Abstract
Background:
Hepatitis C is a public health threat, affecting approximately 1.9% of the Malaysian population.Objectives:
This study demonstrates how a series of initiatives taken by the Ministry of Health (MOH) of Malaysia have impacted the treatment coverage and drug expenditure for hepatitis C patients since 2013, the year in which the first direct-acting antiviral (DAA) was introduced in public health institutions.Methods:
The data were obtained from all the 144 hospitals and 33 primary healthcare centers throughout the country, which were identified to have offered the pharmacological treatment to hepatitis C patients over the last seven years.Results:
The total number of hepatitis C patients treated each year was shown to increase by more than 10 times, reaching 3,116 in 2019. However, the drug expenditure for hepatitis C relative to the overall health expenditure did not significantly increase over time (P = 0.094). The use of DAAs was once limited by its exorbitant cost. A remarkable elevation in the number of patients receiving the treatment only took place as of 2016, particularly following the engagement of the MOH in endeavors driven by non-profit organizations to enhance the accessibility of DAAs and the issuance of a compulsory license to sofosbuvir.Conclusions:
Timely decisions of the MOH and the judicious use of policy tools were shown to have transformed the landscape of hepatitis C management in Malaysia without considerably raising the budgetary pressure. Yet, continuous efforts to massively upscale the screening and treatment of the disease are warranted going forward.Keywords
Antiviral Agents Health Expenditures Malaysia Public Health Hepatitis C
1. Background
Since the discovery of Hepatitis C Virus (HCV) back in 1989, hepatitis C has been a global public health threat (1). Approximately 71 million individuals are currently infected with HCV (2). Despite the possibility of being self-limiting in its acute phase, unresolved HCV infection would eventually progress into Chronic Hepatitis C (CHC) (3). Liver cirrhosis and hepatocellular carcinoma are among the fatal complications of CHC, claiming nearly 400,000 lives annually (2). Although hepatitis C has become a highly curable disease following the advent of a range of effective and yet relatively safe direct-acting antivirals (DAAs), the accessibility of such treatments remains limited, particularly in developing countries, due to its prohibitively high cost (4, 5).
Malaysia, an upper-middle-income country with approximately 1.9% of its adult population infected with HCV (6), has a long history of providing fully subsidized hepatitis C treatment through its public health system. The interferon-ribavirin combination was once the mainstay of hepatitis C treatment in the country until boceprevir, one of the first-generation DAAs, was brought in by the Ministry of Health (MOH) to be used in combination with interferon and ribavirin in 2013 (7). In line with the goal of the World Health Organization (WHO) to eliminate viral hepatitis by 2030, public health institutions under the MOH have also been actively engaging in research on DAAs and endeavors to promote the screening and treatment of hepatitis C, especially those driven by international non-profit organizations (8, 9).
In September 2017, after rounds of unfruitful price negotiations with the pharmaceutical company, Malaysia became the first country invoking the Agreement on the Trade-Related Aspects of Intellectual Right Properties (TRIPS) to issue a Compulsory License (CL) to sofosbuvir, the backbone of many interferon-free DAA regimens (10). Since then, this has enabled the use and procurement of the generic version of sofosbuvir in public health institutions, markedly lowering the treatment cost of hepatitis C in Malaysia. In line with the recommendations of the WHO (11), the use of sofosbuvir in addition to daclatasvir, which was also available in the generic form in Malaysia, was subsequently introduced in public hospitals across the country as the standard treatment for HCV-infected patients.
In early 2019, the MOH also launched a five-year National Strategic Plan (NSP) for hepatitis B and C (12), which serves as a framework for the national response to both diseases. It comprehensively covers all aspects of viral hepatitis management, ranging from capacity building to preventive measures, harm reduction, screening, diagnosis, treatment, and monitoring. Since the availability of less costly DAAs, the MOH has also been pushing for the decentralization of hepatitis C screening and treatment to primary care and community levels. One of the key strategies actively used by the MOH to reach out to key populations, including People Who Inject Drugs (PWID) and People Living with HIV (PLHIV), is through its collaborations with Civil Society Organizations (CSOs) and inter-ministerial partnerships (13).
Irrespective of all the policy decisions and efforts made by the MOH over the years, as summarized in Figure 1, evidence regarding their impacts on hepatitis C management and health expenditure is still lacking.
Summary of initiatives taken by the Ministry of Health to scale up hepatitis C treatment in Malaysia
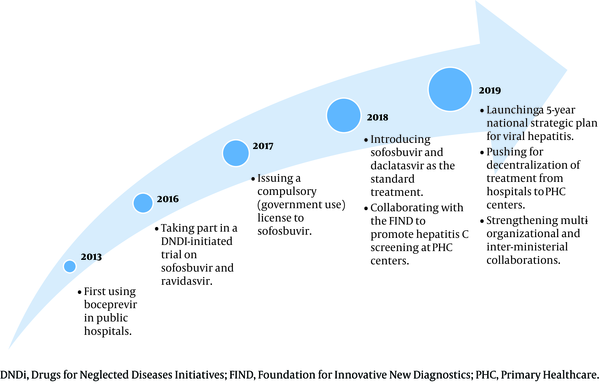
2. Objectives
This study was designed to demonstrate how MOH-led initiatives have changed the treatment coverage and drug expenditure for hepatitis C patients since the first use of DAAs in Malaysia.
3. Methods
This study was approved by the Medical Research Ethics Committee of the MOH under the protocol numbered NMRR-20-275-52850. The findings were generated from the data obtained from all the 144 public hospitals and 33 public primary healthcare (PHC) centers identified to have provided pharmacological treatments for hepatitis C patients between January 1, 2013, and December 31, 2019. Since 2013, the MOH has been requiring each of the public health institutions, mainly through their pharmacy departments, to submit an annual report on the number of hepatitis C patients treated and the corresponding drug expenditure. The patients were treated at the discretion of physicians irrespective of their liver fibrosis stages. In addition to the cases fully subsidized by the MOH, this study also captured the patients who participated in clinical trials, received free treatments from pharmaceutical companies under the Patient Access Scheme, and self-funded their treatment.
The data analysis was performed using SPSS for Windows version 21 (IBM, New York). The treatment coverage was summarized as the percentage of patients who received hepatitis C treatment in a particular year, using the estimated number of HCV-infected individuals in Malaysia at the same time point as the denominator. According to the findings of a nationwide, large-scale screening program, approximately 619,400 Malaysians were infected with HCV in 2019 (1.9% of a 32.6 million population) (6). The annual numbers of hepatitis C patients from 2013 to 2018 were estimated by adding the number of patients who were not yet treated or died from the disease in the preceding years and subtracting the number of patients who were not yet infected or had achieved SVR after receiving treatment in the preceding years from the number of patients in 2019.
Additionally, the corrected total number of hepatitis C patients treated within the seven years was also presented in this study, taking into account the estimated number of interferon-experienced patients who were retreated with DAAs. Using the local data as the guide, all the calculations were grounded on the following estimation made by the Gastroenterology and Hepatology Subspecialty Committee of the MOH: (i) the SVR rates were 55% for interferon-based and 90% for DAA-based regimens, (ii) the annual incidence and mortality rates of hepatitis C were 6.77 and 1.84 per 100,000, respectively, and (iii) the proportions of interferon-experienced patients retreated with DAAs between 2016 and 2019 were one-third in the clinical trial (sofosbuvir and ravidasvir) and 10% in the actual clinical practice (sofosbuvir and daclatasvir).
Meanwhile, the drug expenditure was computed based on the actual consumption and acquisition costs of the medications in a particular year. The findings were presented in both MYR and US$, with the conversion performed based on the mean exchange rate of each year. To make a meaningful comparison with the inflation rate and health budget taken into consideration, the drug expenditure was also presented as the proportion of the total health expenditure in the same year. The trend of drug expenditure relative to the overall health expenditure over time was also evaluated using the linear regression for trend analysis, with a significance level fixed at 5%.
4. Results
Table 1 delineates the trend of treatment coverage and drug expenditure for hepatitis C patients in Malaysia. Between 2013 and 2019, a total of 6,368 courses of pharmacological treatment were provided to hepatitis C patients seeking care from public health institutions throughout the country. Taking into account the interferon-experienced patients retreated with DAAs, the corrected total number of hepatitis C patients who had received treatment until the end of 2019 reached approximately 5,849. A 10-fold increase in the number of patients receiving treatment from 299 in 2013 to 3,116 in 2019 was observed, suggesting that the treatment coverage for hepatitis C patients in the country was constantly expanded over the years. Even though hepatitis C was predominantly managed in hospitals, it is noted that 219 (7.0%) patients treated in 2019 received their treatment from PHC centers. It is also found that an interferon-free DAA regimen, with or without ribavirin, was used to treat most patients (78.1%) throughout the seven-year period.
Treatment Coverage and Drug Expenditure in Hepatitis C Patients in Malaysia With Trend Analysis in 2013-2019
| Regimens | Year | ||||||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | |
| Interferon + ribavirin | |||||||
| Patients, n | 296 | 326 | 388 | 244 | 190 | 29 | 15 |
| Cost, MYR/ US$ | 11,590,421/ 3,691,217 | 10,298,962/ 3,149,530 | 5,934,163/ 1,521,580 | 3,364,433/ 812,665 | 3,241,723/ 753,889 | 351,120/ 87,127 | 128,674/ 31,081 |
| Interferon + ribavirin + boceprevir | |||||||
| Patients, n | 3a | 1 | 4 | 0 | 0 | 0 | 0 |
| Cost, MYR/ US$ | 77,697/ 24,744 | 54,557/ 16,684 | 152,861/ 39,195 | 0/0 | 0/0 | 0/0 | 0/0 |
| Sofosbuvir + daclatasvir | |||||||
| Patients, n | 0 | 0 | 0 | 0 | 0 | 1,105 | 2,375 |
| Cost, MYR/ US$ | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1,591,916/ 395,016 | 2,815,960/ 680,184 |
| Sofosbuvir + daclatasvir + ribavirin | |||||||
| Patients, n | 0 | 0 | 0 | 0 | 0 | 396 | 593 |
| Cost, MYR/ US$ | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 2,888,070/ 716,643 | 4,602,529/ 1,111,722 |
| Sofosbuvir + ravidasvir | |||||||
| Patients, n | 0 | 0 | 0 | 165b | 82b | 0 | 127b |
| Cost, MYR/ US$ | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Other DAA Combinationsc | |||||||
| Patients, n | 0 | 0 | 7d | 4 | 5 | 7e | 6 |
| Cost, MYR/US$ | 0 | 0 | 0 | 535,979/ 129,464 | 785,576/ 182,692 | 339,498/ 84,243 | 634,659/ 153,299 |
| Total | |||||||
| Patients, n (%)f | 299 (0.05%) | 327 (0.05%) | 399 (0.06%) | 413 (0.07%) | 277 (0.05%) | 1,537 (0.25%) | 3,116 (0.50%) |
| Cost, MYR/ US$ (%)g | 11,668,118/ 3,715,961 (0.06%) | 10,353,519/ 3,166,214 (0.05%) | 6,087,024/ 1,560,775 (0.03%) | 3,900,412/ 942,129 (0.02%) | 4,027,299/ 936,581 (0.02%) | 5,170,604/ 1,283,028 (0.02%) | 8,181,822/ 1,976,286 (0.03%) |
Regardless of the availability of the first-generation DAAs, almost all patients were found to receive the interferon-based treatment between 2013 and 2015. The first wave of a larger-scale use of DAAs only took place in 2016 right after the engagement of the MOH in a multi-country clinical trial on sofosbuvir and ravidasvir. In the most recent two years, the sofosbuvir-daclatasvir combination was shown to have taken over interferon and ribavirin as the most common treatment option for hepatitis C in the country.
Most patients (94.0%) had their treatment fully funded by the MOH, while the rest mainly received complimentary treatment through their participation in industry-sponsored clinical trials (5.9%). Between 2013 and 2019, the MOH was found to spend nearly MYR 50 million (US$ 13.6 million) on medications used for hepatitis C treatment. Despite the substantial increase in the number of patients treated, the trend analysis indicated that the drug expenditure on hepatitis C relative to the annual health spending did not significantly increase with time, narrowly ranging between 0.02 and 0.06% (P = 0.094).
Nevertheless, it is worth highlighting that the MOH spent approximately MYR 7.5 million (US$ 1.8 million) on the three-drug combination consisting of sofosbuvir, daclatasvir, and ribavirin for the treatment of fewer than 1,000 patients in 2018 and 2019, which was 70% higher than the spending on the ribavirin-free regimen used for nearly 3,500 patients. Moreover, between 2016 and 2019, an additional annual expenditure in the range of MYR 339,498 to 785,576 (US$ 84,243 to 182,692) was made on a few brand-name DAAs, which were mainly used for a small number of patients with Chronic Kidney Disease (CKD).
5. Discussion
This study represents the first attempt to illustrate, primarily through the changes in the treatment coverage and drug expenditure, how a series of initiatives taken by the MOH and the use of policy tools have transformed the landscape of hepatitis C management in Malaysia. It is encouraging to note that the expenditure on hepatitis C treatment relative to the annual health expenditure did not significantly increase over time, even though the number of patients treated each year had grown by more than 10 times between 2013 and 2019. While the MOH is pushing for the elimination of viral hepatitis by continuously providing free treatments, the findings of this study suggest that the existing model is likely to be sustainable in the near future.
The hepatitis C treatment in Malaysia was, in fact, still limited by the exorbitant cost of interferon and ribavirin in the early 2010s, as clearly indicated in this study. Yet, boceprevir was introduced by the MOH in pursuit of a better treatment outcome for patients. However, it is obvious that the first-generation DAA used as adjunct therapy neither improved the safety profile of the conventional, interferon-based treatment nor lifted the financial burden from the MOH. As a result, very few hepatitis C patients benefited from the new regimen, and the annual number of patients receiving pharmacological treatment still remained below 400 up until 2015.
The situation only shifted in 2016, the year in which the MOH decided to be part of a multi-country trial led by the Drugs for Neglected Diseases initiative (DNDi), a non-profit organization actively engaging in developing new treatments for hepatitis C. The drug expenditure was also found to drop by approximately one-third in 2016 and 2017 as compared to the previous few years, as the DNDi started to offer the patients complimentary treatment with sofosbuvir and ravidasvir within this two-year period. Until December 2019, nearly 400 patients infected with HCV of different genotypes, specifically those who had no cirrhosis or had compensated cirrhosis, were enrolled in the trial. Although the trial is still ongoing, the findings of its first phase are promising, suggesting that the new pan-genotypic dual DAA regimen could yield a Sustained Viral Response (SVR) rate as high as 97% (14).
Although the participation in clinical trials opened the door to large-scale use of DAAs in hepatitis C patients in Malaysia, the MOH fully recognized the need for a long-term solution to ensure DAA accessibility. The issuance of a CL to sofosbuvir in 2017 clearly marked another milestone in the history of hepatitis C management in Malaysia, mainly by reducing the treatment cost by approximately 95%. Consequently, the treatment coverage for hepatitis C patients was greatly expanded in the following two years. More than 4,000 patients were recorded to have received treatment with sofosbuvir and daclatasvir, one of the WHO-recommended standard regimens for hepatitis C (12), at an average cost below MYR 1,500 (US$ 350) per patient between 2018 and 2019. This was also a critical decision made by the MOH to allow for a more competitive market in Malaysia, as more DAAs, especially those with generic versions, were expected to be made available in the country during the three-year validity period of the CL.
Besides the CL, the drastic increase in the number of patients treated in 2018 and 2019 was also attributable to a few important steps taken by the MOH. Together with the Foundation for Innovative New Diagnostics (FIND), another non-profit organization pushing the same agenda as Malaysia, the MOH has been working on the decentralization of the screening and treatment of hepatitis C to 33 PHC centers since 2018. Such a step had further improved DAA accessibility in the country. In addition, the two-fold increase in the number of hepatitis C patients treated between 2018 and 2019 suggests that the partnerships with harm-reduction CSOs helped bring in key populations for screening and treatment.
As much as the MOH is motivated by the above achievement, this study also reveals several major challenges in battling the disease. First, it is obvious that those who had received pharmacological treatment between 2013 and 2019 composed only a small proportion (< 2%) of the estimated HCV-infected population in Malaysia. The MOH feels the urge to vastly expand the treatment coverage in hepatitis C patients, and has, thus, planned to gradually increase the annual target of patients receiving treatment, starting from 10,000 for 2020. It is essential for the MOH to closely monitor the progress toward the WHO’s elimination goal and revisit the annual target periodically. As attention has been disproportionately devoted to patients seeking care from health institutions since DAAs were made available in Malaysia, efforts should also be made to step up the screening and treatment activities at the community level, particularly by extending the services to more public and private primary care centers. Another strategy that could be adopted by the MOH is the micro-elimination approach through systematically screening and treating HCV-infected subpopulations, including patients with advanced liver diseases, prisoners, PWID, PLHIV, hemodialysis patients, and migrants (15-17). As the first step to make micro-elimination possible, the MOH has recently collaborated with the Ministry of Home Affairs, introducing the hepatitis C program in prisons and drug rehabilitation centers throughout the country.
Apart from that, it is found that the use of ribavirin in addition to sofosbuvir and daclatasvir, which is mainly recommended for cirrhotic and treatment-experienced patients of certain HCV genotypes (18), is still posing a financial challenge to the MOH. This is evidenced by the high cost incurred by the ribavirin-containing, three-drug regimen used for less than 1,000 patients in 2018 and 2019, which almost doubled the expenditure on the ribavirin-free regimen used for nearly 3,500 patients over the same period. Another underlying source of the financial burden was the use of a few high-priced brand-name DAAs, particularly for those with CKD. It is hoped that the growing evidence on the alternatives, including daclatasvir and ravidasvir, would eventually enable the use of less costly and yet equally safe DAAs in this particular group of patients. However, as far as the financial implications of scaling up the hepatitis C treatment in Malaysia is concerned, the MOH has started making a special allocation to the hepatitis C program since 2018. Aiming at identifying 90% of HCV-infected individuals and treating 80% of them by 2030, the allocation will be adjusted in line with the elevated annual targets. Given that the hepatitis C program is run using the existing infrastructure and healthcare providers, such special allocations are expected to be used mainly for the screening activities and the acquisition of DAAs.
A few limitations of this study are also worth mentioning. First, this study focused only on drug expenditure. As DAAs generally have a better safety profile than does the conventional treatment, it is conceivable that a lower incidence of adverse drug events might have resulted in the reduced consumption of medical resources. Hence, a comprehensive cost analysis is necessary to provide the whole picture of the economic implications of the government-led initiatives. Moreover, this study does not manage to fully reflect the impact of the initiatives recently taken in 2019, especially the launch of the NSP. Aside from that, the cases managed by the non-MOH institutions, including private and university hospitals, were not captured in this study, and this might have led to a slight underestimation of the treatment coverage in hepatitis C patients.
In conclusion, notwithstanding the escalating budgetary pressure of the public health system, more than 6,300 courses of free pharmacological treatment were provided to hepatitis C patients in Malaysia between 2013 and 2019. The number of patients treated each year has also grown by more than 10 times since the first use of DAA in public health institutions back in 2013. The spending on the hepatitis C treatment relative to the annual health expenditure did not significantly increase over time, and this was attributable to a series of the MOH-led initiatives, including the use of multiple policy tools and inter-organizational partnerships. Nonetheless, aiming at achieving the WHO’s elimination goal by 2030, the MOH is paving a path toward massively scaling up the hepatitis C screening and treatment in the country and increasing the spending on DAAs. Thus, budget planning, close monitoring of the progress toward the goal, and the periodical revision of the annual targets are warranted going forward.
Acknowledgements
References
-
1.
Houghton M. Hepatitis C virus: 30 years after its discovery. Cold Spring Harb Perspect Med. 2019;9(12). [PubMed ID: 31501269]. [PubMed Central ID: PMC6886456]. https://doi.org/10.1101/cshperspect.a037069.
-
2.
WHO. Hepatitis C (fact sheet). Geneva: World Health Organization; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c.
-
3.
Saito T, Ueno Y. Transmission of hepatitis C virus: self-limiting hepatitis or chronic hepatitis? World J Gastroenterol. 2013;19(41):6957-61. [PubMed ID: 24222939]. [PubMed Central ID: PMC3819531]. https://doi.org/10.3748/wjg.v19.i41.6957.
-
4.
Collins LF, Chan A, Zheng J, Chow SC, Wilder JM, Muir AJ, et al. Direct-acting antivirals improve access to care and cure for patients with HIV and chronic HCV infection. Open Forum Infect Dis. 2018;5(1):ofx264. [PubMed ID: 29308413]. [PubMed Central ID: PMC5753271]. https://doi.org/10.1093/ofid/ofx264.
-
5.
Douglass CH, Pedrana A, Lazarus JV, t Hoen EFM, Hammad R, Leite RB, et al. Pathways to ensure universal and affordable access to hepatitis C treatment. BMC Med. 2018;16(1):175. [PubMed ID: 30296935]. [PubMed Central ID: PMC6176525]. https://doi.org/10.1186/s12916-018-1162-z.
-
6.
Md Said R, Mohd Zain R, Chan HK, Soelar SA, Rusli N, Nasir NH, et al. Find the missing millions: Malaysia's experience with nationwide hepatitis c screening campaign in the general population. J Viral Hepat. 2020;27(6):638-43. [PubMed ID: 31997563]. https://doi.org/10.1111/jvh.13267.
-
7.
Wilby KJ, Partovi N, Ford JA, Greanya E, Yoshida EM. Review of boceprevir and telaprevir for the treatment of chronic hepatitis C. Can J Gastroenterol. 2012;26(4):205-10. [PubMed ID: 22506260]. [PubMed Central ID: PMC3354889]. https://doi.org/10.1155/2012/751057.
-
8.
DNDi. Drugs for Neglected Diseases initiative and The BMJ launch a special collection on neglected diseases and innovation in South Asia. DNDi in South-East Asia; 2019, [cited 12 May]. Available from: https://dndi.org/press-releases/2019/dndi-the-bmj-special-collection-neglected-diseases-and-innovation-south-asia/.
-
9.
DNDi. FIND and DNDi team up to support Malaysian MOH efforts to simplify and decentralize hepatitis C screening & treatment. DNDi in South-East Asia; 2018, [cited 12 May]. Available from: https://dndi.org/press-releases/2018/find-dndi-malaysianmoh-efforts-hepatitisc-screening-treatment/.
-
10.
Ooms G, Hanefeld J. Threat of compulsory licences could increase access to essential medicines. BMJ. 2019;365:l2098. [PubMed ID: 31138530]. [PubMed Central ID: PMC6598651 interests and have no relevant interests to declare]. https://doi.org/10.1136/bmj.l2098.
-
11.
WHO. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. World Health Organization; 2018, [cited 12 May]. Available from: https://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2018/en/.
-
12.
Ministry of Health Malaysia. National Strategic Plan for Hepatitis B and C 2019 - 2023. Malaysia: Ministry of Health; 2019, [cited 12 May]. Available from: https://www.moh.gov.my/moh/resources/Penerbitan/Pelan%20Strategik%20/NSP_Hep_BC_2019_2023.pdf.
-
13.
Hassan MRA, Chan HK. Comment on: "Projections of the Healthcare Costs and Disease Burden due to Hepatitis C Infection Under Different Treatment Policies in Malaysia, 2018-2040". Appl Health Econ Health Policy. 2020;18(1):139-40. [PubMed ID: 31853748]. https://doi.org/10.1007/s40258-019-00543-x.
-
14.
DNDi. New affordable hepatitis C combination treatment shows 97% cure rate. in South-East Asia; 2018. Available from: https://dndi.org/press-releases/2018/new-affordable-hepatitis-c-combination-treatment-shows-97-cure-rate/.
-
15.
Morey S, Hamoodi A, Jones D, Young T, Thompson C, Dhuny J, et al. Increased diagnosis and treatment of hepatitis C in prison by universal offer of testing and use of telemedicine. J Viral Hepat. 2019;26(1):101-8. [PubMed ID: 30315691]. https://doi.org/10.1111/jvh.13017.
-
16.
Fernandes ND, Banik S, Abughali N, Sthapit B, Abdullah N, Fragassi P. Hepatitis C Virus Screening Among Adolescents Attending a Drug Rehabilitation Center. J Pediatric Infect Dis Soc. 2020;9(4):437-41. [PubMed ID: 31603512]. https://doi.org/10.1093/jpids/piz065.
-
17.
Busschots D, Toghanian S, Bielen R, Salomonsson S, Koc OM, Hendrickx G, et al. Eliminating viral hepatitis C in Belgium: the micro-elimination approach. BMC Infect Dis. 2020;20(1):181. [PubMed ID: 32106819]. [PubMed Central ID: PMC7045456]. https://doi.org/10.1186/s12879-020-4898-y.
-
18.
European Association for the Study of the Liver. Electronic address EEE. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol. 2017;66(1):153-94. [PubMed ID: 27667367]. https://doi.org/10.1016/j.jhep.2016.09.001.