Zn), and nickel (Ni) in an extremely contaminated soil and reduced the concentrations of potentially toxic elements in soil solution.
3.4. Conclusions
Metal cannot be degraded or lost, it can be only converted from high to low toxic form; therefore, the current study used a two-step approach; 1st transformed the toxicity of metal into other non-available forms using Aspergillus sp., next biochar was added to stabilize these transformed metals for a long time to become non-available to the ecosystem and authors’ field trial is underway. Therefore, this two-step approach may be a valuable method to transform and stabilize the heavy metals in soil.
Acknowledgements
References
-
1.
Volpe A, Pagano M, Mascolo G, Lopez A, Ciannarella R, Locaputo V. Simultaneous Cr(VI) reduction and non-ionic surfactant oxidation by peroxymonosulphate and iron powder. Chemosphere. 2013;91(9):1250-6. [PubMed ID: 23499224]. https://doi.org/10.1016/j.chemosphere.2013.02.012.
-
2.
Zayed AM, Terry N. Chromium in the environment: factors affecting biological remediation. Plant Soil. 2003;249:139-56.
-
3.
Cho DH, Yoo MH, Kim EY. Biosorption of lead (Pb2+) from aqueous solution by Rhodotorula aurantiaca. J Microbiol Biotechnol. 2004;14:250-5.
-
4.
U.S EPA. Air Quality Criteria for Lead. Final Report. U.S. Environmental Protection Agency, Washington, D.C; 2006. p. EPA/600/R-5/144aF-bF.
-
5.
Damek-Proprawa M, Sawicka-Kapusta K. Damage to the liver, kidney and testes with reference to burden of heavy metals in yellow-necked mice from areas around steel workers and zinc smelters in Poland. Toxicolog. 2003;186:1-10.
-
6.
EC Commission Regulation (EC). No. 466/2001 of 8 March 2001. Official J Europ Communit. 2001;1:77.
-
7.
USFDA (United States Food and Drug Administration). Guidelines Document for cadmium in shellfish. Washington, DC: US. Department of Health and Human Services, Public Health Service Office, Office of Seafood (HFS-416); 1993. 44 p.
-
8.
Beesley L, Moreno-Jimenez E, Gomez-Eyles JL, Harris E, Robinson B, Sizmur T. A review of biochars' potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut. 2011;159(12):3269-82. [PubMed ID: 21855187]. https://doi.org/10.1016/j.envpol.2011.07.023.
-
9.
Yuan JH, Xu RK. The amelioration effect of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Management. 2011;27:110-5.
-
10.
Tessier A, Campbell PGC, Bisson M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Analytic Chem. 1979;51:844-51.
-
11.
Cao X, Harris W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour Technol. 2010;101(14):5222-8. [PubMed ID: 20206509]. https://doi.org/10.1016/j.biortech.2010.02.052.
-
12.
Urone PF. Stability of colorimetric agent for chromium s-diphenylcarbazide in various solvent. Anal Chem. 1955;27:1354-5.
-
13.
Adriano DC, Wenzel WW, Vangronsveld J, Bolan NS. Role of assisted natural remediation in environmental cleanup. Geoderma. 2004;122:121-42.
-
14.
Krzyztof L, Danutta W, Irena K. Metal contamination of farming soils affected by industry. Environ Int. 2004;30:159-65.
-
15.
Huang DL, Zeng GM, Jiang XY, Feng CL, Yu HY, Huang GH, et al. Bioremediation of Pb-contaminated soil by incubating with Phanerochaete chrysosporium and straw. J Hazard Mater. 2006;134(1-3):268-76. [PubMed ID: 16343764]. https://doi.org/10.1016/j.jhazmat.2005.11.021.
-
16.
Krishna KR, Philip L. Bioremediation of Cr(VI) in contaminated soils. J Hazard Mater. 2005;121(1-3):109-17. [PubMed ID: 15885411]. https://doi.org/10.1016/j.jhazmat.2005.01.018.
-
17.
Bennett Reuel M, Paul Rodrigo FC, Gershon SB, Gina RD. Reduction of hexavalent chromium using fungi and bacteria isolated from contaminated soil and water samples. Chemist Ecolog. 2013;29:320-8.
-
18.
Kumar R, Singh P, Dhir B, Sharma AK, Mehta D. Potential of Some Fungal and Bacterial Species in Bioremediation of Heavy Metals. J Nuclear Physics Material Sci Radiat Applicat. 2014;1:213-23.
-
19.
Atkinson CJ, Fitzgerald JD, Hipps NA. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils. Rev Plant Soil. 2010;337:1-18.
-
20.
Cui L, Pan G, Li L, Yan J, Zhang A, Bian R, et al. The reduction of wheat Cd uptake in contaminated soil via biochar amendment: A two year field experiment. BioResourc. 2012;7:5666-76.
-
21.
Lu H, Zhang W, Yang Y, Huang X, Wang S, Qiu R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012;46(3):854-62. [PubMed ID: 22189294]. https://doi.org/10.1016/j.watres.2011.11.058.
-
22.
Cao XD, Ma LN, Gao B, Harris W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ Sci Technol. 2009;43:3285-91.
-
23.
Trakal L, Komarek M, Szakova J, Zemanova V, Tlustos P. Biochar application to metal-contaminated soil: Evaluating of Cd, Cu, Pb and Zn sorption behavior using single- and multi-element sorption experiment. Plant Soil Environ. 2011;57:372-80.
-
24.
Choppala GK, Bolan NS, Megharaj M, Chen Z, Naidu R. The influence of biochar and black carbon on reduction and bioavailability of chromate in soils. J Environ Qual. 2012;41(4):1175-84. [PubMed ID: 22751060]. https://doi.org/10.2134/jeq2011.0145.
-
25.
Bolan NS, Choppala G, Kunhikrishnan A, Park J, Naidu R. Microbial transformation of trace elements in soils in relation to bioavailability and remediation. Rev Environ Contam Toxicol. 2013;225:1-56. [PubMed ID: 23494555]. https://doi.org/10.1007/978-1-4614-6470-9_1.
-
26.
Rinklebe J, Shaheen SM, Frohne T. Amendment of biochar reduces the release of toxic elements under dynamic redox conditions in a contaminated floodplain soil. Chemosphere. 2016;142:41-7. [PubMed ID: 25900116]. https://doi.org/10.1016/j.chemosphere.2015.03.067.