1. Context
Airborne particulate matter (PM) threatens the human health (1-4) by increasing the risk of many diseases such as pulmonary disease, asthma, cancer, arrhythmia, and atherosclerosis (5-10). In order to evaluate the impact of aerosols, dynamic and static inhalation exposure systems to humans or animals are applied under controlled conditions (11, 12). These systems include whole-body (WB) and nose/head-only inhalation exposure systems (12). In the WB exposure system, the whole body of the subject is placed in the internal atmosphere of the chamber and the test material naturally reaches the respiratory region, and breathing is performed in a controlled environment containing test material. Unlike the nose/head-only method, inhalation exposure is conducted without any equipment on the head or nose of the animal, similar to the living or occupational environments, and the heat stress and movement restrictions existing in the nose or head-only exposure method are removed (13, 14). Large toxicology laboratories are usually equipped with developed inhalation chambers that control all conditions. However, most toxicological studies are implemented at small labs and performed by technicians inexperienced in the chambers technology, basic aspects of production, and analysis of the test atmospheres (15). Therefore, due to the complex techniques of inhalation exposure (16) and the difficulty in providing homogeneous atmospheres, especially in inhalation exposure tests with aerosols, the lack of attention paid to the technical issues of the exposure systems can bias the results of the studies (17). Heterogeneous distribution of test material in an inhalation exposure system leads to the concentration fluctuations in zones of the chambers and exposure of the same group with different delivery doses (11). Homogeneous distribution of a test material in a WB exposure system depends on several factors such as chamber geometry, rate and type of flow, size of the chamber, particle size, and density of test material (18-23). Recognizing the designs, functions, and factors affecting distribution of test material in exposure chambers can determine the strengths and weaknesses of various chambers based on the specific conditions of each study and provide the proper tool to select a reliable inhalation exposure system for further studies making a reproducible strategy. The current review study aimed at investigating the characteristics, structure, and factors affecting the performance of inhalation exposure chambers, especially for particulate matter and small laboratory animals, describing their advantages, disadvantages, and deficiencies, and introducing the best chamber in terms of design and function.
2. Search Strategy
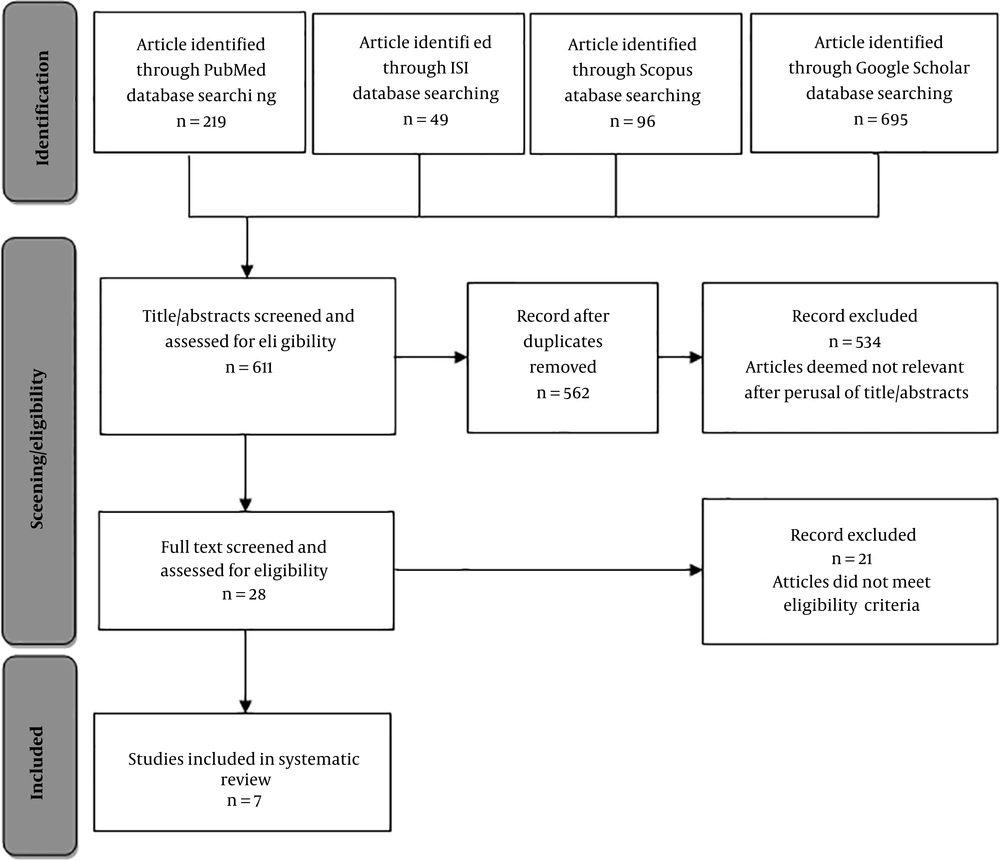
The current review study was conducted in 2017. The published literature on inhalation exposure chambers was searched in Scopus, PubMed, Web of Science, and Google Scholar databases using keywords inhalation exposure, whole body, chamber, design, dust, aerosol, particle, inhalation system, challenge system, and their combined expressions. The retrieved articles were included into the study regardless of the type and the time limits. Subsequently, the enrolled articles were reviewed. The search strategy flowchart of the study is illustrated in Figure 1.
3. Including and Excluding Criteria
Studies performed on inhalation exposure of aerosol and particulate matter in the WB chambers for small laboratory animals, as well as the ones that assessed structure and functionality of the WB chambers for inhalation exposure to particles were included in the current study. The next step was to check the articles fully and precisely; the articles that were available in full text and details in terms of design, chamber structure, fluid used, and particle type and size. Accordingly, in the preliminary study, the total number of extracted published articles was 1059, among which 448 papers were excluded considering the title and abstract, and in addition, 49 duplicate papers extracted from the selected databases were removed. The remaining articles were evaluated and screened according to the inclusion criteria, and 28 articles with available full texts were selected for the next stage. Eventually, seven articles that featured design and technical details of chambers included in the last stage. Subsequently, the results of the review of these seven selected articles are stated from far until the recent time as follows.
4. Results
In 1982, a WB inhalation system was designed and introduced by Barrow with a volume of 391 L (Table 1). The performance assessment of this chamber was conducted using a few gas atmosphere tests and aerosol propylene glycol (PG) experiments with 24 blank cages without laboratory animals. The designers of the chamber stated that they considered the guidelines for including lower than 5% animal exposed and up to 48 rats were put into the chamber (24). However, the system’s poor performance with the presence of PG aerosol was not unexpected, and the system was not able to uniformly distribute aerosols, such as gas atmospheres. The gradient of aerosol concentration in the chamber varied from 55% to 99% of the concentration entering atmosphere (15).
| Property | Barrow (1982) (15) | Cheng (1989) (20) | Kimmel (1997) (25) | Phillpotts (1997) (26) | O'Shaughnessy (2003) (27) | Bhaskar (2003) (28) | Oldham (2004) (29) | |
|---|---|---|---|---|---|---|---|---|
| H1000 | H2000 | |||||||
| Geometry type, sections-level | Cubic, (2 - 1) | Cubic, (1 - 3) | Cubic, (2 - 3) | Cubic with two cones, (9 - 3) | Cubic, (1 - 1) | Cubic, (8 - 2) | Cubic, (1 - 1) | Cubic, (9 - 1) |
| Dimension, volume | 180.3 × 43.8 × 49 cm (391 L) | 1 m3 | 2 m3 | 0.7 m3 | 80 L | 61 × 34 × 32 cm, (65 L) | 30 × 20 × 20 cm | 50 × 27 × 15 cm, (20 L) |
| The material of the chambers | Glass and acrylic plastic | Not stated | Stainless steel | Aluminum | Stainless steel | Polycarbonate | Stainless steel | |
| Subject, capacity | Rats (48) | Rats (6) | Rats (12) | Mice (64), rats (32) | Mice | Mice (40) | Mice (10) | Mice (9) |
| Test material, particle size | Propylene glycol (MMAD: 1.37 - 1.63 µm) | Nickel oxide (1.6 µm), nickel sulfate hexahydrate (3.1 µm) | NaCl aerosols (MMAD: 1.3 μm) | Aerosolized SLE-virus | TiO2 powder (MMAD: 2.56 - 3.14 μm) | E. coli aerosol (5 - 8 µm) | Cigarette smoke (MMAD: 0.6 μm) | |
| Flow rate, L/min | 78 - 130 | 200 | 450 | 162.2 - 170.2 | 8 | 32.5 | Not stated | 3 |
| Direction of flow | Horizontal | Vertical | Vertical | Horizontal | Horizontal | Horizontal | Vertical and horizontal | |
| Number of exchanges, volume air/hour | 12 - 20 | 15 | 14.1 - 14.8 | Not stated | 30 | Not stated | Not stated | |
| Assessment method | Gravimetric analysis | Gravimetric analysis - real-time | CFD nephlometer aerodynamic particle sizer | Sampling with impinger | Particle counting | Colony counting | CFD gravimetric analysis impactor | |
Cheng et al. conducted a study to improve the distribution of aerosols in two types of Hazleton chambers that were similar in geometry, but of different capacities. The H2000 and H1000 chambers of the WB type were designed to expose gases and aerosols (Table 1). The nickel oxide and nickel sulfide hexahydrate particles were used to assess the distribution and determination of mass concentration. For each rat, one point was selected and air sampling was conducted, a probe sample was placed inside the chamber and above the respiratory region of the animal. Furthermore, samples were taken with a Zeflour 25-mm filter for simultaneous sampling. In the consecutive method, a real-time device was used to monitor the mass concentration of particles. The particle size was 1.6 and 3.1 μm, and the spatial variations for H2000 and H1000 were 4.5% to 12.6% and 7.7% to 46.3%, respectively. There was no significant difference in the presence or absence of animal in the chambers (20).
Kimmel and Kirk studied and combined different designs of the WB chambers to improve the performance of the animal WB inhalation exposure chambers. Animal housing and the uniform distribution of toxicant in the chamber were two key factors in the chamber designing. The inside volume of the chamber (the cubic part) was divided into 27 small cubes each with 12.37 L, and concentration sampling and particle size monitoring were implemented in the middle of each cube (Table 1). To assess the performance of the chamber, the computational fluid dynamics (CFD) was used in a two-dimensional, incompressible, and time-dependent flow. The test materials used in their study were polydisperse NaCl particles. The CV of the concentration inside the chamber was 3.5% - 5.2% and the average concentration of particles in the three horizontal chambers was not significantly different (25).
A simple inhalation exposure system was designed and developed to expose small animals to airborne microorganisms by Phillpotts et al. The exposure chamber was made of aluminum sheet and the glass plate on the top of the chamber. In order to access the inside of the chamber, a 20 × 20 glass door was fitted in front of the chamber (Table 1). The entry and exit stream with test material was performed using the tubes embedded on both sides on the lateral and crossover sides of the chamber. Pathogen-laced aerosols were injected into the chamber by the nebulizer, and aerosol sampling was conducted by the impinger every five minutes (26).
O’Shaughnessy et al. designed an aluminum chamber to expose maximum 40 mice. The suspended particles (TiO2 powder) were inserted from the entrance at the top left corner of the chamber and left from the exit at the top right corner through holes with the diameter of a quarter inch. Eight animal cages were separated in two levels, each cage with five mice; thus a total of 40 mice were placed in a chamber. In the outlet, a suction pump was used and placed inside a cabinet to control the noise. Four polyvinyl chloride tubes with a diameter of 4 mm were used to sample the aerosol inside the chamber. Pipes were placed in the center and bottom of the 1st and 4th cages at two levels, and the 5th tube was embedded in the middle of the cages for sampling. The mass median aerodynamic diameter (MMAD) of the used particles was 2.56 and 3.14 μm, and the CVs of concentration were 4.8% and 11.0%, respectively (27).
Bhaskar and Upadhyay designed and assessed a small chamber for animal exposure with infectious aerosols, made of polycarbonate transparent sheet with a size of 30 × 20 × 20 cm and a capacity of 10 mice. The input and output of the chamber were on the upper transparent cap. Inhalation test material (suspended bacteria) was produced by a nebulizer with an output particle size of 5 - 8 μm. To assess performance and the uniform distribution of infectious aerosols inside the exposure chamber, an Escherichia coli DH5α suspension was used and the LB agar sampling plates were placed in the corners and center of the chamber; then aerosol was passed through the chamber for five minutes. In the next step, the plates were incubated overnight at 37°C and the growth of colonies in all plates was investigated. Considering the number of particulate bacteria injected into the system, the number of precipitated bacteria was investigated in the lungs of the mice exposed to this aerosol for five minutes (28).
In the study by Oldham et al., a WB portable exposure system was designed and developed for inhalation exposure to mice with particulate test material (MMAD) smaller than 2.5 μm. This 20-L volume chamber was classified as 3 × 3 and provided nine zones to expose nine mice simultaneously. The chamber was designed to have an animal load less than 5% of the chamber volume. The performance assessment of the exposure system, evaluation of the flow, and the uniform distribution of aerosol were conducted using experimental assessment and the CFD modeling using aerosols sizing 0.5 to 2 μm in diameter. After exposure, the particle concentration in tracheobronchial and various parts of mice lung was also evaluated. They stated that the presence of mice in the chamber was not modeled due to the continuous movement of mice in this section, and it was stated that since the volume occupied by animals was less than 5%, the presence or absence of the mice had no effect on computational results. The test material was cigarette smoke with a particle size of 0.6 μm (MMAD) and the geometric standard deviation (GSD) was determined by an impactor in the middle of the runtime. The numerical simulation results showed the maximum flow velocity in the central zone; the minimums in the 3rd and 7th zones, and moderate in other sections. Moreover, there was no significant difference in concentration between the nine regions, but there was a difference in concentration in various runtimes (29).
5. Discussion
In the WB animal inhalation surveys, two fundamental factors including animal housing and exposure system should be considered by researchers. Large changes and fluctuations in animal housing such as temperature, humidity, oxygen concentration, light, noise, presence of other animals, etc., can affect animal metabolism and stress level, and alter biological responses (11, 30, 31). The recommended temperature to maintain small laboratory animals is 20 - 26°C with a moisture content of 30% - 70% (24). Due to the effect of light on the physiology and behavior of laboratory animals (32-36), some resources are recommending 40 lux of light in the mid cage (37). It is generally preferred that rats and mice be maintained at the lower light intensity (38). The reviewed papers, such as the ones by Barrow and Steinhagen (15), Cheng et al. (20), Phillpotts et al. (26), O’Shaughnessy et al. (27), and Bhaskar and Upadhyay (28), did not mention environmental conditions such as temperature, humidity, and lighting. The drawbacks of these studies were in the design of chambers, but in the studies by Kimmel and Kirk (25), and Oldham et al. (29), the temperature changes were also considered by the researchers.
Furthermore, noise is another factor potentially causing physiological stress in animals (39-42), and thus, noise control should be considered in the design of a chamber and its equipment (43). Most of the animal species can have frequencies listenable to humans (44, 45), and others such as rodents are sensitive to ultrasound noises (46). In the study by O’Shaughnessy et al., establishing noise control received little attention in the designing of chamber. Therefore, it is necessary to design exposure chambers to provide standard conditions for animal housing (24), and using engineering methods to decrease the bias effects of such factors. In WB exposure chambers, animals may be kept individually or in groups, and the ones kept in a group may accumulate over time and receive lower amount of the test material due to this filtration; therefore, it is emphasized that the animal burden of chamber is considered to be less than 5% of the chamber volume. However, some researchers suggest that the control of thermal stress caused by animal metabolism needs the animal burden of 1% - 2% of the chamber (30). Recently, many studies are implemented on determining the space needed for the lab animals housing and its effective factors (47-52). Usually, the space necessary to keep animals is determined based on the weight, age, and gender of the animals (53). In almost all the previous reviewed studies a standard of 5% volume was considered, but it seems that instead of using the volume factor, it is better to use the standard of surface for each animal defined according to their type and characteristics (24), and the height of the chamber can be controlled based on the other variables such as uniform distribution of the test material. In the design of exposure systems, test materials concentration, the production method, and control and uniform distribution of the material are the primary factors (54). Uniformity of the test material distribution in the chamber is a fundamental criterion to confirm its applicability in inhalation toxicology studies (20, 25). Uniformity of distribution depends on the chamber geometry, the type and direction of flow, flow rate, and density, shape, and size of particles. The test materials may be in different phases such as gas, steam, and aerosol, but the behavior of these forms are very different and each of them requires their own production methods and sampling (30). Unlike gases and vapors, the gravitational force of the earth has a greater effect on aerosols and particles; therefore, the best mode of entry and the best direction of the aerosol stream inside the chamber are in line with the gravitational force. This issue was respected in the studies by Kimmel and Kirk, Oldham et al., and Cheng et al., and the coefficient of variations of the concentrations of test materials in the chambers was less than the horizontal states, and the distribution of the materials was more homogeneous (Table 1). On the other hand, another effective factor that could uniform the distribution was the type of the geometry of chambers. In the studies by Kimmel and Kirk, and Cheng et al., chambers were designed vertically with two cones on top and bottom, and without extra equipment could create higher uniform distribution. Oldham et al., used a specific method for the input, output, and distribution of the flow in the chamber. In this method, each section of the chamber is separately fed and ventilated. To distribute the uniform concentration of test material, the combination of vertical input and horizontal methods of flow is employed. The size and shape of an aerosol determines the aerodynamic behavior, amount of penetration, and sedimentation in the lungs and airways (54). Particles > 5 μm usually reach the nasopharyngeal region and the 1 - 5 μm ones often reached the tracheobronchial region (55, 56); particles ≤ 0.5 μm are deposited in the alveolar region (57, 58). In inhalation studies, the size distribution of an aerosol is expressed by a logarithmic normal distribution (30). In all articles reviewed in the current study, MMAD of particles was < 4 μm, except in the study by Bhaskar and Upadhyay that the diameter of the particles of the nebulizer was 5 - 8 μm. This particle size cannot reach the alveolar region. None of the previous reviewed studies stated the shape of particles. The number of air exchanges per hour is an effective factor in the velocity of flow and cold stress of animals. Reeb et al. stated that 30 times air exchange per hour was appropriate to control the humidity, temperature, and ammonium concentration produced by mice (59). The Guide for the care and use of laboratory animals considers the acceptable ventilation rate as 10 - 15 times. More ventilation causes loss of energy in animals, and less ventilation increases the temperature, humidity, and accumulation of the gases generated by animals inside the chamber (24, 60).
On the other hand, further ventilation leads to higher flow rates in the chamber and high-velocity exposure to airflow causes cold stress (61). In the studies by Kimmel and Kirk, and Cheng et al., the ventilation rate was based on the recommendations of the mentioned guide.
In the studies by Oldham et al. and Cheng et al., an internal fan was used to blend the polluting streams and create uniform distribution (20, 29). This distribution is justified only in small chambers, and if the chamber volume becomes greater than a certain volume, this method affects the flow stability and disrupts the principle of uniform and stable distribution over time. With regard to the principles of the fluids dynamics and the aerodynamic behavior of aerosols, more symmetrical form of the chamber leads to more uniformly expected distribution, and symmetry is higher in cylindrical chambers than the cubic ones. None of the previous studies used cylindrical form, but they often designed cubic forms. The gravimetric analysis and real-time method were most commonly used to assess aerosol concentration observed in the reviewed studies. At present, numerical simulation techniques can predict the pattern of flow and test material distribution, and its concentration in different locations (29). In the reviewed articles, Oldham et al. and Kimmel and Kirk used CFD (25, 29).
5.1. Conclusions
According to the two main criteria, animals housing and the uniform distribution of test material in the inhalation chambers, and considering the fluid dynamics and aerodynamic behavior of particles, the best structure was the design by Kimmel and Kirk, since the variations in particle concentration of NaCl injected into the chamber was 3.5% to 5.2%, with the most accurate conditions. The chamber was also made of stainless steel, which can be connected properly to the ground to prevent the accumulation of static electricity in the chamber and distortion in the distribution of particles. In this chamber, the amount of air exchange was designed to be less than 15 times per hour, which was consistent with the standard ventilation defined for exposure chambers (24) and minimized the stress caused by the velocity of flow and consumption of tested materials. The reason for the superiority of this system compared to the other examined chambers was that Kimmel and Kirk, considered comprehensive factors not conducted in other studies to design their chamber and tried to study all the factors affecting the uniform distribution of test material and the recommended environmental conditions for keeping the animals. In addition, in designing and validating the chamber, they used the experimental tests and a powerful instrument (CFD) to predict the microscopic and macroscopic behavior of the particles flow together. Eventually, it can be stated that regarding the dynamics of the fluid, the WB exposure chamber is closer to the cylindrical state and can provide a more uniform distribution. Likewise, the change in the outlet of the flow and embedding it at the bottom of the lower cone of the chamber, with the consideration of the discharge of waste, can prove a more uniform and stable flow in the chamber. In view of the multiplicity of factors affecting the uniform distribution of particulate matter in the WB inhalation exposure chambers, it is suggested that numerical simulation methods should be used to optimize the proposed model by Kimmel and Kirk. This method can save time and cost of the essential empirical tests making reproducible procedure.
5.2. Limitations
The main limitation of the present review study was that only English language documents were included.