Abstract
Background:
Recent studies have indicated that abnormal glucose levels and diabetes are negatively associated with the prognosis of patients with chronic hepatitis C virus (HCV) infection. The genetic polymorphism of the promoter region -1260 of a gene encoding the enzyme 1-alpha-hydroxylase (CYP27B1-1260) has been shown to have an impact on the signaling pathways involved in insulin secretion.Objectives:
The aim is to investigate the effect of CYP27B1-1260 polymorphism on the fasting plasma glucose (FPG) levels in patients with chronic HCV undergoing antiviral therapy.Patients and Methods:
A total of 461 patients with chronic HCV infection and 300 volunteers without HCV infection were enrolled in an observational cohort study in the China-Japan Union hospital of Jilin University and the Second Hospital of Daqing, Changchun, Jilin Province. Both groups were further divided into normal and abnormal FPG subgroups. The frequencies of the three CYP27B1-1260 genotypes (AA, AC, and CC) were determined in each subgroup. FPG levels were monitored at baseline in HCV and control participants, and both during and after antiviral therapy in HCV infected patients. The frequency of each genotype was determined. Logistic regression analysis was performed to evaluate the risk factors associated with abnormal FPG levels in HCV infected patients undergoing antiviral therapy.Results:
In HCV infected patients with abnormal FPG levels, the frequency of the genotype CC was significantly higher than that in patients with normal FPG levels (19% vs. 7%, P < 0.001). In contrast, in the control participants, the CC genotype was not significantly different between FPG groups. At baseline, the CC genotype was associate with four times more risk of IFG after adjusting for multiple variables (OR: 4.11; 95%CI: 1.98 - 8.52, P = 0.0001). During 24 weeks of anti-HCV treatment, 38 HCV participants developed newly-diagnosed impaired fasting glucose. The CC genotype markedly increased the risk for newly developed IFG (OR: 26.54; 95%CI: 7.80 - 90.32, P < 0.0001). Other risk factors included age and body mass index.Conclusions:
CYP27B1-1260 polymorphism is associated with abnormal glucose metabolism in HCV infected patients. HCV infected individuals with CYP27B1-1260 genotype CC appeared to have an increased risk of developing abnormal FPG levels.Keywords
Single Nucleotide Polymorphism Impaired Fasting Glucose Hepatitis C Virus Interferon Therapy
1. Background
Recent studies have suggested that impaired fasting glucose (IFG) and type 2 diabetes mellitus (T2DM) are not only are associated with chronic hepatitis C virus (HCV) infection, but also aggravate liver damage, thereby adversely affecting the prognosis in these patients (1, 2). The risk factors for IFG and T2DM, and the underlying molecular mechanisms by which HCV infection influences the susceptibility of patients to T2DM, remain largely unknown (3, 4). Increased fasting blood sugar levels during interferon therapy in patients with chronic hepatitis C have attracted considerable interest. Enhanced autoimmune reactions to interferon treatment have been suggested as a contributing factor for impaired pancreatic function and insulin resistance, but definitive evidence of this association has yet to be obtained (5, 6). Interferon therapy is the primary treatment regimen for HCV infection in China, and its association with IFG and T2DM requires further investigation (7).
With the advances in genome-wide association technology, significant progress has been made in isolating the genetic determinants of susceptibility to hyperglycemia. Over two dozen T2DM-associated genes have been identified, but the crucial ones that initiate the pathogenesis of T2DM have yet to be discovered (4, 8-10). Recently, the active form of vitamin D3 (1,25(OH)2D3) was shown to be closely associated with the development and prognoses of several diseases (11). Studies have shown that 1,25(OH)2D3 can increase the cytoplasm Ca2+ levels in islet β cells, enhance the binding of calmodulin to insulin receptor substrate 1 (IRS-1), regulate the PI3K/AKT signaling pathway, and stimulate insulin secretion, indicating that 1,25(OH)2D3 deficiency may play a role in the pathogenesis of T2DM (12-15). Renal mitochondrial enzyme 1-alpha-hydroxylase, converting 25(OH)D3 to 1,25(OH)2D3, is encoded by gene CYP27B1. Polymorphisms of CYP27B1 have been associated with the plasma levels of 1,25(OH)2D3 (16). However, whether this gene polymorphism is also associated with the impaired fasting glucose in patients with HCV infection remains unclear.
2. Objectives
As a result of these observations, in the present study, we aimed to investigate the effects of CYP27B1-1260 polymorphism on fasting blood glucose in patients with chronic hepatitis C, both before and after treatment with pegylated interferon alfa-2a and ribavirin (Peg-IFNα-2a/RBV) therapy.
3. Patients and Methods
3.1. Patients
Patients with chronic hepatitis C who were admitted to the China-Japan Union hospital of Jilin University and the second hospital of Daqing in the period between 2009 and 2013 were enrolled in this observational cohort study. Diagnosis of chronic hepatitis C was conducted as per the clinical guidelines of the American association for the study of liver diseases in 2004 (17). IFG were diagnosed as per the American Diabetes Association guidelines from 2009 (18).
3.2. Inclusion Criteria
The inclusion criteria consisted of: 1) confirmed HCV infection with an HCV viral load > 50 IU/ml; 2) no history of antiviral therapy. The exclusion criteria were: 1) pregnancy; 2) liver cirrhosis; 3) patients with mental illness, uncontrolled epilepsy, autoimmune diseases, symptomatic or severe heart disease, and impaired kidney function; 4) alcohol or substance abuse; 5) infection with hepatitis A, B, D or E, EBV, CMV, or HIV infection; 6) presence of gastrointestinal bleeding, hepatic encephalopathy, primary liver cancer, and other complications.
A total of 491 patients with chronic hepatitis C qualified for the selection criteria and were enrolled in the study. Thirty patients withdrew from the study due to complications from interferon therapy or loss during the follow-up process (18 with either neutropenia or thrombocytopenia; three with decreased hemoglobin; two with thyroid dysfunction; one with interstitial pneumonia, and six lost during the follow-up). Therefore, a total of 461 patients were included in the final analysis. Meanwhile, 300 volunteers without HCV infection from the same geographical area were enrolled in the control group. Informed consent was obtained from all study subjects. The study was approved by the Ethics Committee at the China-Japan Union Hospital of Jilin University and the second hospital of Daqing, Changchun, Jilin province (study code: CHICTR-ONRC-12002207.
3.3. Study Design
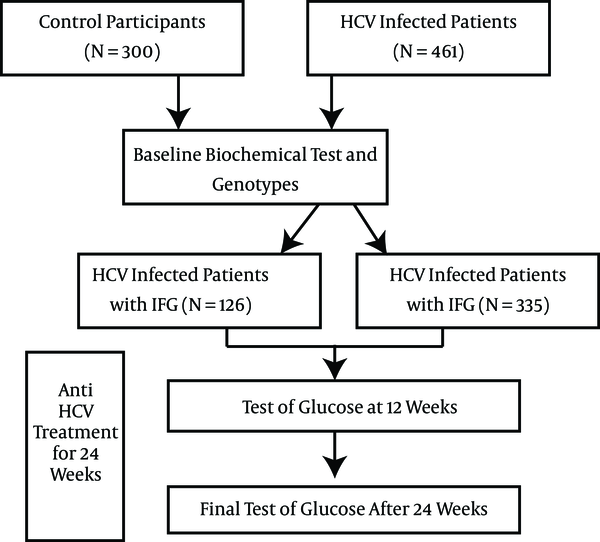
HCV-infected patients and healthy controls were divided into FPG normal and abnormal groups according to the baseline FPG levels. All HCV patients were administered Peg-IFNα-2a/RBV treatments for 24 weeks. Correlations between the CYP27B1-1260 genotypes and abnormal FPG at baseline and after therapy were assessed. Risk factors affecting impaired fasting glucose were also investigated through backward logistic regression. A schematic illustration of the study design is shown in Figure 1.
Schematic Illustration of the Study Design
3.4. Treatment Regimen and Efficacy
In patients diagnosed with T2DM, insulin was administered to control the FPG levels prior to the initiation of Peg-IFNα-2a/RBV treatment. The Peg-IFNα-2a dosage was 180 μg/w and the duration of treatment was 48 weeks for patients with HCV genotype 1 infection, while the duration was 24 weeks for those with other HCV genotypes.
The RBV dosages were as follows: 1) for patients with HCV genotype 1 infection, 1200 mg/d for those weighing > 75 kg, and 1000 mg/d for those weighing ≤ 75 kg; 2) for patients with infection with other HCV genotypes, 1000 mg/d for those weighing > 75 kg, and 800 mg/d for those weighing ≤ 75 kg.
During treatment, insulin dosage was adjusted and the Peg-IFNα-2a dosage was reduced gradually to 135 and 90 μg/w in the event of an increase in FPG level to 7.8 - 10.0 mmol/L. If the FPG level approached ≥ 10.0 mmol/L, the insulin dosage was titrated accordingly, and the Peg-IFNα-2a was reduced further to 67.5 μg/w or discontinued (19). In the event of the development of leucopenia, the Peg-IFNα-2a dosage was reduced as described above. If pancytopenia occurred, cell growth-stimulating factor (C-GSF) and erythropoietin were administered, and the Peg-IFNα-2a dosage was reduced or discontinued. Upon resolution of the adverse effects, the Peg-IFNα-2a dosage was gradually restored to the standard level. An FPG level ≥ 5.6 mmol/L indicated IFG, while the FPG was considered as having improved if the level was < 6.1 mmol/L following reduced dosage or discontinuation of insulin treatment. Insulin treatment was considered to be ineffective if FPG level was ≥ 6.1 mmol/L even with an appropriate dose of insulin (18).
3.5. Clinical and Laboratory Parameters
Age, gender, and body mass index (BMI) were collected during the participants’ first clinical visits by professional staff. Both the weight and height of each participant were recorded on a scale. BMI was than calculated by height (m) divided by weight (kg)2.
Laboratory parameters were assessed in patients prior to the initiation of antiviral therapy. These included: FPG, liver function, anti-HCV antibodies, HCV RNA quantification, HCV genotyping, IL-28B genotypes, and CYP27B1-1260 genotypes. During the first month of Peg-IFNα-2a/RBV treatment, routine blood tests were performed at weekly intervals. Afterwards, the test was performed at monthly intervals until the sixth month, followed thereafter by once every three months. Liver function and FPG were examined once every month during the course of treatment, and once every three months post-treatment. The HCV RNA levels were measured at weeks 4, 12, 24, and 48 of treatment, and at 6 months post-treatment. Patients with abnormal pre-treatment thyroid function were monitored at monthly intervals.
The routine blood test was performed using the XE-5000 Hematology Analyzer (Sysmex, Kobe, Japan). Liver function and FPG were measured by using an automatic biochemistry analyzer with the relevant reagents obtained from Beckman Coulter (Pasadena, CA, USA). Serum HCV RNA levels were determined using a real-time quantitative polymerase chain reaction (RT-qPCR), the reagents for which were obtained from Da’an Gene of Yat-Sen University (Guangzhou, Guangdong, China). The HCV genotype was determined using the PCR-oligo hybridization-ELISA kit obtained from Lanxing Biotech (Guangzhou, Guangdong, China). The IL-28B genotype was determined using an ABI3730 sequencer obtained from Sangon Biotech (Shanghai, China) following the manufacturer’s instructions. The CYP27B1-1260 polymorphism was examined through sequencing by using the primers: F 5’-GGTGGCGTATGCCTGTAGTG-3’, R 5’-TGTTCCCTAAGTGTTGTCTCTGG -3’. The results were analyzed with 3730 fast system image analytic software V1.3.1 (Applied Biosystems, Foster City, CA, USA).
3.6. Statistical Analysis
Quantitative data were expressed as mean ± standard deviation (SD); inter-group differences were assessed using a t test or nonparametric statistical methods. Qualitative data were expressed as percentages (%); a Pearson chi-square test was performed to assess differences in genotypes and allele frequencies. The allele frequency of the CYP27B1-1260 rs10877012 A/C polymorphism was calculated with the Hardy-Weinberg Equilibrium test. Stepwise logistic regression analysis was performed, and odds ratios (OR) with 95% confidence intervals (CI) were calculated. Post-hoc power was 85.3% based on the estimated relative risk for CC genotype as four times higher than AA or AC. A P value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistical Analysis Software Version 19.0 (SPSS Inc., Chicago, IL, USA).
4. Results
4.1. Baseline Characteristic in Chronic Hepatitis C Patients
A total of 461 patients (251 males, 210 females; male: female = 1.19:1) with chronic hepatitis C were recruited for the study. The average age of the patients was 44.6 ± 7.1 years (range 31 - 72 years). Baseline characteristics are outlined in Table 1. Patients with higher fasting plasma glucose were significantly older (P < 0.001), had higher body mass index (P < 0.001), and had higher ALT levels (P < 0.001). More patients in the abnormal FPG group had positive family histories for diabetes (P < 0.001). More importantly, the CYP27B1-1260 genotype frequencies were significantly different between the FPG groups (P = 0.001), whereas the IL-28B genotypes were similar between groups (P = 0.375). There were no significant differences in gender distribution, HCV genotype, or HCV RNA load.
Baseline Characteristics of HCV Patients Grouped by Different Fasting Blood Glucose Level Status (N = 461)a
| Characteristic | Abnormal FPG (N = 126) | Normal FPG (N = 335) | P Value |
|---|---|---|---|
| Male gender | 66 (52.4) | 185 (55.2) | 0.775 |
| Age | 48.9 ± 6.9 | 43.7 ± 5.3 | < 0.001 |
| BMI | 26.0 ± 2.2 | 23.5 ± 1.9 | < 0.001 |
| ALT | 93.3 ± 37.6 | 63.6 ± 22.8 | < 0.001 |
| HCV type 1 | 113 (89.7) | 274 (81.8) | 0.290 |
| HCV RNA load > 4 × 105 IU/mL | 70 (55.6) | 210 (62.7) | 0.441 |
| CYP27B1-1260 Genotype AA | 48 (38) | 144 (43) | 0.001 |
| AC | 54 (43) | 168 (50) | |
| CC | 24 (19) | 23 (7) | |
| IL-28B genotype CC | 96 (76.2) | 279 (83.3) | 0.375 |
| Family history of diabetes | 102 (81.0) | 149 (44.5) | < 0.001 |
4.2. CYP27B1-1260 and IL-28B Genotypes and Allele Frequencies
The genotype and allele frequencies of CYP27B1-1260 were compared among the four subgroups (Table 2). In the HCV patients, the CC genotypes were significantly higher in patients with abnormal FPG than ones with normal FPG (P < 0.001), whereas in the healthy controls, no such difference was observed (P = 0.805). There was no difference in the C allele frequencies between patients with or without normal FPG in either the HCV or healthy participants. When comparing the genotype frequencies to those of the control participants, no sufficient difference was observed (Table 2).
Genotype and Allele Frequencies of CYP27B1-1260 and IL28 in HCV Infected Patients Before Antiviral Treatment and in the Non-Infected Controlsa,b,c,d
| HCV Infected Patients | Non-Infected Controls | P Valuec | P Valued | ||||
|---|---|---|---|---|---|---|---|
| Abnormal FPG (N = 126) | Normal FPG (N = 335) | P Valueb | Abnormal FPG (N = 91) | Normal FPG (N = 209) | |||
| CYP27B1-1260 Genotype | |||||||
| AA | 48 (38) | 143 (43) | 45 (49) | 90 (43) | 0.135 | ||
| AC | 54 (43) | 168 (50) | 0.909 | 38 (42) | 106 (51) | 0.239 | 0.712 |
| CC | 24 (19) | 23 (7) | < 0.001 | 8 (9) | 13 (4) | 0.805 | 0.436 |
| Allele frequency | |||||||
| A | 60 | 68 | 70 | 68 | 0.920 | ||
| C | 40 | 32 | 0.864 | 30 | 32 | 0.966 | 0.884 |
| IL-28B genotype | |||||||
| CC | 96 (76.2) | 279 (83.3) | |||||
| CT | 30 (23.8) | 56 (16.7) | 0.375 | ||||
IL-28B genotype analysis showed that 375 patients were found to have the CC genotype, while 86 patients had the CT genotype, and no patients had the TT genotype. There was no difference in IL-28B genotypes between the FPG groups (P = 0.375).
4.3. Proportions of Impaired Fasting Glucose Throughout Antiviral Treatment
In the 461 HCV-infected patients, the accumulated proportions of IFG are presented in Figure 2. At baseline, 50% of the CC patients already had higher fasting glucose, while in week 12 of the antiviral treatment, 56.3% of the CC patients were diagnosed with IFG through the fasting glucose test. At the end of the treatment process, over 80% of CC carriers had higher fasting glucose. The proportions of IFG at the three time points were all significantly higher than those of the AA or AC participants (all P < 0.001).
Proportions of Impaired Fasting Glucose in Different CYP27B1-1260 Genotypes Throughout Anti-HCV Treatment
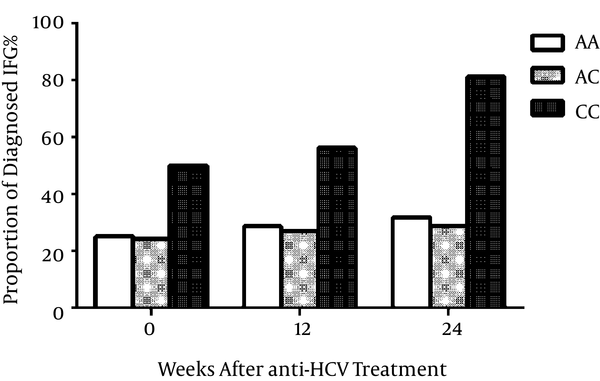
4.4. Associations Between Genotype Polymorphism and Risk of Impaired Fasting Glucose
At baseline, associations between CYP27B1-1260 and IL-28B genotypes and IFG risk are presented in Table 3. The CC genotype was significantly associated with a higher risk of IFG in a multivariable regression model (OR: 4.11, 95%CI: 1.98 - 8.52), whereas the IL-28B genotype CT was not associated with a risk of impaired glucose (OR: 1.04; 95%CI: 0.60 - 1.81).
Association Between Genotype Polymorphism and the Risk of Baseline Impaired Fasting Glucose in HCV Infected Patients (N = 461)a
| P for Trend | ||||
|---|---|---|---|---|
| CYP27B1-1260 Genotypes | ||||
| AA | AC | CC | ||
| Unadjusted | 1.00 | 0.96 (0.61 - 1.50) | 2.98 (1.55 - 5.72) | 0.016 |
| Multivariable adjusted | 1.00 | 1.82 (1.07 - 3.09) | 4.11 (1.98 - 8.52) | 0.0001 |
| IL-28B Genotype | ||||
| CC | CT | |||
| Unadjusted | 1.00 | 0.95 (0.57 - 1.59) | 0.858 | |
| Multivariable adjusted | 1.00 | 1.04 (0.60 - 1.81) | 0.882 | |
After 24 weeks of anti-HCV treatment, 38 patients experienced newly-developed higher fasting glucose. A backward logistic regression model was used to analyze the risk factors associated with the increased likelihood of newly-diagnosed IFG (Table 4). The CYP27B1-1260 CC genotype was significantly associated with an increased likelihood for newly-developed IFG as compared to the AA genotype (OR: 26.12; 95%CI: 7.85 - 86.92; P < 0.0001). Other risk factors included age and BMI. Multi-variable regression showed a similar trend (OR: 26.54; 95%CI: 7.80 - 90.32; P for trend < 0.0001; Table 5). The IL-28B genotype was not associated with newly developed IFG either before or after multi-variable adjustment.
Risk Factors Associated With Newly-Diagnosed Impaired Fasting Glucose After 24-Week Anti-Viral Treatment in HCV Infected Patients (N = 335)a
| Variables | Odds Ratios (95% CI) | P Value |
|---|---|---|
| Age | 0.96 (0.93 - 1.00) | 0.036 |
| BMI | 1.17 (1.01 - 1.37) | 0.043 |
| Liver fibrosis | 0.48 (0.22 - 1.08) | 0.076 |
| CYP27B1-1260 genotypes AA | 1.00 | |
| AC | 1.03 (0.39 - 2.76) | 0.953 |
| CC | 26.12 (7.85 - 86.92) | < 0.0001 |
Association Between Genotype Polymorphism and the Risk of Newly-Diagnosed Impaired Fasting Glucose After 24-Week Anti-Viral Treatment in HCV Infected Patients (N = 335)a
| P for Trend | ||||
|---|---|---|---|---|
| CYP27B1-1260 Genotypes | ||||
| AA | AC | CC | ||
| Unadjusted | 1.00 | 0.63 (0.27 - 1.49) | 16.67 (6.11 - 45.49) | < 0.0001 |
| Multivariable adjusted | 1.00 | 1.02 (0.37 - 2.80) | 26.54 (7.80 - 90.32) | < 0.0001 |
| IL-28B Genotype | ||||
| CC | CT | |||
| Unadjusted | 1.00 | 0.70 (0.28 - 1.74) | 0.438 | |
| Multivariable adjusted | 1.00 | 0.54 (0.21 - 1.40) | 0.204 | |
5. Discussion
This study demonstrated that the CC genotype of CYP27B1-1260 was associated with a higher risk of impaired fasting glucose in HCV infected patients, especially during anti-viral treatment. To our knowledge, this was the first study investigating genetic polymorphism and hyperglycemia in Chinese HCV-infected patients.
Chronic HCV infection complicated by diabetes could aggravate liver injury (2). The incidence of T2DM during chronic HCV infection has been reported to be 13.8% in a case control study (20), which was higher than the rate known to be induced by other chronic liver diseases (1). In our study, we observed 27.3% of HCV patients with impaired fasting glucose at baseline, whereas 11.3% of HCV patients developed IFG during 24 weeks of anti-viral therapy. Previous studies also suggested that HCV patients were more susceptible to developing insulin resistance (21) and impaired glucose tolerance (22). Therefore, our results reinforced the evidence that chronic HCV infection and diabetes were inter-related.
In our study, we also observed a higher risk of IFG in HCV patients with the CC genotype of CYP27B1-1260. Current studies on genetic factors associated with T2DM primarily focus on the genes implicated in insulin resistance and the dysfunction of islet β cells (4). Nevertheless, vitamin D levels were shown to be closely associated with insulin secretion and insulin resistance. Vitamin D deficiency has also induced insulin resistance in the skeletal muscles, as demonstrated in mouse models (23). The islet β cells express vitamin D receptors on the cell surface, and also produce the enzyme 1-alpha-hydroxylase, which converts 25(OH)D3 into its bioactive form 1,25(OH)2D3. Evidence has shown that the reduction in 1-alpha-hydroxylase activity may have led to vitamin D deficiency, which subsequently leads to decreased interaction with vitamin D receptors and further results in β cell dysfunction and impaired insulin production (12, 24, 25).
Investigation of the gene CYP27B1 encoding 1-alpha-hydroxylase showed that the polymorphism in its promoter region-1260 has a substantial impact on the enzymatic activity of 1-alpha-hydroxylase and thus influences the serum 1,25(OH)2D3 level (16). Further studies have found that HCV infected individuals carrying the genotype CC had significantly lower serum 1,25(OH)2D3 levels and showed poorer sustained virological responses to interferon therapy when compared with those carrying the AA and AC genotypes (16). Besides its impact on HCV infection, previous research has suggested a possible relationship between CYP27B1-1260 polymorphism and the persistence of, but not susceptibility to, hepatitis B virus (HBV) infection (26). In patients with type 1 diabetes mellitus (T1DM), the mRNA levels of CYP27B1 in the peripheral blood mononuclear cells of those with CC genotype have been reported to be much lower than those in the healthy controls (27). Consequently, the CYP27B1-1260 genotype CC has been considered to be a risk factor for T1DM (27, 28). All of these results, including our own, imply that, as a factor influencing the level of active vitamin D in the human body, the genotype CC may contribute to the development of hyperglycemia and T2DM.
In the present study, the CYP27B1-1260 genotypes were examined in patients with both chronic HCV infection and in controls at baseline. The marked higher frequency of the genotype CC in the HCV infected patients with abnormal FPG levels was not observed in the uninfected controls. Therefore, it appears that the genotype CC is more frequently associated with abnormal FPG in patients with chronic hepatitis C. During antiviral therapy, the genotype CC was consistently associated with a higher risk of IFG in HCV patients. Previous studies suggested that the genotype CC of CYP27B1-1260 was a potential risk factor for T1DM, T2DM, Hashimoto's thyroiditis, Graves’ disease, chronic hepatitis B, and hepatitis C (29). However, contrasting results have been reported from studies on some other diseases including multiple sclerosis. In some cases, the CYP27B1-1260 polymorphism does not seem to be associated with disease progression (30). We therefore think that the role of 1-alpha-hydroxylase might be disease-specific, and that the involvement of CYP27B1-1260 polymorphism as a genetic susceptibility factor in pathogenesis should be defined individually for different disease types. Although our results require further validation through clinical studies, they still provide a new direction for the investigation of the relationship between chronic HCV infection and impaired glucose metabolism.
The strength of our study was the employment of a prospective cohort design to assess the records of HCV patients with newly developed abnormal fasting glucose. There were also certain limitations in our study, which ought to be highlighted. First, the functions mediated by vitamin D are also in part related to the gene polymorphisms of the vitamin D receptor and the vitamin D-binding protein. We did not examine the genotype distribution of these genes in our patients, nor did we evaluate the levels of vitamin D in the participants. Furthermore, insulin was frequently administered in patients who showed abnormal FPG levels during the antiviral treatment; however, the extent of its influence on the results of our study remains largely unclear. These issues should be addressed in future investigations.
In conclusion, the CYP27B1-1260 polymorphism is associated with abnormal glucose metabolism in HCV-infected patients. HCV patients with CYP27B1-1260 genotype CC appear to be at an increased risk of developing abnormal FPG levels both during and after antiviral treatment.
References
-
1.
Naing C, Mak JW, Ahmed SI, Maung M. Relationship between hepatitis C virus infection and type 2 diabetes mellitus: meta-analysis. World J Gastroenterol. 2012;18(14):1642-51. [PubMed ID: 22529694]. https://doi.org/10.3748/wjg.v18.i14.1642.
-
2.
Elkrief L, Chouinard P, Bendersky N, Hajage D, Larroque B, Babany G, et al. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology. 2014;60(3):823-31. [PubMed ID: 24841704]. https://doi.org/10.1002/hep.27228.
-
3.
Petit JM, Bour JB, Galland-Jos C, Minello A, Verges B, Guiguet M, et al. Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J Hepatol. 2001;35(2):279-83. [PubMed ID: 11580152].
-
4.
Staiger H, Machicao F, Fritsche A, Haring HU. Pathomechanisms of type 2 diabetes genes. Endocr Rev. 2009;30(6):557-85. [PubMed ID: 19749172]. https://doi.org/10.1210/er.2009-0017.
-
5.
Fabris P, Betterle C, Greggio NA, Zanchetta R, Bosi E, Biasin MR, et al. Insulin-dependent diabetes mellitus during alpha-interferon therapy for chronic viral hepatitis. J Hepatol. 1998;28(3):514-7. [PubMed ID: 9551692].
-
6.
Betterle C, Fabris P, Zanchetta R, Pedini B, Tositti G, Bosi E, et al. Autoimmunity against pancreatic islets and other tissues before and after interferon-alpha therapy in patients with hepatitis C virus chronic infection. Diabetes Care. 2000;23(8):1177-81. [PubMed ID: 10937518].
-
7.
Welch NM, Jensen DM. Pegylated interferon based therapy with second-wave direct-acting antivirals in genotype 1 chronic hepatitis C. Liver Int. 2015;35 Suppl 1:11-7. [PubMed ID: 25529082]. https://doi.org/10.1111/liv.12715.
-
8.
Florez JC, Jablonski KA, Bayley N, Pollin TI, de Bakker PI, Shuldiner AR, et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med. 2006;355(3):241-50. [PubMed ID: 16855264]. https://doi.org/10.1056/NEJMoa062418.
-
9.
Groves CJ, Zeggini E, Minton J, Frayling TM, Weedon MN, Rayner NW, et al. Association analysis of 6,736 U.K. subjects provides replication and confirms TCF7L2 as a type 2 diabetes susceptibility gene with a substantial effect on individual risk. Diabetes. 2006;55(9):2640-4. [PubMed ID: 16936215]. https://doi.org/10.2337/db06-0355.
-
10.
Zhang C, Qi L, Hunter DJ, Meigs JB, Manson JE, van Dam RM, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene and the risk of type 2 diabetes in large cohorts of U.S. women and men. Diabetes. 2006;55(9):2645-8. [PubMed ID: 16936216]. https://doi.org/10.2337/db06-0643.
-
11.
McGrath JJ, Saha S, Burne TH, Eyles DW. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2010;121(1-2):471-7. [PubMed ID: 20363324]. https://doi.org/10.1016/j.jsbmb.2010.03.073.
-
12.
Bourlon PM, Faure-Dussert A, Billaudel B. Modulatory role of 1,25 dihydroxyvitamin D3 on pancreatic islet insulin release via the cyclic AMP pathway in the rat. Br J Pharmacol. 1997;121(4):751-8. [PubMed ID: 9208144]. https://doi.org/10.1038/sj.bjp.0701204.
-
13.
Hoenderop JG, Dardenne O, Van Abel M, Van Der Kemp AW, Van Os CH, St -Arnaud R, et al. Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3-1alpha-hydroxylase knockout mice. FASEB J. 2002;16(11):1398-406. [PubMed ID: 12205031]. https://doi.org/10.1096/fj.02-0225com.
-
14.
Munshi HG, Burks DJ, Joyal JL, White MF, Sacks DB. Ca2+ regulates calmodulin binding to IQ motifs in IRS-1. Biochemistry. 1996;35(49):15883-9. [PubMed ID: 8961953]. https://doi.org/10.1021/bi962107y.
-
15.
Li Z, Joyal JL, Sacks DB. Binding of IRS proteins to calmodulin is enhanced in insulin resistance. Biochemistry. 2000;39(17):5089-96. [PubMed ID: 10819975].
-
16.
Lange CM, Bojunga J, Ramos-Lopez E, von Wagner M, Hassler A, Vermehren J, et al. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J Hepatol. 2011;54(5):887-93. [PubMed ID: 21145801]. https://doi.org/10.1016/j.jhep.2010.08.036.
-
17.
Strader DB, Wright T, Thomas DL, Seeff LB, American Association for the Study of Liver D. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39(4):1147-71. [PubMed ID: 15057920]. https://doi.org/10.1002/hep.20119.
-
18.
American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32 Suppl 1:S62-7. [PubMed ID: 19118289]. https://doi.org/10.2337/dc09-S062.
-
19.
Arase Y, Kobayashi M, Suzuki F, Suzuki Y, Kawamura Y, Akuta N, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57(3):964-73. [PubMed ID: 22991257]. https://doi.org/10.1002/hep.26087.
-
20.
Elhawary EI, Mahmoud GF, El-Daly MA, Mekky FA, Esmat GG, Abdel-Hamid M. Association of HCV with diabetes mellitus: an Egyptian case-control study. Virol J. 2011;8:367. [PubMed ID: 21791087]. https://doi.org/10.1186/1743-422X-8-367.
-
21.
Sciacqua A, Perticone M, Tassone EJ, Cimellaro A, Caroleo B, Miceli S, et al. Renal function is impaired in normotensive chronic HCV patients: role of insulin resistance. Intern Emerg Med. 2016;11(4):553-9. [PubMed ID: 26597876]. https://doi.org/10.1007/s11739-015-1349-7.
-
22.
Kaur H, Singh P, Pannu HS, Sood A, Jain NP, Bhoday HS. To study the prevalence of impaired glucose tolerance in patients with hepatitis C virus related chronic liver disease. J Clin Diagn Res. 2015;9(3):OC16-20. [PubMed ID: 25954649]. https://doi.org/10.7860/JCDR/2015/11444.5665.
-
23.
Chen S, Villalta SA, Agrawal DK. FOXO1 Mediates Vitamin D Deficiency-Induced Insulin Resistance in Skeletal Muscle. J Bone Miner Res. 2016;31(3):585-95. [PubMed ID: 26462119]. https://doi.org/10.1002/jbmr.2729.
-
24.
Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol. 1994;267(3 Pt 1):E356-60. [PubMed ID: 7943215].
-
25.
Bland R, Markovic D, Hills CE, Hughes SV, Chan SL, Squires PE, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89-90(1-5):121-5. [PubMed ID: 15225758]. https://doi.org/10.1016/j.jsbmb.2004.03.115.
-
26.
Zhu Q, Li N, Han Q, Li Z, Zhang G, Li F, et al. Single-nucleotide polymorphism at CYP27B1-1260, but not VDR Taq I, is possibly associated with persistent hepatitis B virus infection. Genet Test Mol Biomarkers. 2012;16(9):1115-21. [PubMed ID: 22963605]. https://doi.org/10.1089/gtmb.2012.0148.
-
27.
Bailey R, Cooper JD, Zeitels L, Smyth DJ, Yang JH, Walker NM, et al. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes. 2007;56(10):2616-21. [PubMed ID: 17606874]. https://doi.org/10.2337/db07-0652.
-
28.
Ramos-Lopez E, Kahles H, Weber S, Kukic A, Penna-Martinez M, Badenhoop K, et al. Gestational diabetes mellitus and vitamin D deficiency: genetic contribution of CYP27B1 and CYP2R1 polymorphisms. Diabetes Obes Metab. 2008;10(8):683-5. [PubMed ID: 18476984]. https://doi.org/10.1111/j.1463-1326.2008.00879.x.
-
29.
Lopez ER, Zwermann O, Segni M, Meyer G, Reincke M, Seissler J, et al. A promoter polymorphism of the CYP27B1 gene is associated with Addison's disease, Hashimoto's thyroiditis, Graves' disease and type 1 diabetes mellitus in Germans. Eur J Endocrinol. 2004;151(2):193-7. [PubMed ID: 15296474].
-
30.
Jensen CJ, Stankovich J, Van der Walt A, Bahlo M, Taylor BV, van der Mei IA, et al. Multiple sclerosis susceptibility-associated SNPs do not influence disease severity measures in a cohort of Australian MS patients. PLoS One. 2010;5(4). ee10003. [PubMed ID: 20368992]. https://doi.org/10.1371/journal.pone.0010003.