Abstract
Background:
The prevalence of newly-diagnosed cases of chronic hepatitis is decisive for the overall incidence rate of hepatitis B observed in Poland.Objectives:
We aimed to determine the chronic hepatitis B incidence trends in Poland, taking into consideration the ages, genders, and environments of the patients.Methods:
The study is based on aggregated data from Polish descriptive epidemiological studies for the period of 2005 to 2013 (i.e., hepatitis B incidence numbers and ratios, including mixed HBV and HCV infections) as published in the annual bulletins Choroby zakazne i zatrucia w Polsce (Infectious diseases and poisonings in Poland] drawn up by the laboratory for the monitoring and analysis of epidemiological status of the department of epidemiology at the national institute of public health - national institute of hygiene (NIPH-NIH). Poland, a central European country situated in the humid continental climate zone, is classified as a highly developed country. In the analyzed period, the Polish population consisted of about 38 million people, more than 19 million of whom were women, and more than 18 million of whom were men. Among European countries, Poland has the smallest number of national and ethnic minorities. For the purposes of epidemiological supervision, a special definition of acute hepatitis B was adopted in Poland in 2005, which facilitated separate registration of acute and chronic cases.Results:
A significantly increasing chronic hepatitis B incidence trend was observed in the population of Poland, with considerable increases in incidence rates for both men and women alike. The incidence rates for inhabitants of both urban and rural areas also showed an increasing tendency. Chronic hepatitis B occurred more frequently in men and in urban areas. For each of the five-year age groups encompassing patients between 20 and 54 years of age, the increase in the incidence rate proved to be significant.Conclusions:
The registered increase in the incidence rate of chronic hepatitis B in Poland is a consequence of the new registration of cases of chronic hepatitis B acquired in the past. The problem of chronic hepatitis B can be solved by improving epidemiological supervision, enhancing the detection of frequently asymptomatic infections, and by providing easier access to optimized therapies.Keywords
1. Background
Despite effective vaccination programs which have led to a considerable decline in the number of new cases, chronic hepatitis B still remains a serious global epidemiological problem. According to the current WHO estimates, more than two billion people have been infected with the hepatitis B virus (HBV) all over the world. There are 350 - 400 million chronic virus carriers, whereas about 600,000 to one million people die each year of complications related to HBV infection (1-6).
The data published by WHO in March of this year indicate a decrease in the number of chronically-infected people (estimated at 240 million) and approximately 780,000 deaths per year, out of which 130,000 were due to acute hepatitis (7). Chronic hepatitis B may exhibit itself in the form of inactive carrier status, or lead to the development of serious complications related to cirrhosis and hepatocellular cancer, which constitute the remaining 650,000 deaths resulting from HBV infections each year (3, 7-9). The new cases of hepatitis B that have been reported in Poland in recent years have been dominated by the newly diagnosed cases of chronic HBV, which account for the increase in overall hepatitis B incidence rates (10-18).
2. Objectives
We aimed to determine the chronic hepatitis B incidence rate trends observed in Poland, taking into consideration the various age groups, gender, and environment of the patients.
3. Methods
The study is based on aggregated data from Polish descriptive epidemiological studies for the years 2005 to 2013 (referring to hepatitis B incidence numbers and ratios, including mixed HBV and HCV infections) as published in the annual bulletins Choroby zakazne i zatrucia w Polsce [Infectious diseases and poisonings in Poland] drawn up by the Laboratory for the Monitoring and Analysis of Epidemiological Status of the Department of Epidemiology at the National Institute of Public Health- National Institute of Hygiene (NIPH-NIH) (18, 19). Poland, a Central European country situated in the humid continental climate zone, is classified as a highly developed country. In the analyzed period, the Polish population consisted of about 38 million people, approximately 19 million of whom were women, and 18 million of whom were men. Among European countries, Poland has the smallest number of national and ethnic minorities (Table 1).
Total Population by Gender and Age Groups in Poland in 2005 and 2013
| Age Groups | 2005 | 2013 | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| 0 - 4 | 915133 | 865498 | 1024298 | 969798 |
| 4 - 9 | 1016667 | 965947 | 998173 | 948298 |
| 10 - 14 | 1240021 | 1185909 | 938818 | 892041 |
| 15 - 19 | 1467263 | 1402020 | 1086683 | 1037626 |
| 20 - 24 | 1686937 | 1629090 | 1331665 | 1278777 |
| 25 - 29 | 1576368 | 1531961 | 1544623 | 1496695 |
| 30 - 34 | 1394972 | 1359571 | 1639758 | 1595440 |
| 35 - 39 | 1204078 | 1177496 | 1521716 | 1482557 |
| 40 - 44 | 1252692 | 1243114 | 1284484 | 1261764 |
| 45 - 49 | 1486330 | 1522049 | 1169458 | 1164110 |
| 50 - 54 | 1448284 | 1542563 | 1281345 | 1317943 |
| 55 - 59 | 1174259 | 1309013 | 1412715 | 1524115 |
| 60 - 64 | 671598 | 812399 | 1214608 | 1405543 |
| 65 - 69 | 659541 | 882389 | 792048 | 997610 |
| 70 - 74 | 559808 | 834433 | 511569 | 720932 |
| 75+ | 699904 | 1439748 | 877574 | 1752922 |
| Total | 18 453 855 | 19 703 200 | 18 629 535 | 19 866 124 |
For the purposes of epidemiological supervision, a special definition of acute hepatitis B was adopted in Poland in 2005, which facilitated separate registration of acute and chronic cases (newly detected and diagnosed in earlier registered carriers of HBsAg) (12, 16).
In the period of our study, the cases of chronic hepatitis B in Poland were registered based on the diagnosis of the reporting physician who, unlike with cases of acute hepatitis B, had not applied the case classification and detailed qualification criteria to define the case as chronic (16). All the reported cases were subject to formal and substantive verification by the registrars who monitored the registration of HBV cases (20).
The statistical analysis was carried out in two stages. In the first stage, the linear trend of chronic hepatitis B incidence rates among the population of Poland was determined, and the rates according to gender and place of residence were also determined with the use of Pearson’s correlation coefficient. The values of the incidence rates (test for independent proportions) and the rates which illustrated the studied trends among men and women were also compared, as well as the rates among the inhabitants of urban versus rural areas.
In the second stage, an in-depth analysis of the trends was performed with the use of multiple regression time-dependent analysis and a generalized estimating equation (GEE). The independent impact of the year on the value of the incidence rates was studied after adjustment for gender, place of residence, and age (as the confounding variables), as well as controlling for the interaction between age and year.
The criterion of statistical significance was arbitrarily adopted at the level of P < 0.05, and for the purpose of multiple comparisons, it was modified according to the Bonferroni correction to the value of P < 0.00333. The calculations were performed using PQStat version 1.6.0. software package, and the GEE analysis was conducted using STATA version 14.
4. Results
In the years from 2005 to 2013, 1,337 to 1,727 cases of hepatitis B in total were registered in Poland, of which 1.9% to 5.3% were diseases caused by mixed infections of HBV and HCV (10-18). The last three years of follow-up were characterized by a stable incidence rate of hepatitis B in general (1,583, 15,83, and 1,540, respectively) (16-18). Most of the newly reported cases were chronic hepatitis B.
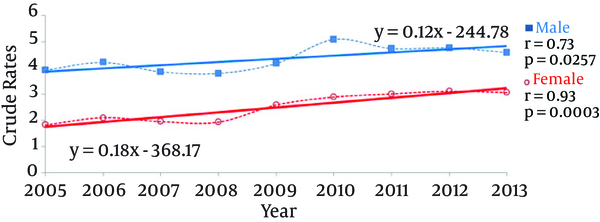
In the population of Poland, the incidence of chronic hepatitis B caused by the rise in incidence rates for both genders showed a substantially increasing trend (P = 0.0025) (Figure 1). Significant rising trends were also observed in both the populations of men and women (P = 0.0257 and P = 0.003, respectively) (Figure 2). Both trends were characterized with a similar slope (P = 0.2619) and correlation coefficient (P = 0.2210) (Table 2).
Chronic Hepatitis B Incidence Trends in Poland for the Years 2005 to 2013
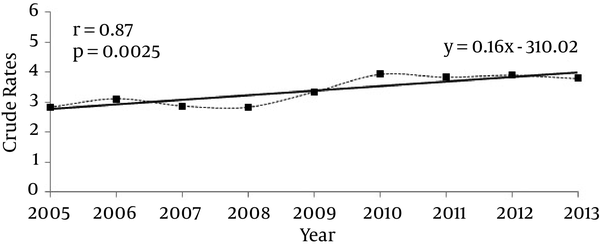
Chronic Hepatitis B Incidence Trends Among Men and Women in Poland for the Years 2005 to 2013
Comparison of Slope Coefficient Rates and Correlation Trends of Chronic Hepatitis B Incidence Rates in Poland for the Years 2005 to 2013
| Crude Rates Comparison | Comparison of the Linear Time Trends | |||||
|---|---|---|---|---|---|---|
| Crude Rate | P-Value | Slope Coefficient of Linear Trends | P Value | Pearson’s Correlation Coefficient | P Value | |
| Male | 4.33 | < 0.0001 | 0.12 | 0.2619 | 0.73 | 0.2210 |
| Female | 2.49 | 0.18 | 0.93 | |||
| City | 3.76 | < 0.0001 | 0.17 | 0.4393 | 0.84 | 0.8054 |
| Village | 2.78 | 0.13 | 0.88 | |||
In the period subject to analysis, the new cases of chronic hepatitis B occurred significantly more frequently in the population of men (P < 0.0001) (Table 2).
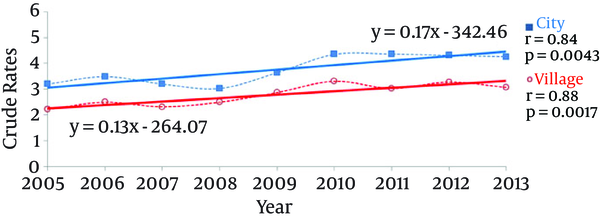
Both areas of residence (urban vs. rural) showed a significant rising trend in chronic hepatitis B incidence rates (for cities: P = 0.0043, and for rural areas: P = 0.0017), and both trends were characterized by a similar slope coefficient (P = 0.4393) and correlation (P = 0.8054) (Figure 3, Table 2). The cases of newly registered chronic hepatitis B in the period of 2005 to 2013 occurred more frequently in the urban population rather than the rural areas, and that difference was statistically significant (P < 0.0001) (Table 2).
Chronic Hepatitis B Incidence Trends Among Urban and Rural Areas in Poland for the Years 2005 to 2013
The significant growing trend in chronic hepatitis B incidence rates in Poland was persistent irrespective of confounding variables such as gender, place of residence, or age (Table 3). Furthermore, a statistically significant correlation between the age of the patients and the year was observed (Table 3). Additionally, a substantial increase in incidence rates occurred in all five-year age groups encompassing patients aged between 20 and 54, but a significant declining tendency was observed in the age group of 10 to 14 years, as well as in the oldest group, aged 75 and older (Table 4).
Analysis of Linear Trends of Chronic Hepatitis B Incidence Rates in Poland Among Specific Age Groupsa,b
| Age Group | R | P Value |
|---|---|---|
| 0 - 4 | -0.19 | 0.6455 |
| 5 - 9 | -0.43 | 0.2540 |
| 10 - 14 | -0.87 | 0.0023 |
| 15 - 19 | 0.21 | 0.5850 |
| 20 - 24 | 0.88 | 0.0016 |
| 25 - 29 | 0.93 | 0.0003 |
| 30 - 34 | 0.87 | 0.0023 |
| 35 - 39 | 0.91 | 0.0006 |
| 40 - 44 | 0.96 | < 0.0001 |
| 45 - 49 | 0.93 | 0.0002 |
| 50 - 54 | 0.95 | < 0.0001 |
| 55 - 59 | 0.78 | 0.0137 |
| 60 - 64 | -0.09 | 0.8155 |
| 65 - 74 | -0.77 | 0.0145 |
| 75+ | -0.86 | 0.0029 |
The significance level for the P-value in the tabulation above had been modified according to the Bonferroni correction and was set to 0.0033.
5. Discussion
As mentioned above, between 2005 and 2013, there was a total of 1,337 to 1,727 cases of hepatitis B registered in Poland, out of which 1.9% to 5.3% were cases caused by HBV and HCV mixed infections (10-18). The last three years under observation were characterized by an overall stable level of hepatitis B incidence rates (1,583, 1.583, and 1540, respectively) (16-18). In the period subject to analysis, the newly detected chronic hepatitis B cases constituted from 62.4% (1,078) in 2005 to 95% in 2012 and 2013 (1,505 and 1,459, respectively) of the total number of patients with hepatitis B. Until 2012, this percentage had been growing systematically year by year (10-19).
In the WHO European Region, about 14 million people have been chronically infected with HBV, and 36,000 people die each year as a consequence of the infection (4, 21). The observations carried out by the European Liver Patients Association indicate that as many as 90% of infected individuals are unaware of their conditions. Among the diagnosed patients, approximately 20% had not heard of viral hepatitis until they were actually diagnosed, and 27% did not know that they were in a group of elevated risk of infection (4, 22).
According to the information provided by 13 EU/EEA countries (Austria, Denmark, Estonia, Finland, Ireland, Latvia, Holland, Slovakia, Slovenia, Sweden, the UK (excluding Scotland), and Romania) which have been maintaining records of chronic hepatitis cases, the number of new cases and the incidence rates in 2012 were generally higher and showed greater diversity than the corresponding rates for acute cases (23). The number of new cases of chronic hepatitis B reported ranged from 26 in Slovenia to 7,368 in Great Britain. The prevalence rates were the lowest in Romania, amounting to 0.1/100,000, and the highest in Sweden at 14.9/100,000 (23). In the years from 2006 to 2012, there was an increase in the values of chronic hepatitis B prevalence rates from 1.4/100,000 to 2.5/100,000. According to the data from nine countries where both acute and chronic hepatitis cases have been registered (Denmark, Estonia, Ireland, Norway, Slovenia, Sweden, and the UK excluding Scotland), a decline in the incidence rates of acute hepatitis B has been observed, while the number of chronic hepatitis cases has been growing (23).
This upward tendency across Europe corresponded to the observable rise in the incidence rates in Poland in the years from 2005 to 2013. The significantly increasing rates in the population of Poland were the result of a considerable increase in the incidence rates of chronic hepatitis B in both men and women. Even though the incidence rates among men were higher than those among women, both were characterized by similar slope coefficients and correlations.
With respect to the incidence rates of chronic viral hepatitis B in most European countries (besides Romania and Denmark), the dominant group was men. The male to female ratio in the case of inflammatory conditions turned out to be lower (0.6 to 2.3) when compared to cases of acute hepatitis B (0.5 to 5.2) (24). However, according to the data provided by the Danish National Board of Health, the estimated incidence rate of chronic hepatitis B in the population under 16 years of age registered prior to 31 December 2007 (including undiagnosed patients) was 0.24, and the rate was a little higher among men (25). The results of studies from Iran confirm the gender differences revealed earlier in the incidence rates of chronic carrier states of the HBs antigen to the disadvantage of men (26). Thus, male gender has been quoted as an independent risk factor having an impact on the incidence rates of hepatitis B (exposure to HBV) besides other variables such as family size, type of job (small business), and family history involving HBV (27). This is further corroborated by the fact that in the retrospective studies performed in Kerala (India), a predominant number of male patients was observed in all age groups and for all hepatitis types except HCV (28).
About one-third (33.4%) of the registered hepatitis B cases in total in 28 EU/EEA countries in 2012 were among the 25 to 34 age group, and 16.9% of chronic hepatitis cases were diagnosed in patients younger than 25 (23). In Poland from 2005 to 2013, chronic hepatitis cases were most frequently diagnosed in the 15 to 39 age group, reaching the highest level among 15 to 19 year olds (10-19).
The prevailing high level of chronic hepatitis B incidence rates was thought to be the result of higher detectability of hepatitis contracted in the 1980s and the beginning of the 1990s, which was the time of the highest incidence rate of the illness in Poland prior to the introduction of infant and other risk group immunization programs (13, 14). In those decades in Poland, the incidence rate among small children up to 4 years of age was 40/100,000 (13). The age group of those from 15 to 19 included adolescents born before the introduction of the compulsory infant vaccination programme which started in the years 1994 to 1996 (10, 13). In the years to come, a decline in the incidence rate in this age group is forecasted because this group will now include the people vaccinated as newborns, and the peak incidence rate will be moved to older age groups. Among 15 to 19-year-olds, a decline in the incidence rate was observed in 2011 in comparison with 2010, and this falling tendency was maintained in 2012 and 2013 (16-18).
This tendency is confirmed in our study, in which a significant growing trend of chronic hepatitis B cases was observed in all five-year age intervals encompassing 20 to 54-year-old individuals, i.e., excluding 15 to 19-year-olds. A significant decrease was noted in 10 to 14 year olds, the majority of whom had surely been vaccinated as infants.
Besides gender and age, another factor strongly differentiating the incidence rates of chronic hepatitis B in Poland was the patients’ places of residence. The incidence rates proved to be higher among the inhabitants of urban areas in comparison with those in the countryside (10-19). The significant growing trends in the incidence rates of chronic hepatitis B observed in both environments indicated approximate slopes and correlation coefficients.
The available studies on the relationship between the occurrence of HBV and place of residence are scarce. Higher incidence rates of acute (not chronic) hepatitis B have been reported by the Dutch in the border rural areas in the north-western part of the country. Most of the patients were men, with a high probability of belonging to the men who have sex with men (MSM) category or men for whom the likely route of transmission was not established (29). Iranian studies indicated a significant correlation between positive HBsAg and family history of hepatitis, as well as the countryside being a place of residence as another independent risk factor (30).
The incidence of chronic hepatitis B (diagnosis based on the serological markers) in Europe indicates geographic diversification. The HBs antigen carrier status is geographically diversified across Europe as well; the incidence is low in northern and western Europe (< 2% carriers) and medium in eastern and southern European countries (2% - 7%) (31, 32). According to the meta-analysis performed for the years from 2001 to 2009, the estimated number of chronically infected people varied from 4,466 in Ireland to 3,718,889 in Turkey (31).
The rate of carriers in the overall population of Europe varies from 0.5% - 0.7%, with the lowest level observed in Ireland and Holland (0.1% - 0.2%) and the highest in some regions of Turkey (7%) (21, 22, 29). In the population of Bulgaria, the estimated percentage of HBs antigen carriers is 4%, while in Romania it is 5.6% - 6.0% (22, 33). The percentages of carriers in Poland, the Czech Republic, Belgium, Lithuania, Italy, and Germany were determined to be at the level between 0.5% and 1.5% (34). In Italy, a fall in the number of chronic HBsAg carriers in the overall population was observed, dropping from 3% in 1980 to 1% in 2001 (33).
The decline in the incidence of acute hepatitis B in many regions of the world related to the free immunization programmes for children does not correspond to a decrease in the incidence of chronic hepatitis B. One should also bear in mind that not all countries have introduced vaccination programmes, thus maintaining multiple HBV infection and transmission options (35).
The rise in the number of chronic hepatitis B cases may be associated with the increased number of tests performed, but also with a growing migration of hepatitis cases from the countries with high hepatitis B incidence rates, which has been reported in many European countries and the USA (23, 25, 33, 34, 36, 37).
The strengths of this study include the presentation of the incidence rates through establishing trends not only in male and female populations and according to the places of residence, but also among 15 age groups. This type of presentation has not been found in any other work. Most often the epidemiological situation with regard to the incidence rates of hepatitis B in different countries is described by using either crude or standardized rates employing a different standard population and thus making it difficult to compare results between countries. Another advantage of this study is that the determination of coefficient trends allows for observation of the direction of changes and their stability in the incidence rates or mortality from the disease over a long period of time. Analysis carried out in this manner prevents drawing conclusions which depend on the random variation of coefficients (fluctuations).
A weakness of this study is the relatively short follow-up period necessitated by the introduction of the case definition of acute hepatitis B only in 2005, which only then allowed for separate registration of acute and chronic cases.
To sum up, the decline in the incidence rates of acute hepatitis B, which is the result of the growth in the number of vaccinated people and the improved sanitary conditions at medical care outlets, has had a positive impact on the overall epidemiological situation related to hepatitis B in Poland. The registered increase in the incidence of chronic hepatitis B in Poland is a consequence of the new registration of chronic hepatitis B cases acquired in the past. A weak point of the registration system in our country is the recognition of chronic hepatitis B based only on physicians’ diagnoses without the more precise application of the definitions of a chronic state of the disease, which may lead to multiple reporting of the same cases.
The solution to the problem of chronic hepatitis B can be found in the improvement of epidemiological surveillance, enhancement of the detection of frequently asymptomatic infections (the related screening tests performed in Poland encompass only pregnant women and blood donors), as well as providing easier access to optimized therapies.
References
-
1.
Janahi EM. Prevalence and risk factors of hepatitis B virus infection in Bahrain, 2000 through 2010. PloS one. 2014;9(2):87599.
-
2.
McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatol. 2009;49(5):45-55.
-
3.
Paganelli M, Stephenne X, Sokal EM. Chronic hepatitis B in children and adolescents. J Hepatol. 2012;57(4):885-96. [PubMed ID: 22634122]. https://doi.org/10.1016/j.jhep.2012.03.036.
-
4.
Hatzakis A, Wait S, Bruix J, Buti M, Carballo M, Cavaleri M, et al. The state of hepatitis B and C in Europe: report from the hepatitis B and C summit conference. J Viral Hepat. 2011;18(s1):1-16.
-
5.
Rossi C, Schwartzman K, Oxlade O, Klein MB, Greenaway C. Hepatitis B screening and vaccination strategies for newly arrived adult Canadian immigrants and refugees: A cost-effectiveness analysis. PloS one. 2013;8(10):78548.
-
6.
Ergunay K, Balaban Y, Cosgun E, Alp A, Simsek H, Sener B, et al. Epidemiologic trends in HBV infections at a reference centre in Turkey: an 11-year retrospective analysis. Ann Hepatol. 2012;11(5):672-8. [PubMed ID: 22947528].
-
7.
Hepatitis B. 2015. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/.
-
8.
Wozniak TM, Smith M, Maher L. Hepatitis B. N S W Public Health Bull. 2013;24(2):94. [PubMed ID: 24195855]. https://doi.org/10.1071/NB12094.
-
9.
Cadranel JF, Lahmek P, Causse X, Bellaiche G, Bettan L, Fontanges T, et al. Epidemiology of chronic hepatitis B infection in France: risk factors for significant fibrosis--results of a nationwide survey. Aliment Pharmacol Ther. 2007;26(4):565-76. [PubMed ID: 17661760]. https://doi.org/10.1111/j.1365-2036.2007.03400.x.
-
10.
Czarkowski MP, Rosinska M. [Hepatitis B in Poland in 2005]. Przegl Epidemiol. 2007;61(2):273-9. [PubMed ID: 17956042].
-
11.
Czarkowski MP, Bobel D. [Hepatitis B in Poland in 2006]. Przegl Epidemiol. 2008;62(2):317-24. [PubMed ID: 18807474].
-
12.
Rosinska M, Czarkowski MP. [Hepatitis B in Poland in 2007]. Przegl Epidemiol. 2009;63(2):245-50. [PubMed ID: 19799254].
-
13.
Stepien M, Czarkowski MP. [Hepatitis B in Poland in 2008]. Przegl Epidemiol. 2010;64(2):239-44. [PubMed ID: 20731229].
-
14.
Stepien M, Czarkowski MP. [Hepatitis B in Poland in 2009]. Przegl Epidemiol. 2011;65(2):259-64. [PubMed ID: 21913471].
-
15.
Stepien M, Czarkowski MP. [Hepatitis B in Poland in 2010]. Przegl Epidemiol. 2012;66(2):277-85. [PubMed ID: 23101217].
-
16.
Stepien M, Czarkowski MP. Hepatitis B in Poland in 2011. Przegl Epidemiol. 2013;67(2):239-45. 349-52. [PubMed ID: 24040725].
-
17.
Stepien M, Piwowarow K. Hepatitis B in Poland in 2012. Przegl Epidemiol. 2014;68(2):257-63. 363-7. [PubMed ID: 25135511].
-
18.
Choroby zakazne i zatrucia w Polsce 2013. Warszawa; 2014.
-
19.
Choroby zakazne i zatrucia w Polsce. Warszawa; 2006-2013.
-
20.
2015. Available from: http://www.pzh.gov.pl/oldpage/epimeld/index_p.html.
-
21.
Hatzakis A, Damme P, Alcorn K, Gore C, Benazzouz M, Berkane S, et al. The state of hepatitis B and C in the Mediterranean and Balkan countries: report from a summit conference. Viral Hepatitis J. 2013;20(s2):1-20.
-
22.
Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58(3):593-608. [PubMed ID: 23419824]. https://doi.org/10.1016/j.jhep.2012.12.005.
-
23.
Duffell EF, Laar MJW, Amato‐Gauci AJ. Enhanced surveillance of hepatitis B in the EU, 2006-2012. Viral Hepatitis J. 2015;22(7):581-9.
-
24.
Hepatitis B and C surveillance in Europe. Stockholm; 2012.
-
25.
Hansen N, Hay G, Cowan S, Jepsen P, Bygum Krarup H, Obel N. Hepatitis B prevalence in Denmark-an estimate based on nationwide registers and a national screening programme, as on 31 December 2007. Euro Surveill. 2013;18:20637.
-
26.
Poustchi H, Katoonizadeh A, Ostovaneh MR, Moossavi S, Sharafkhah M, Esmaili S, et al. Cohort profile: golestan hepatitis B cohort study- a prospective long term study in northern iran. Middle East J Dig Dis. 2014;6(4):186-94. [PubMed ID: 25349681].
-
27.
Chen CJ, Yang HI. Natural history of chronic hepatitis B REVEALed. J Gastroenterol Hepatol. 2011;26(4):628-38. [PubMed ID: 21323729]. https://doi.org/10.1111/j.1440-1746.2011.06695.x.
-
28.
Antony J, Celine T. A Hospital-based Retrospective Study on Frequency and Distribution of Viral Hepatitis. J Glob Infect Dis. 2014;6(3):99-104. [PubMed ID: 25191049]. https://doi.org/10.4103/0974-777X.138499.
-
29.
Soetens LC, van Benthem B, Urbanus A, Cremer J, Benschop K, Rietveld A, et al. Ongoing transmission of hepatitis b virus in rural parts of the netherlands, 2009 - 2013. PloS one. 2015;10(2):117703.
-
30.
Keyvani H, Sohrabi M, Zamani F, Poustchi H, Ashrafi H, Saeedian F, et al. A population based study on hepatitis B virus in northern iran, amol. Hepat Mon. 2014;14(8). e20540. [PubMed ID: 25237372]. https://doi.org/10.5812/hepatmon.20540.
-
31.
Hahne SJ, Veldhuijzen IK, Wiessing L, Lim TA, Salminen M, Laar M. Infection with hepatitis B and C virus in Europe: a systematic review of prevalence and cost-effectiveness of screening. BMC Infect Dis. 2013;13:181. [PubMed ID: 23597411]. https://doi.org/10.1186/1471-2334-13-181.
-
32.
Oh JK, Weiderpass E. Infection and cancer: global distribution and burden of diseases. Ann Glob Health. 2014;80(5):384-92. [PubMed ID: 25512154]. https://doi.org/10.1016/j.aogh.2014.09.013.
-
33.
Sagnelli E, Sagnelli C, Pisaturo M, Macera M, Coppola N. Epidemiology of acute and chronic hepatitis B and delta over the last 5 decades in Italy. World J Gastroenterol. 2014;20(24):7635-43. [PubMed ID: 24976701]. https://doi.org/10.3748/wjg.v20.i24.7635.
-
34.
Zacharakis G, Papoutselis M, Vafiadis N, Tzara F, Pouliou E. Changes in the epidemiology of hepatitis b virus infection following the implementation of immunization programmes in northeastern greece. Euro Surveill. 2009;14(32):1-6.
-
35.
Hwang EWM, Cheung R. Global epidemiology of hepatitis b virus (hbv) infection. N A J Med Sci. 2011;4(1):7-13.
-
36.
Chu J, Wörmann T, Popp J, Pätzelt G, Akmatov MK, Krämer A, et al. Changing epidemiology of hepatitis b and migration: A comparison of six northern and north-western european countries. Eur J Public Health. 2013;23(4):642-7.
-
37.
Kim W. Epidemiology of hepatitis B in the United States. Hepatol. 2009;49(S5).