Abstract
Background:
After treatment cessation, a high prevalence of relapse was reported in chronic hepatitis B (CHB) patients in China, especially in nucleot(s)ide analogues (NUCs)-experienced patients. Re-treatment for these patients remains unsolved.Objectives:
This study aims to evaluate the efficacy of PEGylated interferon in HBeAg positive patients with exposure to antiviral therapy.Patients and Methods:
A total of 55 treatment-experienced, HBeAg positive Chinese patients were enrolled in this study. Of these patients, 33 were NUCs-experienced and 22 were interferon-experienced. PEGylated interferon was administered to 34 patients; and 21 patients were retreated with conventional interferon.Results:
Of the 34 treatment-experienced patients who received PEGylated interferon, 52.9% achieved virologic response, and 41.2% achieved HBeAg loss and seroconversion. Patients who were treated with PEGylated interferon for 48 weeks achieved higher virologic response (80%); HBeAg loss (60%); HBeAg seroconversion (60%); and HBsAg loss (5%) than patients treated for 24 weeks with PEGylated interferon. Their responses were also higher than those who were treated with conventional interferon. HBeAg seroconversion in treatment-experienced patients was independently associated with 48-week PEGylated interferon therapy duration.Conclusions:
PEGylated interferon was effective in treatment-experienced patients with HBeAg positive CHB, and showed higher rates of virological response, HBeAg loss, and seroconversion. The results provide important information regarding the role of re-treatment with PEGylated interferon in treatment-experienced HBeAg positive patients.Keywords
Chronic Hepatitis B PEGylated Interferon Treatment-Experienced Nucleot(s)ide Analogues
1. Background
Approximately 350 million people are infected with the hepatitis B virus (HBV), and from 15% to 40% of infected patients will also develop cirrhosis, hepatic failure, and hepatocellular carcinoma (1). Nucleot(s)ide analogues (NUCs) and interferon are approved for the treatment of chronic hepatitis B (CHB) (2-4). Furthermore, patients successfully treated for chronic hepatitis B are less likely to develop cirrhosis, hepatic failure, and hepatocellular carcinoma (5).
The ideal endpoint of antiviral therapy is sustained off-therapy HBsAg loss. And a satisfactory endpoint is sustained off-therapy HBV DNA undetectability, normalization of alanine aminotransferase, and seroconversion to anti-HBe antibody. However, long-term, sometimes even life-long, use of NUCs, drug-related side effects, and a high incidence of drug-resistance mutations limit adherence to antiviral therapy. The HBV replication will likely rebound once the antiviral therapy ceases; in fact, several studies have reported high relapse rates after discontinuation of antiviral therapy (6-9). The efficacy of entecavir (ETV) in treatment-experienced patients was also explored, and ETV proved to be efficient in treatment-experienced patients. The efficacy was not influenced by prior treatment with adefovir, although it was decreased in patients with lamivudine-resistance (10). Currently, data on re-treatment for those treatment-experienced patients are limited.
PEGylated interferon (Peg-IFN), with double mechanisms of direct-acting antiviral and immune modulatory effect, has a prolonged half-life and augmented therapeutic efficiency. Additionally, the advantages of Peg-IFN treatment over nucleos(t)ide analogues are not only is the patient free of drug resistance, but also experiences definite therapy duration (11). And of the HBeAg positive patients, a full 32% achieved HBeAg seroconversion at 6 months following 48 weeks of Peg-IFN therapy (12, 13). And perhaps most significantly, for HBeAg negative patients, less than 20,000 copies/mL of HBV DNA were indicated in 43% of the patients, and less than 400 copies/mL of HBV DNA were indicated in 19% of the patients at 6 months post-therapy (14).
2. Objectives
This study aims to evaluate the efficacy of Peg-IFN in HBeAg positive CHB patients with prior antiviral therapy and explore the potential factors associated with HBeAg seroconversion.
3. Patients and Methods
3.1. Patients
Between June 2004 and June 2014, 55 treatment-experienced Chinese patients who received interferon therapy (Peg-IFN or conventional interferon) as per the patients’ willingness, visited the hepatitis clinic of the Second Affiliated Hospital of Chongqing Medical University were retrospectively analyzed.
All of the fifty-five Chinese patients were older than 18 years of age, had failed in previous antiviral therapy due to poor adherence, and had been HBsAg positive for at least 24 weeks. They were also HBeAg positive and anti-HBe antibody negative, with a serum HBV DNA of greater than 3 log10 copies/mL before receiving interferon or Peg-IFN therapy (at baseline). The patients had a completed therapy duration on interferon or Peg-IFN for at least 24 weeks, as well as a follow up time, calculated from end of treatment (EOT) through last visit, of at least 24 weeks. Patients with HBeAg negative at baseline (n = 14), HBV DNA of 3 log10 copies/mL or less at baseline (n = 8), and a follow up time of less than 24 weeks (n = 2) were excluded.
The study was conducted in accordance with the ethical guidelines of the declaration of Helsinki, and was approved by the institutional review boards (IRB) for human subject review at the second affiliated hospital of Chongqing medical university. In accordance with the IRB, no informed consent was required because the data was going to be analyzed anonymously.
3.2. Laboratory Tests
Serum alanine aminotransferase (ALT) was quantified by using the HITACHI 7600 analyzer (HITACHI High-Technologies, Tokyo, Japan). The Hepatitis B surface antigen, antibodies against HBsAg, hepatitis B e antigen, and antibody against HBeAg were measured by the Cobas E601 immunoassay analyzer (Roche Diagnostics GmbH, Mannheim, Germany) and by the Abbott Architect i2000 immunoassay analyzer (Abbott Diagnostics, Abbott Park, IL, USA). Serum HBV DNA levels were detected by real-time polymerase chain reactions, with a detection limit = 1000 copies/mL (Shanghai Fosun Long March Medical Science Co., Ltd., China).
3.3. Endpoints
HBeAg seroconversion (defined as a loss of HBeAg and presence of anti-HBe antibody) at 24 weeks after EOT was the primary endpoint. Secondary endpoints included virologic responses (VR, defined in terms of HBV DNA <3 log10 copies/mL by PCR assay) and serological responses, including HBeAg and HBsAg loss at 24 weeks after EOT.
3.4. Safety Analysis
The frequency and type of adverse events both within clinical laboratory parameters and in vital signs were evaluated.
3.5. Statistical Analysis
All statistical analyses were performed using SPSS statistics 17.0 set α at 0.05 and all P values are two-sided. Continuous variables were expressed as means with a standard deviation (SD) or median. Categorical variables were presented as numbers (percentages). Comparisons were performed by the Mann-Whitney U test for continuous variables, and the Chi-square test or Fisher’s exact test were used, when appropriate, for categorical variables.
Logistic regression analysis was conducted to identify the potential predictors for HBeAg seroconversion at 6-months post-treatment. All prognostic factors were stratified, and odds ratios (OR), confidence intervals (95% CI), and P values were used to define the results. And finally, variance inflation factors were used to assess co-linearity.
4. Results
4.1. Patient Characteristics
A total of 55 treatment-experienced patients were enrolled the study; of these, 33 were NUCs-experienced patients and 22 were IFN-experienced patients. Most of the patients were men (70.9%) and the average age was 27.8 ± 7.5 years.
Of the total, 20 patients received Peg-IFN for 48 weeks; 14 patients received Peg-IFN for 24 weeks; and 21 patients received conventional interferon (cIFN) for at least 24 weeks. The median therapy duration was 36 weeks, ranging from 24 weeks to 48 weeks for the cIFN group. Data at baseline were comparable (data were not shown).
4.2. Virological Response
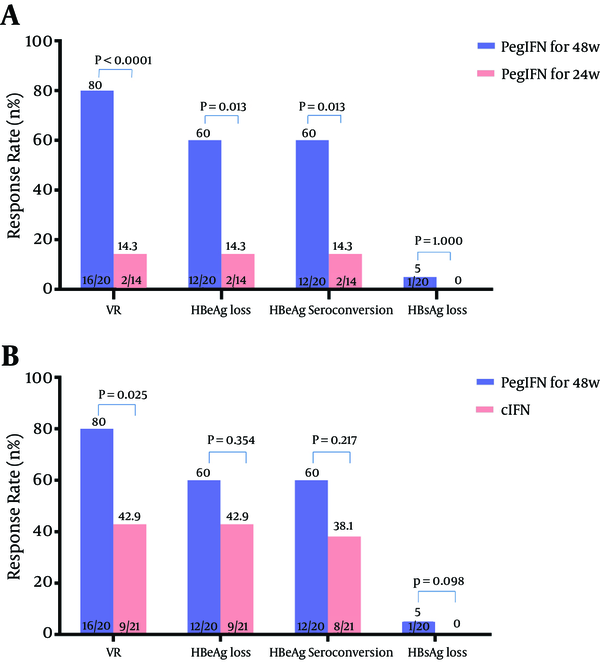
Virological responses (VR) were achieved by 27 of 55 patients (49.1%) at 24 weeks after EOT. The rate of VR was 80% (16/20) in patients with 48-week Peg-IFN therapy, 14.3% (2/14) in patients with 24-week Peg-IFN therapy, and 42.9% (9/21) in patients with cIFN. The differences were both statistically significant (80% vs. 14.3%, P < 0.0001, 80% vs. 42.9%, P = 0.025, respectively) (Figure 1A and B).
A, Comparison of Response Rates of Retreatment With PegIFN for 48 Weeks and 24 Weeks; and B, Comparison of Response Rates of Retreatment With PegIFN for 48 Weeks and cIFN
4.3. HBeAg Loss and Seroconversion
Six months after EOT, 41.8% (23/55) of all patients achieved HBeAg loss. And the HBeAg loss rate was higher in patients treated with 48-week Peg-IFN (60%) than those treated with 24-week Peg-IFNPeg-IFN therapy (14.3%) (Figure 1A), as well as those treated with cIFN (42.9%) (Figure 1B).
The HBeAg seroconversion rate at 24 weeks after EOT was significantly higher in patients with 48-week Peg-IFN therapy (60%) than that of 24-week Peg-IFN therapy (14.3%, P = 0.013) (Figure 1A).
4.4. HBsAg Loss
HBsAg loss occurred in 1 patient who received 48-week Peg-IFN therapy, while none occurred in patients with 24-week Peg-IFN therapy and cIFN therapy. None of the patients developed anti-HBs antibody.
4.5. Prediction for HBeAg Seroconversion at 24 Weeks After EOT in Peg-IFN-Treated Patients
Logistic regression analysis was performed to identify the potential predictors for HBeAg seroconversion at 24 weeks after EOT in Peg-IFN treated patients (n = 34). Age, gender, Peg-IFN therapy duration, prior treatment schedules, baseline levels of HBV DNA and ALT, decrease of HBV DNA and ALT from baseline to week 12 were included in univariate analysis. Five factors (gender, Peg-IFN therapy duration (48 weeks), baseline levels of HBV DNA and ALT, decrease of HBV DNA from baseline to week 12) were included in finial multivariate logistic regression analysis.
By multivariate logistic regression analysis, 48-week Peg-IFN therapy duration was the only predictive factor for HBeAg seroconversion (OR 10.67, 95% CI 1.663 - 68.46, P = 0.013). Baseline ALT ≥ 3ULN tended to be the potential predictive factor for HBeAg seroconversion (OR 6.22, 95% CI 0.904 - 42.805, P = 0.063).
4.6. Safety Assessment
Pyrexia occurred in 38.2% (13/34) of the Peg-IFN-treated patients, and 61.9% (13/21) of the cIFN-treated patients. Alopecia was another frequent side-effect identified at 32.7%. Other adverse effects included myalgia (14.5%), dose modification (12.7%), and headache (5.4%). Depression was not observed in any of the patients.
Univariate and Multivariate Predicting Analysis of HBeAg Seroconversion at 6 Months After EOT in 34 Peg-IFN-Treated Patients
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Gender, male | 0.200 | 0.032 - 1.240 | 0.097 | |||
| Age, ≥ 40 y | 0.436 | 0.041 - 4.689 | 0.627 | |||
| 48-week Peg-IFN therapy duration | 9.000 | 1.57 - 51.476 | 0.013 | 10.67 | 1.663-68.46 | 0.013 |
| LAM-experienced | 0.679 | 0.167 - 2.765 | 0.728 | |||
| ADV-experienced | 0.409 | 0.086 - 1.945 | 0.295 | |||
| IFN-experienced | 0.917 | 0.231 - 3.633 | 1.000 | |||
| LDT-experienced | 3.167 | 0.258 - 38.345 | 0.745 | |||
| Baseline ALT, ≥ 3 ULN | 4.909 | 0.864 - 27.88 | 0.076 | |||
| Baseline DNA, < 8 lg copies/mL | 2.571 | 0.435 - 15.19 | 0.422 | |||
| HBV DNA decrease, ≥ 4 lg copise/mLa | 5.667 | 1.129 - 28.45 | 0.054 | |||
| ALT decrease, ≥ 1 ULNa | 3.056 | 0.71 - 13.107 | 0.171 | |||
5. Discussion
This study explored the efficacy of Peg-IFN in HBeAg positive patients with exposure to antiviral therapy and identified factors associated with HBeAg seroconversion. Results from our study demonstrated that Peg-IFN was efficient in the re-treatment of HBeAg positive CHB patients.
Peg-IFN therapy duration of 48 weeks was shown to be more efficacious in achieving virologic response, HBeAg loss, and HBeAg seroconversion. The antiviral efficacy of Peg-IFN was not influenced by prior treatment schedules. Studies have shown that seroconversion to anti-HBe antibody was indicated in 32% of treatment-naive patients with 48 weeks Peg-IFN therapy (12, 13). This study revealed the satisfactory response rate of Peg-IFN in treatment-experienced HBeAg positive patients. The rate of HBeAg seroconversion was not decreased by prior antiviral schedules in the study, and for patients receiving 48-week Peg-IFN therapy, the efficacy was significantly improved (60%).
Results in the present study although similar, were superior to those in previous studies, where the rates of HBeAg seroconversion ranged from 14.6% to 34.9% (15-17). One possible reason for the differences might be that patients in their studies were refractory (non-responders or with drug-resistance). Additionally, 48-week Peg-IFN therapy proved to have superior results for patients with 24-week Peg-IFN therapy, which was also confirmed in a previous study performed in treatment-naive patients (18). Further investigations are required to confirm the long-term efficacy of 48-week Peg-IFN in the re-treatment of CHB patients.
Of the 34 Peg-IFN treated patients, 4 (11.8%) experienced virologic relapse in the current study. The relapse rate in the long-term follow-up of Peg-IFN re-treatment in CHB patients should be clarified through more prospective and extensive investigations. Pre-treatment ALT and HBV DNA level have been proven to be associated with response at 24 weeks’ post-treatment (12, 13, 15, 19). In this study, the 48-week Peg-IFN therapy duration remains the only predictive parameter for HBeAg seroconversion in Peg-IFN treated patients. Baseline ALT ≥ 3ULN tended to be the potential predictive factor for HBeAg seroconversion at 6 months post-therapy. The small sample size limits the statistical analysis.
There were some limitations to this study. First, the study was retrospectively designed, thus parameters such as HBeAg titer at baseline were incomplete, which is a critical parameter and should be monitored throughout the IFN re-treatment. Second, the follow up period was relatively short, and thus studies with longer follow-up periods are needed. Third, the study population was so small that we couldn’t come to any solid conclusions. Therefore, in the future, large-scale and prospective studies are required to identify the best potential strategies for patients with a prior history of antiviral therapy.
5.1. Conclusion
Peg-IFN was efficient in treatment-experienced HBeAg positive patients. Compared with patients who received 24-week Peg-IFN or cIFN therapy, those receiving 48-week Peg-IFN could reach superior response rates and had an increased probability of achieving HBeAg seroconversion.
Acknowledgements
References
-
1.
Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97-107. [PubMed ID: 14996343].
-
2.
European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167-85. [PubMed ID: 22436845]. https://doi.org/10.1016/j.jhep.2012.02.010.
-
3.
Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-2. [PubMed ID: 19714720]. https://doi.org/10.1002/hep.23190.
-
4.
Liaw YF, Kao JH, Piratvisuth T, Chan HL, Chien RN, Liu CJ, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6(3):531-61. [PubMed ID: 26201469]. https://doi.org/10.1007/s12072-012-9365-4.
-
5.
Lai CL, Yuen MF. Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy. Hepatology. 2013;57(1):399-408. [PubMed ID: 22806323]. https://doi.org/10.1002/hep.25937.
-
6.
Santantonio T, Mazzola M, Iacovazzi T, Miglietta A, Guastadisegni A, Pastore G. Long-term follow-up of patients with anti-HBe/HBV DNA-positive chronic hepatitis B treated for 12 months with lamivudine. J Hepatol. 2000;32(2):300-6. [PubMed ID: 10707871].
-
7.
Shouval D, Lai CL, Chang TT, Cheinquer H, Martin P, Carosi G, et al. Relapse of hepatitis B in HBeAg-negative chronic hepatitis B patients who discontinued successful entecavir treatment: the case for continuous antiviral therapy. J Hepatol. 2009;50(2):289-95. [PubMed ID: 19070393]. https://doi.org/10.1016/j.jhep.2008.10.017.
-
8.
Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med. 2005;352(26):2673-81. [PubMed ID: 15987916]. https://doi.org/10.1056/NEJMoa042957.
-
9.
Hadziyannis S, Sevastianos V, Rapti I. 18 Outcome OF HBeAg-Negative Chronic Hepatitis B (CHB) 5 Years After Discontinuation of Long Term Adefovir Dipivoxil (ADV) Treatment. J Hepatol. 2009;50:S9-S10. https://doi.org/10.1016/s0168-8278(09)60020-9.
-
10.
Reijnders JG, Deterding K, Petersen J, Zoulim F, Santantonio T, Buti M, et al. Antiviral effect of entecavir in chronic hepatitis B: influence of prior exposure to nucleos(t)ide analogues. J Hepatol. 2010;52(4):493-500. [PubMed ID: 20185191]. https://doi.org/10.1016/j.jhep.2010.01.012.
-
11.
Perry CM, Jarvis B. Spotlight on peginterferon-alpha-2a (40KD) in chronic hepatitis C. BioDrugs. 2002;16(3):213-7. [PubMed ID: 12102649].
-
12.
Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365(9454):123-9. [PubMed ID: 15639293]. https://doi.org/10.1016/S0140-6736(05)17701-0.
-
13.
Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352(26):2682-95. [PubMed ID: 15987917]. https://doi.org/10.1056/NEJMoa043470.
-
14.
Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351(12):1206-17. [PubMed ID: 15371578]. https://doi.org/10.1056/NEJMoa040431.
-
15.
Flink HJ, Hansen BE, Heathcote EJ, Feinman SV, Simsek H, Karayalcin S, et al. Successful treatment with peginterferon alfa-2b of HBeAg-positive HBV non-responders to standard interferon or lamivudine. Am J Gastroenterol. 2006;101(11):2523-9. [PubMed ID: 17029610]. https://doi.org/10.1111/j.1572-0241.2006.00812.x.
-
16.
Sun J, Hou JL, Xie Q, Li XH, Zhang JM, Wang YM, et al. Randomised clinical trial: efficacy of peginterferon alfa-2a in HBeAg positive chronic hepatitis B patients with lamivudine resistance. Aliment Pharmacol Ther. 2011;34(4):424-31. [PubMed ID: 21692822]. https://doi.org/10.1111/j.1365-2036.2011.04750.x.
-
17.
Zhang XQ, Zhang HY, You JP, Mao Q. Efficacy of pegylated interferon alpha2a in patients without HBeAg loss after the withdrawal of long-term lamivudine therapy. Virol J. 2013;10:21. [PubMed ID: 23320822]. https://doi.org/10.1186/1743-422X-10-21.
-
18.
Liaw YF, Jia JD, Chan HL, Han KH, Tanwandee T, Chuang WL, et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology. 2011;54(5):1591-9. [PubMed ID: 22045673]. https://doi.org/10.1002/hep.24555.
-
19.
Buster EH, Hansen BE, Lau GK, Piratvisuth T, Zeuzem S, Steyerberg EW, et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology. 2009;137(6):2002-9. [PubMed ID: 19737568]. https://doi.org/10.1053/j.gastro.2009.08.061.