Abstract
Background:
Chronic hepatitis B (CHB) remains a major public health problem worldwide, and the prevalence of CHB patients with hepatic steatosis is gradually increasing. Noninvasive approaches for the assessment of hepatic steatosis have been developed as alternatives to liver biopsy.Objectives:
This study evaluated the diagnostic performance of the fat attenuation parameter (FAP) measured by transient elastography (FibroTouch) and a new algorithm to assess hepatic steatosis in CHB patients, in comparison to liver biopsy as the gold standard.Methods:
Two hundred fifty-four CHB patients underwent simultaneous liver biopsy, biochemical blood testing, and FibroTouch examination. A new algorithm based on four factors (FAP; body mass index, BMI; high-density lipoprotein, HDL; apolipoprotein B, APOB) was defined as follows: fatty index = 10*ep/ (1+ep), and P = -2.75 + 0.028 ln FAP (dB/m) + 0.409 ln BMI (Kg/m2) - 2.482 ln HDL (mmol/L) + 1.979 ln APOB (g/L). The performances of FAP and fatty index were assessed by area under the ROC curve (AUROC).Results:
The difference in FAP was significant (P < 0.001) between CHB-only patients and CHB patients with hepatic steatosis. The cytokeratin 18 fragment (CK18-M65) level was significantly higher in CHB patients with non-alcoholic steatohepatitis (NASH) compared with CHB patients without NASH (P < 0.05). The optimal cutoff FAP values for hepatic steatosis of > 0, ≥ 5%, ≥ 10%, ≥ 20%, and ≥ 30% were 224.1, 230.6, 235.5, 246.9, and 261.1 dB/m, and AUROCs were 0.833, 0.801, 0.915, 0.917, and 0.972, respectively. The optimal cutoff value of fatty index for the diagnosis of hepatic steatosis was 1.5 and the AUROC was 0.807.Conclusions:
FAP is an accurate, reliable, and noninvasive approach that can also be combined with other metabolic biomarkers to comprehensively detect and quantify hepatic steatosis.Keywords
Chronic Hepatitis B Hepatic Steatosis Transient Elastography Noninvasive Diagnosis Fatty Index
1. Background
Hepatitis B virus (HBV) has now infected more than two billion people worldwide. Diseases or conditions induced by HBV infections, such as liver decompensation, liver cirrhosis, and hepatocellular carcinoma (HCC), are the leading causes of liver-related morbidity and death (1, 2). There is a high prevalence of HBV infection in China; an epidemiological survey carried out in 2005 demonstrated that it was as high as 7.8%, and approximately 22% of infected patients develop chronic hepatitis B (CHB). CHB remains a major public health problem in China and worldwide (3). With improved living standards and lifestyle changes, the incidences of hypertension, diabetes, obesity, hyperlipidemia, and other manifestations of metabolic syndrome (MS) increase every year. Non-alcoholic fatty liver disease (NAFLD) as the hepatic manifestation of MS is a metabolic-stress-induced liver injury that is closely related to insulin resistance (IR) and genetic susceptibility. NAFLD includes simple non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH), as well as liver cirrhosis and hepatocellular carcinoma. The NAFLD prevalence in European countries is approximately 15% - 30% in the general population, while it is as high as 70% - 90% in the obese and type 2 diabetic population (4). Currently, the NAFLD prevalence in developed areas of China, such as Guangzhou, Shanghai, and Hong Kong, is approximately 12% - 17% (5, 6). A survey indicated that the diagnosis rate of hepatic steatosis in CHB patients ranges from 5% to 76% (average 28%) (7). An investigation in China showed that the prevalence of hepatic steatosis was 14% in 1,915 CHB patients with no excessive alcohol intake, confirmed by liver biopsy (8). With improvements in living standards, the prevalence of CHB patients with hepatic steatosis will gradually rise. A trend analysis revealed that the annual prevalence of CHB patients with hepatic steatosis has risen from 8.2% to 31.8% (x2 = 51.657, P < 0.001) (9). However, further studies are needed to explore more accurate and noninvasive approaches for the assessment of hepatic steatosis in CHB patients. Yilmaz et al. reported that the aspartate transaminase-to-platelet ratio index (APRI) has an acceptable accuracy for predicting and staging the extent of liver fibrosis in patients with CHC and NAFLD, but not in those with CHB (10). In addition, a disadvantage of APRI in future clinical usage could be that the upper limits of normal (ULN) for aspartate transaminase can be neither standardized nor reproducible in different laboratories (11).
Transient Elastography FibroScan® (Echosens, Paris, France) is a globally used noninvasive method for the detection and quantification of liver fibrosis and hepatic steatosis using the liver stiffness measurement (LSM) and the controlled attenuation parameter (CAP), respectively (12-15). FibroTouch, a new generation of transient elastography that integrates a two-dimensional (2D)-image-guided system for precise positioning, has been available for clinical use in China since 2013 and possesses independent intellectual property rights. Similarly, LSM and the fat attenuation parameter (FAP) can be synchronously measured for the quantitative assessment of liver fibrosis and hepatic steatosis, using a dynamic broadband ultrasound probe that can overcome errors encountered in obesity, automatically adjusting the thickness of subcutaneous fat in all body shapes, including those of children, adults, and obese patients. FibroTouch has shown good diagnostic performance compared to liver biopsy and FibroScan (16-20). Benefiting from the creative probe, FibroTouch has a higher rate of successful detection than FibroScan (100% vs. 97%) (17).
2. Objectives
The main aim of this study was to analyze the diagnostic performance of FAP in the assessment of hepatic steatosis in CHB patients, compared to liver biopsy as the gold standard. We also developed a new algorithm (fatty index) that combined FAP and metabolic biomarkers to assess hepatic steatosis.
3. Methods
The human ethics committee of the third affiliated hospital of Sun Yat-sen University approved this study. The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
3.1. Patients
The subjects were selected from among CHB patients who received liver biopsies and transient elastography (FibroTouch) between March 2013 and March 2015 at the department of infectious diseases, the third affiliated hospital of Sun Yat-sen University. The inclusion criteria were as follows: (i) 18 - 65 years of age and (ii) a diagnosis of CHB with fatty liver, meeting the criteria of both the Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2012 update and the diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American association for the study of liver diseases, American college of gastroenterology, and the American gastroenterological association, according to which the patient must be HBsAg-seropositive for > 6 months and have evidence of hepatic steatosis by histology (21, 22). The exclusion criteria were as follows: (i) the presence of hepatitis C virus (HCV), hepatitis D virus (HDV), human immunodeficiency virus (HIV) or other types of hepatotropic viral infection; (ii) other liver diseases, such as alcoholic liver disease, Wilson’s disease, or autoimmune liver disease; (iii) other end-stage diseases or cancer; (iv) contraindications for FibroTouch examination (i.e. ascites, heart pacemakers and other implanted materials, right upper abdominal non-healing wounds, and pregnancy); (v) success rates of < 60% with an interquartile range (IQR) of > 30% of LSM and FAP; and (vi) failure to sign an informed consent form.
3.2. Clinical Assessment
We collected demographic data from the eligible patients, including age, gender, medication history, alcohol-drinking history, and body mass index (BMI). Routine blood tests and liver biochemical tests (ALT, AST, ALP, γ-GGT, CHE, etc.), as well as hepatitis B virus biomarkers and other routine tests, were collected within four weeks of liver biopsy. The experimental instruments used were the Olympus AU 640 automatic biochemical analyzer (Olympus Diagnostic Systems, Tokyo, Japan), and the ABI 5700 real-time PCR system (Applied Biosystems, CA, USA). Five milliliters of serum were collected to measure cytokeratin 18 (CK18) fragments with the CK18-M30 ELISA kit and the CK18-M65 ELISA kit (PEVIVA, Bromma, Sweden).
3.3. Liver Biopsy and Histological Assessment
Liver biopsies were performed under ultrasonographic guidance with a 16-gauge needle used to obtain tissue samples measuring at least 15 mm, which were formalin-fixed and paraffin-embedded. Five-micrometer-thick sections were stained with hematoxylin and eosin (H&E) and Masson trichrome. Liver fibrosis, inflammatory activity, and steatosis stage were evaluated according to the nonalcoholic steatohepatitis clinical research network scoring system; the evaluations were performed independently by two senior liver pathologists who were blinded to the clinical and biological data (23, 24). NAFL was defined as the absence of lobular inflammation, ballooning, or fibrosis, but the presence of steatosis of > 33%. If the steatosis did not reach the grade of 33%, it was only defined as hepatic steatosis (23).
3.4. Transient Elastography
Transient elastography was performed by two trained and certified physicians blinded to the patients’ clinical data, using FibroTouch according to the operations manual. LSM was expressed in kiloPascals (kPa) and FAP was expressed in dB/m. The LSM and FAP were considered reliable only if 10 successful measurements were obtained, with an IQR/median of LSM and FAP (IQR/M) of < 30% and a success rate of > 60% (25).
3.5. Statistical Analysis
Statistical analysis was performed using IBM SPSS 20.0 (IBM Corp., NY, USA). Quantitative variables were expressed as mean ± standard deviation (SD), or median and IQR. The independent risk factors for fatty liver were screened by a multiple logistic regression model, and a new algorithm (fatty index) was established. The diagnostic performance of FAP and fatty index were assessed by the areas under the receiver-operating characteristic (AUROC) curves according to DeLong’s test using MedCalc version 11.4.2.0 (MedCalc Software, Mariakerke, Belgium), while considering liver biopsy as the gold standard, albeit imperfect, and its 95% confidence intervals. The optimal cutoff values of FAP and fatty index were obtained by using the Youden Index and were determined to maximize the sum of sensitivity (Se) and specificity (Sp), and the corresponding positive predictive value (PPV), negative predictive value (NPV), negative likelihood ratio (LR−), and positive likelihood ratio (LR+) were computed for these cutoff values. A two-sided P value of < 0.05 was considered significant.
4. Results
4.1. Characteristics of the Study Population
Two hundred fifty-four patients were studied (204 men, 80.3%) with a mean age at the time of liver biopsy of 35.27 ± 9.55 years, including a group of CHB patients without hepatic steatosis (CHB group; 136 men, 76%) with a mean age of 35.04 ± 10.03 years, and a group of CHB patients with hepatic steatosis (CN group; 68 men, 90.7%) with a mean age of 35.81 ± 8.36 years. The proportion of males between the two groups was significantly different (P = 0.009), but age was not (P = 0.252). BMI, albumin (ALB), cholinesterase (CHE), triglyceride (TRIG), low-density lipoprotein (LDL), apolipoprotein B (APOB), and FAP in the CN group were significantly higher than in the CHB group, whereas high-density lipoprotein (HDL), APOA, and HBV DNA levels were significantly lower in the CN group (all P < 0.05). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), gamma-glutamyl transferase (GGT), the proportion of HBeAg+, and LSM were not significantly different between the two groups (all P > 0.05) (Table 1).
| Total (n = 254) | CHB (n = 179) | CN (n = 75) | P Valueb | |
|---|---|---|---|---|
| Age, y | 35.27 ± 9.55 | 35.04 ± 10.03 | 35.81 ± 8.36 | 0.252 |
| Male gender | 204 (80.3) | 136 (76) | 68 (90.7) | 0.009 |
| BMI, kg/m2, (n = 171) | 22.3 (20.1 - 24.6) | 21.13 (19.5 - 22.9) | 24.7 (23.6 - 26.8) | < 0.01 |
| < 18.5 | 113 (66.1) | 98 (85.2) | 15 (26.8) | |
| 18.5 - 27.9 | 50 (29.2) | 16 (13.9) | 34 (60.7) | |
| > 28 | 8 (4.7) | 1 (0.9) | 7 (12.5) | |
| AST, U/L | 32 (25 - 50) | 33 (25 - 58) | 29 (25 - 38) | 0.096 |
| ALT, U/L | 42 (27 - 67.2) | 44 (27 - 78) | 41 (28 - 62) | 0.819 |
| TBIL, umol/L | 13.3 (10.7 - 17.4) | 13.1 (10.7 - 17.5) | 13.9 (10.7 - 17.3) | 0.618 |
| ALB, g/L | 43.71 ± 3.75 | 43.27 ± 3.84 | 44.75 ± 3.35 | 0.004 |
| GGT, U/L | 32 (20 - 52) | 29 (19 - 51) | 34 (22 - 52) | 0.092 |
| CHE, U/L | 8002.6 ± 1904.8 | 7683.4 ± 1833.6 | 8764.5 ± 1866.3 | < 0.001 |
| TRIG, mmol/L | 0.97 (0.73 - 1.31) | 0.93 (0.68 - 1.03) | 1.31 (0.99 - 1.43) | < 0.001 |
| HDL, mmol/L | 1.22 (1.06 - 1.36) | 1.28 (1.1 - 1.46) | 1.08 (0.98 - 1.16) | < 0.001 |
| LDL, mmol/L | 2.87 ± 0.84 | 2.81 ± 0.88 | 3.03 ± 0.71 | 0.048 |
| APOA, g/L | 1.4 (1.33 - 1.53) | 1.43 (1.34 - 1.56) | 1.34 (1.29 - 1.45) | < 0.001 |
| APOB, g/L | 0.95 (0.88 - 1.10) | 0.94 (0.85 - 1.03) | 1.1 (1.04 - 1.21) | < 0.001 |
| PLT, 109/L | 194.63 ± 57.33 | 194.31 ± 60.54 | 195.40 ± 49.21 | 0.891 |
| HBeAg+ (n = 211), % | 49.8 | 50 | 49.3 | 0.92 |
| HBV-DNA, IU/mL | 2.8E5 (2.8E3 - 1.4E7) | 3.6E5 (5.4E3 - 2.1E7) | 2.6E4 (1E2 - 7.3E6) | 0.031 |
| FAP, dB/m | 222.9 (208.8 - 239.7) | 214.86 (203.1 - 228.4) | 238.64 (226.7 - 263.7) | 0.001 |
| LSM, kPa | 6.5 (4.7 - 11.5) | 6.4 (4.8 - 11.8) | 6.8 (4.7 - 10.9) | 0.839 |
4.2. Pathologic Findings
The results of the histopathological analysis of 254 liver biopsies are summarized in Table 2. Among all of the CHB patients, 29 (11.4%) were classified as fibrosis stage F0 (without fibrosis), 99 (39.0%) as F1, 60 (23.6%) as F2, 35 (13.8%) as F3, and 31 (12.2%) as F4, while 179 (70.5%) were classified as steatosis stage S0 (< 5%), 50 (19.5%) as S1 (5%~< 10%), 13 (5.1%) as S2 (10%~< 20%), 7 (2.8%) as S3 (20%~< 30%), and 5 (2.0%) as S4 (≥ 30%).
| Total | CHB (n = 44) | CN (n = 37) | P Valueb | |
|---|---|---|---|---|
| CK18 Fragment | ||||
| CK18-M30, U/L | 178.24 (130.49 - 311.185) | 169.35 (128.34 - 297.14) | 199.81 (130.33 - 342.75) | 0.363 |
| CK18-M65, U/L | 70.63 (43.82 - 141.83) | 69.78 (40.74 - 144.20) | 81.92 (46.22 - 136.77) | 0.626 |
| CK18 Fragment | Non-NASH (n = 31) | NASH (n = 5) | P Valuec | |
| CK18-M30, U/L | 199.81 (135.1 - 273.7) | 702.7 (143.9 - 901.4) | 0.224 | |
| CK18-M65, U/L | 68.9 (44.6 - 120.8) | 363.7 (72.9 - 1183.4) | 0.047 |
4.3. FAP in Different Hepatic Steatosis Stages
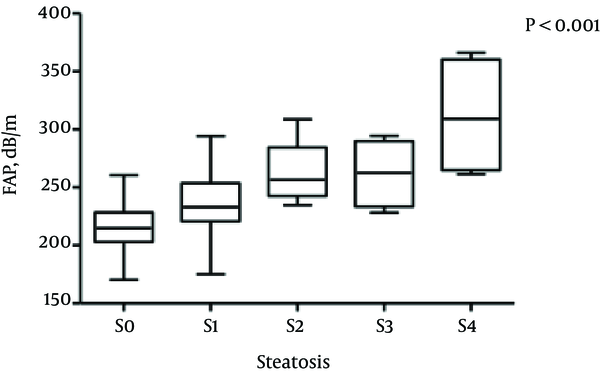
The median FAP was 237.25 dB/m (range, 225.97 - 263.2 dB/m) for patients with stage S0 steatosis, 231.3 dB/m (range, 219.0 - 246.9 dB/m) for S1, 256.5 dB/m (range, 242.6 - 284.4 dB/m) for S2, 262.69 dB/m (range, 233.44 - 289.89 dB/m) for S3, and 309.16 dB/m (range, 264.85 - 360.35 dB/m) for S4, respectively. The differences among the median FAPs for hepatic steatosis stages were statistically significant (Kruskal-Wallis H test, all P < 0.001) (Figure 1).
Boxplots Showing FAP According to Hepatic Steatosis Stage
4.4. Cytokeratin 18 (CK18) Fragment Levels
Cytokeratin 18 fragment (CK18-M30 and CK18-M65) levels were tested in 81 patients, including 44 in the CHB group and 37 in the CN group. The median CK18-M30 level was 169.35 U/L in the CHB group and 199.81 U/L in the CN group; the median CK18-M65 level was 69.78 U/L in the CHB group and 81.92 U/L in the CN group. The CK18 fragment levels were not significantly different between the two groups (both P > 0.05) (Table 2 and Figure 2).
Boxplots Showing CK18-M30 and CK18-M65 in the CHB and CN Groups
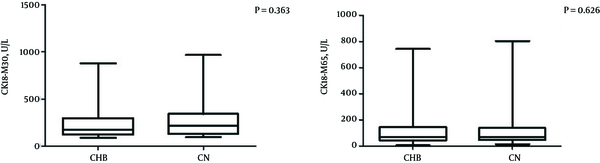
Thirty-six patients in the CN group were classified into a non-NASH subgroup (CHB patients without NASH, n = 31) and a NASH subgroup (CHB patients with NASH, n = 5) using the NAFLD activity score (NAS). The median CK18-M30 level was 199.81 U/L in the non-NASH subgroup and 702.7 U/L in the NASH subgroup, which was not a significant difference (P = 0.224). The median CK18-M65 levels were significantly higher in the NASH subgroup than in the non-NASH subgroup (363.7 U/L and 68.9 U/L, respectively; P < 0.05) (Table 2).
4.5. Independent Predictors for Hepatic Steatosis in CHB Patients
BMI, AST, ALT, cholesterol (CHOL), TRIG, HDL, LDL, APOA, APOB, FAP, LSM, G, and S were included in a univariate binary regression analysis, taking CHB patients with or without hepatic steatosis as an outcome variable. The factors of FAP (OR = 1.062, P < 0.001), BMI (OR = 1.729, P < 0.001), TRIG (OR = 3.443, P < 0.001), HDL (OR = 0.1, P < 0.001), APOA (OR = 0.292, P = 0.046), and APOB (OR = 21.316, P < 0.001) were significant predictors for hepatic steatosis. In a multivariate logistic regression analysis (forward stepwise method), FAP (OR = 1.028, P = 0.039), BMI (OR = 1.506, P = 0.001), and APOB (OR = 7.236, P = 0.041) were independent risk factors for CHB patients with hepatic steatosis, and HDL (OR = 0.084, P = 0.002) was a protective factor for CHB patients with hepatic steatosis (Table 3).
Multivariate Logistic Regression Analysis for Hepatic Steatosis in CHB Patients
| Factor | B | OR | 95% CI | P Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| FAP | 0.028 | 1.062 | 1.001 | 1.055 | 0.039 |
| BMI | 0.409 | 1.506 | 1.206 | 1.880 | 0.001 |
| HDL | -2.482 | 0.084 | 0.017 | 0.406 | 0.002 |
| APOB | 1.979 | 7.236 | 1.084 | 48.325 | 0.041 |
According to the multiple regression analysis, a new algorithm was defined for the assessment of hepatic steatosis: fatty index = 10*ep/ (1 + ep), which was based on four factors (FAP, BMI, HDL, and APOB) and P = -2.75 + 0.028 ln FAP (dB/m) + 0.409 ln BMI (kg/m2) - 2.482 ln HDL (mmol/L) + 1.979 ln APOB (g/L) (ln = loge, natural logarithm). This was converted to an exponential model with scores from 0 to 10.
4.6. Diagnostic Performance of FAP and Fatty Index
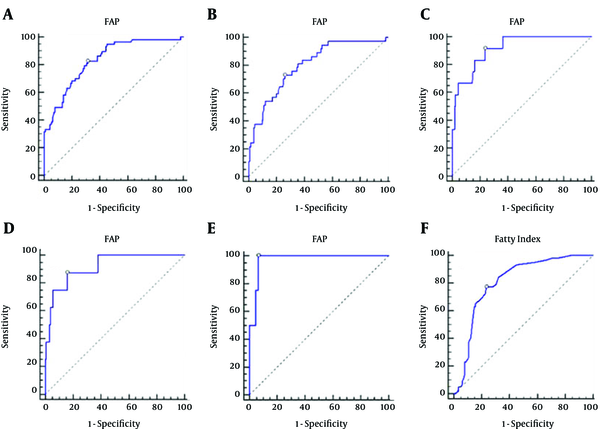
The diagnostic performance and corresponding ROC curves of FAP and fatty index are shown in Table 4 and Figure 3. The optimal cutoff FAP values were 224.1 dB/m for hepatic steatosis of > 0 (AUROC 0.833, Se 82.46%, Sp 68.70%, PPV 56.60%, and NPV 88.80%), 230.6 dB/m for ≥ 5% (AUROC 0.801, Se 72.97%, Sp 74.07%, PPV 43.50%, and NPV 90.90%), 235.5 dB/m for ≥ 10% (AUROC 0.915, Se 91.67%, Sp 76.25%, PPV 22.40%, and NPV 99.20%), 246.9 dB/m for ≥ 20% (AUROC 0.917, Se 87.50%, Sp 84.15%, PPV 21.20%, and NPV 99.30%), and 261.1 dB/m for ≥ 30% (AUROC 0.972, Se 100.00%, Sp 93.45%, PPV 26.70%, and NPV 100.00%; all P < 0.0001) (Table 4, Figure 3).
ROC Curves of FAP for the Diagnosis of Hepatic Steatosis of A, > 0; B, ≥ 5%, C, ≥ 10%; D, ≥ 20%; E, ≥ 30% ROC Curves of Fatty Index for the Diagnosis of Hepatic Steatosis of F, > 0
Diagnostic Performance of FAP and Fatty Index
| Steatosis, % | AUROC | 95% CI | P Value | Cutoff Valuea | Se, % | Sp, % | LR+ | LR- | PPV, % | NPV, % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FAP | ≥ 5 | 0.833 | 0.768 - 0.885 | < 0.0001 | > 224.1 | 82.46 | 68.70 | 2.63 | 0.26 | 56.6 | 88.8 |
| ≥ 10 | 0.925 | 0.874 - 0.959 | < 0.0001 | > 234.3 | 94.44 | 77.27 | 4.16 | 0.072 | 32.7 | 99.2 | |
| ≥ 20 | 0.917 | 0.865 - 0.954 | < 0.0001 | > 246.9 | 87.50 | 84.15 | 5.52 | 0.15 | 21.2 | 99.3 | |
| ≥ 30 | 0.972 | 0.935 - 0.991 | < 0.0001 | > 261.1 | 100.00 | 93.45 | 15.27 | 0.001 | 26.7 | 100.0 | |
| Fatty index | > 0 | 0.807 | 0.740 - 0.863 | < 0.0001 | 1.5 | 77.19 | 76.52 | 3.29 | 0.30 | 62.0 | 87.1 |
The optimal cutoff value of the fatty index for the diagnosis of hepatic steatosis was 1.5, AUROC was 0.807 (95% CI: 0.740 - 0.863, P < 0.001), sensitivity was 77.19% (64.2% - 87.3%), specificity was 76.52 % (67.7% - 83.9%), LR+ was 3.29 (2.8 - 3.9), LR− was 0.30 (0.2 - 0.5), PPV was 62.0% (49.6% - 73.3%), and NPV was 87.1% (79.0% - 93.0%) (Table 4, Figure 3).
5. Discussion
This study enrolled 254 CHB patients, and the prevalence of hepatic steatosis was 29.53% (n = 75). BMI, TRIG, LDL, APOB, and uric acid (UA) in the CN group were significantly higher than in the CHB group (P < 0.001), whereas HDL and APOA, as protective factors, were significantly lower in the CN group (both P < 0.05). These results are consistent with the current opinion that CHB patients with hepatic steatosis are primarily associated with metabolic factors. In this study, we found that HBV DNA levels were significantly lower in the CN group (P < 0.05), but the proportion of HBeAg+ patients was not significantly different between the two groups (P > 0.05). A previous study involving 3,212 cases showed that virologic factors, including HBV DNA levels and the proportion of HBeAg+ status, were decreased in CHB patients with hepatic steatosis compared to a CHB-only group (9).
CK18 is one of the most commonly used serum biomarkers for the diagnosis of NASH. Two CK18 epitopes, M65 and M30, have been reported to distinguish between overall (necrotic) and apoptotic cell death, respectively. A meta-analysis indicated that the AUROC of CK18 for the diagnosis of NASH was 0.71 - 0.93 (sensitivity 66%, specificity 82%) (26). In this study, we compared the levels of CK18-M30 and CK18-M65 in the CHB group and the CN group, and found that there were no significant differences between the two groups. The 36 patients with NAS scores were divided into two groups, non-NASH and NASH. There was no significant difference in CK18-M30 levels between the two groups (P = 0.224); however, the difference in CK18-M65 levels was statistically significant (P = 0.047). These results possibly indicate that liver injury in CHB patients with NASH is primarily due to necrosis, and simple hepatic steatosis is not sufficient to cause large changes in levels of both CK18-M30 and CK18-M65. The cell apoptosis and necrosis status of CHB patients with NASH still requires further research. It is also possible that because the degree of NASH in the CN group was mild, the inflammatory activity was similar to the non-NASH CHB patients.
Several studies have indicated that CAP, evaluated with transient elastography FibroScan®, is a novel method for the noninvasive quantitative assessment of hepatic steatosis, with a good diagnostic performance in patients with chronic liver disease who were mainly affected by BMI and the grade of hepatic steatosis, not by the causes of liver disease (27). FibroTouch, a new generation of transient elastography, is similar to FibroScan®, which employs FAP to assess the grade of hepatic steatosis quantitatively and noninvasively (19, 20). Sasso et al. concluded that there was a significant correlation between CAP and the grade of hepatic steatosis in patients with CHC; the AUROC was 0.91 for patients with hepatic steatosis of > 10% and 0.95 for hepatic steatosis of > 30%, respectively (28). The optimal cutoff CAP value was 222 dB/m for hepatic steatosis of > 10% (sensitivity 76%, specificity 71%) (13). Referring to research published by Macaluso et al., this study evaluated the diagnostic performance of FAP for the staging of hepatic steatosis of > 0, ≥ 5%, ≥ 10%, ≥ 20%, and ≥ 30%, and found that the optimal cutoff FAP values were 224.1 dB/m (AUROC 0.833, 95% CI: 0.768 - 0.885, P < 0.0001), 230.6 dB/m (AUROC 0.801, 95% CI: 0.733 - 0.857, P < 0.001), 235.5 dB/m (AUROC 0.915, 95% CI: 0.863 - 0.952, P < 0.001), 246.9 dB/m (AUROC 0.917, 95% CI: 0.865 - 0.954, P < 0.001), and 261.1 dB/m (AUROC 0.972, 95% CI: 0.935 - 0.991, P < 0.001), respectively (29). These results indicate that FAP can accurately assess the grade of hepatic steatosis in CHB patients; in particular, it has a better diagnostic performance in patients with > 10% hepatic steatosis. A multi-center study in China evaluated the diagnostic performance of CAP and found that its optimal cutoff value was 244.5 dB/m for ≥ 5% hepatic steatosis (AUROC 0.853); after adjusting by BMI (≥ 25 kg/m2), the optimal cutoff CAP value was 269.5 dB/m for ≥ 5% hepatic steatosis (AUROC 0.835) (30). Currently, there is a consensus that CAP is not significantly correlated with the causes of liver disease, but is independently associated with BMI. Considering that the subjects enrolled in the previous study had more severe hepatic steatosis and higher BMIs than in our study, it is logical that the optimal cutoff CAP value was higher in that study. Therefore, BMI and other related factors should be taken into consideration when assessing the grade of hepatic steatosis.
Based on CHB combined with or without hepatic steatosis and a multivariate logistic regression analysis (forward stepwise method), this study identified four independent factors (FAP, BMI, HDL, and APOB) that were associated with hepatic steatosis, and a new noninvasive diagnostic model was defined: fatty index = 10*ep/(1 + ep), P = −2.75 + 0.028 ln FAP (dB/m) + 0.409 ln BMI (kg/m2) - 2.482 ln HDL (mmol/L) + 1.979 ln APOB (g/L). The AUROC of the fatty index was 0.807 (95% CI: 0.740 - 0.863, P < 0.0001) and the optimal cutoff value of the fatty index was 1.5 with a sensitivity of 77.19% (64.2% - 87.3%) and a specificity of 76.52% (67.7% -83.9%). Due to the small population included in this study, this model requires further validation with follow-up studies. Currently, noninvasive algorithms for assessment of hepatic steatosis, including SteatoTest, the fatty liver index (FLI), the hepatic steatosis index (HSI), and the NAFLD liver fat score, each have advantages and disadvantages (31-34). In addition, some biomarkers involved in these algorithms are difficult to test in common clinical practice. Our new algorithm contained four factors (FAP, BMI, HDL, and APOB) that can be applied to evaluate the metabolic status of patients, and the liver fibrosis stage can be comprehensively assessed by LSM at the same time as the assessment of hepatic steatosis by FAP using FibroTouch.
In summary, FAP by transient elastography (FibroTouch) showed excellent diagnostic performance for the assessment of hepatic steatosis in CHB patients. It is an accurate, reliable, and completely noninvasive approach. FAP also can be combined with other metabolic biomarkers to comprehensively detect and quantify hepatic steatosis.
Acknowledgements
References
-
1.
Liaw YF, Chu CM. Hepatitis B virus infection. The Lancet. 2009;373(9663):582-92.
-
2.
European Association For The Study Of The L. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167-85. [PubMed ID: 22436845]. https://doi.org/10.1016/j.jhep.2012.02.010.
-
3.
Chinese Society of H, Chinese Society of Infectious Diseases CMA. [The guideline of prevention and treatment for chronic hepatitis B (2010 version)]. Zhonghua Liu Xing Bing Xue Za Zhi. 2011;32(4):405-15. [PubMed ID: 21569677].
-
4.
Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99-S112. [PubMed ID: 16447287]. https://doi.org/10.1002/hep.20973.
-
5.
Zhou YJ, Li YY, Nie YQ, Ma JX, Lu LG, Shi SL, et al. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol. 2007;13(47):6419-24. [PubMed ID: 18081233].
-
6.
Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F, et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43(3):508-14. [PubMed ID: 16006003]. https://doi.org/10.1016/j.jhep.2005.02.042.
-
7.
Fan JG, Chitturi S. Hepatitis B and fatty liver: causal or coincidental? J Gastroenterol Hepatol. 2008;23(5):679-81. [PubMed ID: 18410599]. https://doi.org/10.1111/j.1440-1746.2008.05361.x.
-
8.
Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50(1):204-10. [PubMed ID: 19014878]. https://doi.org/10.1016/j.jhep.2008.10.010.
-
9.
Wang MM, Wang GS, Shen F, Chen GY, Pan Q, Fan JG. Hepatic steatosis is highly prevalent in hepatitis B patients and negatively associated with virological factors. Dig Dis Sci. 2014;59(10):2571-9. [PubMed ID: 24838496]. https://doi.org/10.1007/s10620-014-3180-9.
-
10.
Yilmaz Y, Yonal O, Kurt R, Bayrak M, Aktas B, Ozdogan O. Noninvasive assessment of liver fibrosis with the aspartate transaminase to platelet ratio index (APRI): Usefulness in patients with chronic liver disease: APRI in chronic liver disease. Hepat Mon. 2011;11(2):103-6. [PubMed ID: 22087126].
-
11.
Mao Y, Huang J. Commentary on: Aspartate transaminase to platelet ratio index (APRI): A simple noninvasive index assessing liver fibrosis in patients with chronic liver diseases. Hepat Mon. 2011;11(5):376-7. [PubMed ID: 22087165].
-
12.
Wong GL. Update of liver fibrosis and steatosis with transient elastography (Fibroscan). Gastroenterol Rep (Oxf). 2013;1(1):19-26. [PubMed ID: 24759663]. https://doi.org/10.1093/gastro/got007.
-
13.
Sasso M, Tengher-Barna I, Ziol M, Miette V, Fournier C, Sandrin L, et al. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan((R)): validation in chronic hepatitis C. J Viral Hepat. 2012;19(4):244-53. [PubMed ID: 22404722]. https://doi.org/10.1111/j.1365-2893.2011.01534.x.
-
14.
Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29(7):1470-6. [PubMed ID: 24548002]. https://doi.org/10.1111/jgh.12557.
-
15.
Ledinghen V, Wong GL, Vergniol J, Chan HL, Hiriart JB, Chan AW, et al. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31(4):848-55. [PubMed ID: 26514665]. https://doi.org/10.1111/jgh.13219.
-
16.
Yuan L, Shao J, Hao M, Li C, Wang G, Wang T, et al. [Correlation between liver hardness testing results obtained by FibroTouch and FibroScan and liver pathological stage]. Zhonghua Gan Zang Bing Za Zhi. 2014;22(6):425-9. [PubMed ID: 25203705]. https://doi.org/10.3760/cma.j.issn.1007-3418.2014.06.006.
-
17.
Ou X, Wang X, Wu X, Kong Y, Duan W, Zhou J, et al. [Comparison of FibroTouch and FibroScan for the assessment of fibrosis in chronic hepatitis B patients]. Zhonghua Gan Zang Bing Za Zhi. 2015;23(2):103-6. [PubMed ID: 25880975]. https://doi.org/10.3760/cma.j.issn.1007-3418.2015.02.006.
-
18.
Wang R, Ren W, Zhao S, Niu X, Tan P, Du H, et al. [Clinical study on FibroTouch and multi-parameter model for diagnosis of hepatic fibrosis in patients with chronic liver disease]. Zhonghua Gan Zang Bing Za Zhi. 2015;23(4):265-9. [PubMed ID: 26133817]. https://doi.org/10.3760/cma.j.issn.1007-3418.2015.04.007.
-
19.
Mao CS, B NH, Yin H. The value of FibroTouch fat attenuation parameter in patients with chronic hepatitis B complicated with nonalcoholic fatty liver disease. Chin J Infect Dis. 2015;33(6):339-42.
-
20.
Li JB, liu S, Wen B, Gao N, Wang BY. Clinical significance of FibroTouch, ultrasound, and computed tomography in diagnosis of fatty liver disease: a comparative analysis. J Clin Hepatol. 2016;32(3):459-62.
-
21.
Liaw YF, Kao JH, Piratvisuth T, Chan HL, Chien RN, Liu CJ, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6(3):531-61. [PubMed ID: 26201469]. https://doi.org/10.1007/s12072-012-9365-4.
-
22.
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005-23. [PubMed ID: 22488764]. https://doi.org/10.1002/hep.25762.
-
23.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313-21. [PubMed ID: 15915461]. https://doi.org/10.1002/hep.20701.
-
24.
Scheuer PJ, Standish RA, Dhillon AP. Scoring of chronic hepatitis. Clin liver Dis. 2002;6(2):335-47.
-
25.
Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705-13. [PubMed ID: 14698338].
-
26.
Kwok R, Tse YK, Wong GL, Ha Y, Lee AU, Ngu MC, et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease--the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014;39(3):254-69. [PubMed ID: 24308774]. https://doi.org/10.1111/apt.12569.
-
27.
Kumar M, Rastogi A, Singh T, Behari C, Gupta E, Garg H, et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis: does etiology affect performance? J Gastroenterol Hepatol. 2013;28(7):1194-201. [PubMed ID: 23425053]. https://doi.org/10.1111/jgh.12134.
-
28.
Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36(11):1825-35. [PubMed ID: 20870345]. https://doi.org/10.1016/j.ultrasmedbio.2010.07.005.
-
29.
Macaluso FS, Maida M, Camma C, Cabibbo G, Cabibi D, Alduino R, et al. Steatosis affects the performance of liver stiffness measurement for fibrosis assessment in patients with genotype 1 chronic hepatitis C. J Hepatol. 2014;61(3):523-9. [PubMed ID: 24815874]. https://doi.org/10.1016/j.jhep.2014.04.045.
-
30.
Shen F, Zheng R, Mi Y, Shi J, Chen G, Chen J, et al. [A multi-center clinical study of a novel controlled attenuation parameter for assessment of fatty liver]. Zhonghua Gan Zang Bing Za Zhi. 2014;22(12):926-31. [PubMed ID: 25654287]. https://doi.org/10.3760/cma.j.issn.1007-3418.2014.12.010.
-
31.
Poynard T, Ratziu V, Naveau S, Thabut D, Charlotte F, Messous D, et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4:10. [PubMed ID: 16375767]. https://doi.org/10.1186/1476-5926-4-10.
-
32.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [PubMed ID: 17081293]. https://doi.org/10.1186/1471-230X-6-33.
-
33.
Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503-8. [PubMed ID: 19766548]. https://doi.org/10.1016/j.dld.2009.08.002.
-
34.
Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137(3):865-72. [PubMed ID: 19524579]. https://doi.org/10.1053/j.gastro.2009.06.005.