Abstract
Background:
Olynyk et al. studied the liver iron concentration (LIC) by MRI of 52 consecutive patients of northern European origin who were referred to a tertiary hospital for hyperferritinemia (HF). They described three groups according to HFE mutations and the transferrin saturation index (TSI) (group A: no predisposing mutations (PM) for hereditary hemochromatosis (HH) and TSI > 45 %, group B: PM for HH and TSI > 45 %; group C: no PM for HH and normal TSI). In the Basque country, HH predisposing mutations differ, with prevalence of the H63D/H63D mutation.Objectives:
To study the importance of HFE mutations and the TSI in determining LIC of HF patients attending the outpatient clinic at a secondary hospital.Methods:
Prospective study of 132 consecutive patients with HF. In 120 HFE study was available. In 79 LIC was obtained by MRI. In 71 patients values of HFE mutations, TSI, and LIC by MRI were available.Results:
Mean age: 55.68 ± 14.26 (23 - 83), 55 men and 16 women. The mean LIC in men was 35.66 ± 36.85; women 38.81 ± 29.75. The mean LIC in group A: 38.80 ± 45.18 (5 - 210), group B: 48.96 ± 37.51 (15 - 160), group C: 28.12 ± 18.85 (5 - 75). We compared the LIC mean values of the 3 groups with no significant differences.Conclusions:
LIC values are similar in different groups of patients referred to a secondary hospital for HF, despite variable predisposition to HH.Keywords
HFE Gene Hyperferritinemia MRI Liver Iron Concentration Hemochromatosis
1. Background
Hyperferritinemia (HF) is very common in clinical practice. This biochemical abnormality is often discovered during the evaluation of patients with potential liver iron overload (IO) disorders, such as hereditary hemochromatosis (HH). HF is also found in patients with other chronic liver diseases and non-hepatic inflammatory disorders. In the general population, up to 12% have HF (1). In Australia, Olynyk et al. (1) studied the prevalence of liver IO in patients of northern European origin with HF. They studied 52 consecutive patients who were referred to a tertiary hospital for HF, who were divided in three groups taking into account HFE mutations and TSI. They concluded that an increase in LIC of greater than three times the ULN is highly unlikely in HF patients who do not have HFE-related HH or causes of secondary IO.
Mediterranean populations have shown that the prevalence of C282Y homozygosity in HH is lower than in northern European populations (2, 3) with a north to south gradient. There is evidence that the H63D mutation contributes to IO, increasing serum iron and transferrin, and an independent relationship with hemochromatosis has also been demonstrated in the presence of C282Y (4-6). The H63D/H63D genotype is responsible for HF (2, 4-6). It has been shown that H63D leads to liver IO in homozygosis or in compound heterozygosis with C282Y. The causative role of the H63D/H63D mutation in HH or IO has been demonstrated (4, 7, 8), but with less penetrance and a considerable variation of phenotypic expression. The H63D polymorphism is highly prevalent in caucasian populations (15% - 20%). The highest allele frequency, (> 30%), has been reported in the Basque country in Spain (2, 4).
2. Objectives
Our objective was to study the importance of HFE mutations and the TSI in determining the LIC of HF patients attending the outpatient clinic at a secondary hospital. We evaluate the proposed groups from the publication of Olynyk et al. (1), with the genotypic variations present in the Basque Country, to verify if the conclusions are applicable to a southern European population.
3. Methods
In Australia, Olynyk et al. (1) analyzed in 2009 the LIC by MRI of 52 consecutive patients who were referred for HF to a tertiary hospital for the evaluation of liver IO, either in the presence or absence of increased TSI. They described three different groups according to HFE mutations and TSI: Group A: no predisposing mutations (PM) for HH and TSI > 45%; Group B: PM for HH: C282Y/C282Y; C282Y/H63D, and TSI > 45 %; Group C: no PM for HH and normal TSI.
In the Basque country, PM differ, with prevalence of the H63D/H63D PM (2, 6) and a less important role of the C282Y/C282Y mutation than in Australia (1).
This was a prospective study including patients from January 2010 to December 2010 and was conducted in Mendaro hospital, a secondary hospital in the Basque health service, with a total catchment of 70,000 people. Other objectives of the study have been recently published (4, 9). The study protocol conforms to the 1975 ethical guidelines of the declaration of Helsinki, with the understanding and the consent of the patients. This study was approved by the ethical committee of the Mendaro hospital.
Inclusion criteria: consecutive outpatients referred for HF (serum ferritin (SF) > 200 µmol/g in women; >300 µmol/g in men, in accordance with the WHO criteria (10).
Exclusion criteria: systemic inflammation, infections, and renal or neoplastics diseases (excluded by clinical, laboratory and radiologic methods); history of transfusion or iron supplements; patients younger than 18 years. Patients were studied and treated according to the best practice.
We have studied 132 consecutive patients referred to a secondary hospital in Gipuzkoa, Basque country, Spain, with HF (4) for evaluation of possible IO, either in the presence or absence of a raised TSI.
In our study, we obtained 132 HF patients, with 120 HFE gene mutation studies, and 79 LIC determinations by MRI (SIR method) (5). In 71 patients, we had HF and the TS, HFE and LIC by MRI values.
To form the three groups, the predisposing genotypes for HH in group B were recorded according to the results obtained from epidemiological studies performed in Basque country (2, 6) and from a recent publication (7), including H63D homozygosity as a PM (C282Y/C282Y; C282Y/H63D; H63D/H63D).
3.1. LIC by MRI
The MRI technique used (SIR method) was that proposed by Alustiza et al. (5). This method has a high correlation with biochemical measurements (r = 0.937). MR images were obtained on a 1.5-Tesla imaging unit (Philips Intera, Osatek, Donostia) (5). The images were studied, blinded, by the same radiologist (JMA). Liver steatosis was discarded by assessing systematically T1-weighted in-phase and opposed-phase images (11). Fat may interfere in signal intensity measurements. To avoid this problem, we performed all the LIC sequences as in-phase sequences (11).
The LIC was considered normal if it was lower than 36 µmol/g (0-36 µmol/g). IO was diagnosed when the LIC was between 36 and 80 µmol/g. High IO was defined as a LIC ≥ 80 µmol/g.
3.2. Laboratory Analysis
DNA was extracted from the blood samples and HFE gene analysis was carried out by multiplex real-time PCR using lightcycler technology (LC 1.0). Simultaneous study of the HFE C282Y, H63D and S65C mutations was performed in a single capillary using LC-Red 640, LC-Red 705 and fluorescein-labelled hybridization probes (Tibmolbiol, Berlin, Germany). Melting curve analysis was performed to distinguish wild type and mutant alleles in each case (2, 4).
SF and the transferrin saturation index (TSI) were obtained from blood samples obtained during fasting from all patients included in the study (4, 9). Normal values from our laboratory were: SF in women (15 - 200 µg/L); SF in men (30 - 300 µg/L); TSI (15% - 45%) (4, 9).
3.3. Statistics
SPSS 15.0 software (SSPS Inc., Chicago, IL, USA) was used to perform the appropriate statistical analyses. Mean values with range and standard deviation were calculated for continuous variables and frequencies and percentages for categorical variables.
We compared the LIC mean values of the three groups using ANOVA. A P value < 0.05 was considered statistically significant.
4. Results
This was a prospective study of 132 consecutive patients with HF. In 120 patients the HFE study was available. In 79 patients LIC was obtained by MR. In 71 patients data on HFE mutations, TSI, and LIC by MRI were available (Figure 1). LIC was measured in µmol / g (normal ≤ 36 µmol/g) by MRI. The period of inclusion was from January to December 2010.
Flow Chart of the Patients Included in the Study
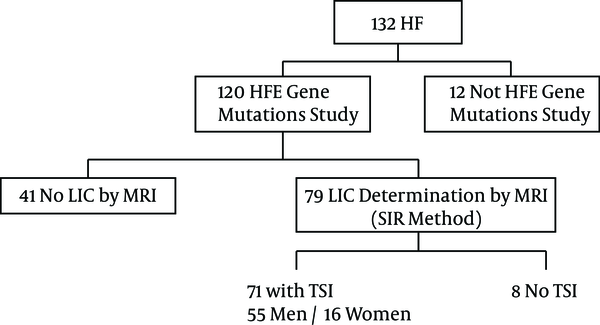
The mean age was 55.68 ± 14.26 (23 - 83). There were 55 men and 16 women (77.5%, 22.5%). The mean age for men was 53.07 ± 13.61 and the mean age for women 64.63 ± 13.14. The mean LIC by MRI in men was 35.66 ± 36.85 and 38.81 ± 29.75 in women. The age and sex of the patients did not have a significant influence on the mean value of LIC (P > 0.05).
Metabolic syndrome was present in the 55.67% of the 132 patients and 89% of the patients were overweight or obese. High IO was present in 5% and IO in 33% of the patients (4).
The HFE mutation (120/132) analysis of the study group (4) revealed no C282Y/C282Y cases, 6 C282Y/wt patients, 6 C282Y/H63D, 21 H63D/H63D, 38 H63D/wt, 47 wt/wt, and 2 S65C/wt. There were 27 patients with known PM in Basque Country (21 H63D/H63D/ 6 C282Y/H63D). Twenty of these patients had TSI > 45% (20/27), and 19 LIC determined by MR.
Laboratory studies revealed a SF mean value of 579.54 ± 296.57 µg/L (206 - 1668) and mean TSI value of 43.87 ± 14.10 % (12 - 95).
The TSI and SF values did not have an statistically significant influence on LIC (P > 0.05).
Of the 79 patients with LIC determination by MR, two patients were excluded due to the absence of TSI data and six patients were excluded because they had a PM but normal TSI.
With the obtained data, we made the three groups recommended by Olynyk et al. (1). Clinical and laboratory data from the three groups are described in Table 1.
Clinical and Laboratory Data from the Three Groups of Patients with Hyperferritinemia Proposed by Olynyk et al. (1) According to HFE Mutations and TSI Valuesa
| Group A | Group B | Group C | |
|---|---|---|---|
| N | 21 | 19 | 31 |
| Age (years) | 55.28 ± 14.87 | 59.94 ± 15.40 | 52 ± 13.01 |
| Male/ Female | 17/4 | 11/8 | 27/4 |
| SF (µg/L) | 610.42 ± 322.56 | 503.42 ± 200.49 | 703.03 ± 317.88 |
| TSI (%) | 57.68 ± 10.13 | 55.20 ± 6.50 | 34.63 ± 7.45 |
| HFE gene PM | 0 | 19 | 0 |
| C282Y/H63D | 0 | 4 | 0 |
| H63/H63D | 0 | 15 | 0 |
| LIC (µmol/g) | 38.80 ± 45.18 | 48.96 ± 37.51 | 28.12 ± 18.85 |
Group A: 21 patients, 14 with normal LIC and 7 patients with raised LIC (8 were wt/wt: 5 normal LIC, 3 raised LIC; 11 H63D/wt: 7 normal LIC, 4 raised LIC; 2 C282Y/wt: 2 normal LIC); the 33.3% (7/21) of this group had IO.
Group B: 19 patients (PM: H63D/H63D; C282Y/H63D), 11 with normal LIC, 8 with raised LIC; 7 H63D/H63D with raised LIC and 8 with normal LIC; 1 C282Y/H63D with raised LIC and 3 with normal LIC. The 42.1% had IO (8/19).
Group C: 31 patients, 23 with normal LIC, 8 with raised LIC (12 were wt/wt: 9 normal LIC, 3 raised LIC; 17 H63D/wt: 12 normal LIC, 5 raised LIC; 2 S65C/wt: normal LIC); the 25.5% had IO (8/31).
The mean LIC in group: A 38.80 ± 45.18 (5 - 210), group B: 48.96 ± 37.51 (15 - 160), group C: 28.12 ± 18.85 (5 - 75).
We compared the LIC mean values of the three groups using ANOVA, with no significant differences (P = 0.0816).
5. Discussion
HF is a very common finding in clinical practice, generally leading to the study of possible IO by general practitioners and hepatologists. SF may be raised due to different and nonspecific causes, and significant IO will not often be present (1).
Olynyk et al. (1) recently studied in Australia the prevalence of hepatic IO in individuals with HF and northern European origin. They considered three groups: group A with increased transferrin saturation and no significant HFE mutations (N: 17), group B with increased TS and C282Y homozygosity or C282Y/H63D compound heterozygosity (n: 22); and group C with normal TS and no significant HFE mutations (N: 13). LIC was determined by MRI (relaxometry). LIC was significantly higher in group B (123 ± 22 µmol/g) compared with group A and C (39 ± 4; 36 ± 5) P < 0.01. Nine of the 22 patients from group B has an increase in their LIC greater than three times the ULN compared with none from groups A and C.
Our objective was to evaluate the proposed groups in the Basque country, taking into account the genotypic variations of our country, and to verify if the conclusions of the previous study are valuable in southern Europe. In the Basque country, HFE gene mutations analysis in HH patients and in the general population is different from other populations (2). The frequency of C282Y homozygotes in HH patients is only of 57%; it is 90% in other populations (2). In the Basque country, H63D/H63D mutation is present in the 7.76% of the general population and in 2.1% in other populations (6).
The H63D polymorphism is highly prevalent in caucasian population (15% - 20%). The highest allele frequency (30%) has been reported in the Basque country in Spain (2). There is evidence that the H63D mutation contributes to IO, increasing serum iron and transferrin, and has an independent relationship with hemochromatosis in the presence of C282Y (12-14). The causative role of the H63D/H63D mutation in HH or IO has been demostrated (8).
When we compared the SF values between the three groups, group C had the highest value with 703.03 µg/L, group A 610.42 and group B 503.42. There were not statistically significant differences between the three groups.
When the LIC values were determined by MRI, group B had 48.96 µmol/g, group A 38.80 µmol/g and group C 28.12 µmol/g. The LIC values obtained in the different groups were compared and there were no statistical significant differences. Given the observed difference between group B and group C LIC mean, one could argue that a study with a bigger sample size will end up showing statistical differences if the observed LIC means hold. One of the problems in this study is that there were no C282Y/C282Y patients in the series. It is well-known that the liver IO in HH produced by the C282Y/C282Y mutation is higher than that produced by other mutations, such as C282Y/H63D or H63D/H63D (6, 15). The causative role of C282Y/H63D and H63D/H63D mutations in HH or IO has been demonstrated, but with less penetrance and a considerable phenotypic variability (6, 15). The absence of C282Y homozygotic patients in our study may have been why no differences were detected. In a previous study by our group, no statistically significant differences were found concerning the LIC values between three predisposing genotypes in a retrospective HH cohort (6).
We can conclude that, in this study performed in the Basque country in Spain, with the genotypic special characteristics that have been exposed, there are no differences in LIC between the three proposed groups, and we cannot predict liver IO in patients with HF with HFE mutations and TSI values alone. A larger series, with the presence of other PM, such as the C282Y/C282Y, may obtain different results.
References
-
1.
Olynyk JK, Gan E, Tan T. Predicting iron overload in hyperferritinemia. Clin Gastroenterol Hepatol. 2009;7(3):359-62. [PubMed ID: 19095082]. https://doi.org/10.1016/j.cgh.2008.11.010.
-
2.
de Juan D, Reta A, Castiella A, Pozueta J, Prada A, Cuadrado E. HFE gene mutations analysis in Basque hereditary haemochromatosis patients and controls. Eur J Hum Genet. 2001;9(12):961-4. [PubMed ID: 11840200]. https://doi.org/10.1038/sj.ejhg.5200731.
-
3.
Bauduer F, Scribans C, Degioanni A, Renoux M, Dutour O. Distribution of the C282Y and H63D polymorphisms in hereditary hemochromatosis patients from the French Basque Country. Ann Hematol. 2005;84(2):99-102. [PubMed ID: 15503019]. https://doi.org/10.1007/s00277-004-0957-5.
-
4.
Castiella A, Zapata E, Zubiaurre L, Ma Alustiza J, De Juan MD, Iribarren A, et al. Impact of H63D mutations, magnetic resonance and metabolic syndrome among outpatient referrals for elevated serum ferritin in the Basque Country. Ann Hepatol. 2015;14(3):333-9. [PubMed ID: 25864213].
-
5.
Alustiza JM, Artetxe J, Castiella A, Agirre C, Emparanza JI, Otazua P, et al. MR quantification of hepatic iron concentration. Radiology. 2004;230(2):479-84. [PubMed ID: 14668426]. https://doi.org/10.1148/radiol.2302020820.
-
6.
Castiella A, Zapata E, de Juan MD, Otazua P, Fernandez J, Zubiaurre L, et al. Significance of H63D homozygosity in a Basque population with hemochromatosis. J Gastroenterol Hepatol. 2010;25(7):1295-8. [PubMed ID: 20594259]. https://doi.org/10.1111/j.1440-1746.2010.06247.x.
-
7.
Adams PC. Epidemiology and diagnostic testing for hemochromatosis and iron overload. Int J Lab Hematol. 2015;37 Suppl 1:25-30. [PubMed ID: 25976957]. https://doi.org/10.1111/ijlh.12347.
-
8.
Castiella A, Zapata E. Proven: The causative role of homozygous H63D mutation in hereditary haemochromatosis. Gastroenterol Hepatol. 2016;39(7):494-5. [PubMed ID: 26906094]. https://doi.org/10.1016/j.gastrohep.2015.12.004.
-
9.
Castiella A, Zapata E, Zubiaurre L, Iribarren A, Alustiza JM, Otazua P. Liver iron concentration is not raised in patients with dysmetabolic hyperferritinemia. Ann Hepatol. 2016;15:540-4.
-
10.
Licata A, Nebbia ME, Cabibbo G, Iacono GL, Barbaria F, Brucato V, et al. Hyperferritinemia is a risk factor for steatosis in chronic liver disease. World J Gastroenterol. 2009;15(17):2132-8. [PubMed ID: 19418586].
-
11.
Alustiza JM, Castiella A. Liver fat and iron at in-phase and opposed-phase MR imaging. Radiology. 2008;246(2):641. [PubMed ID: 18227554]. https://doi.org/10.1148/radiol.2462071131.
-
12.
de Diego C, Opazo S, Murga MJ, Martinez-Castro P. H63D homozygotes with hyperferritinaemia: Is this genotype, the primary cause of iron overload? Eur J Haematol. 2007;78(1):66-71. [PubMed ID: 17042772]. https://doi.org/10.1111/j.1600-0609.2006.00775.x.
-
13.
Aguilar-Martinez P, Bismuth M, Picot MC, Thelcide C, Pageaux GP, Blanc F, et al. Variable phenotypic presentation of iron overload in H63D homozygotes: are genetic modifiers the cause? Gut. 2001;48(6):836-42. [PubMed ID: 11358905].
-
14.
Fairbanks VF, Brandhagen DJ, Thibodeau SN, Snow K, Wollan PC. H63D is an haemochromatosis associated allele. Gut. 1998;43(3):441-2. [PubMed ID: 9863493].
-
15.
Cheng R, Barton JC, Morrison ED, Phatak PD, Krawitt EL, Gordon SC, et al. Differences in hepatic phenotype between hemochromatosis patients with HFE C282Y homozygosity and other HFE genotypes. J Clin Gastroenterol. 2009;43(6):569-73. [PubMed ID: 19359997]. https://doi.org/10.1097/MCG.0b013e3181919a33.