Abstract
Background:
Chemotherapy for lymph nodes cancer is often composed of several drugs that are used in a treatment program.Objectives:
The aim of this study was to perform a cost-utility analysis of IEV regimen (ifosfamide, epirubicin and etoposide) versus ESHAP regimen (etoposide, methylprednisolone, high-dose cytarabine, and cisplatin) in patients with lymphoma in the south of Iran.Patients and Methods:
This was a cost-utility analysis done as a cross-sectional study in the south of Iran. Using decision tree, expected costs, quality -adjusted life years (QALYs) and the incremental cost-effectiveness ratio (ICER) were estimated. In addition, the robustness of results was examined by sensitivity analysis.Results:
The results of this study indicated that the total lymphoma patients were about 65 people that 27 patients received IEV regimen and 38 patients ESHAP (43 patients with Hodgkin’s and 22 with non-Hodgkin lymphoma). The results of decision tree showed that in the IEV arm, the expected cost was $20952.93 and the expected QALYs was 3.89 and in the ESHAP arm, the expected cost was $31691.74 and the expected QALYs was 3.86. Based on the results of the study, IEV regimen was cost-effective alternative to the ESHAP regimen.Conclusions:
According to the results of this study, it is recommended that oncologists use IEV instead of ESHAP in the treatment of patients with lymphoma and because of high costs of IEV drug costs, it is suggested that IEV drugs should be covered by insurance.Keywords
Hodgkin Lymphoma Non-Hodgkin’s Lymphoma Cost-Utility Analysis IEV ESHAP
1. Background
14 million cases of cancer are diagnosed annually and the number of cancer patients will reach 24 million people in the world by the year 2035 (1). In 2012, in the United States, the death rate from lymphoma, both among women and men, included 3% of all cases of death (2). In 2008, in Canada, 7,000 new cases were detected and 3,100 deaths occurred approximately (3). The non-Hodgkin’s lymphomas are the second cancer rising, both in terms of incidence and mortality, and its incidence has doubled since 1970 (4). Non-Hodgkin’s lymphomas are the most common hematologic malignancy among adults in the world (5) and the sixth type of malignant neoplasm in the United States (6). The incidence of Hodgkin’s lymphomas in the United States and England is 2.7 to 2.8 per 100,000, and 1,700 new cases are identified annually (7).
In Iran, cancer is the third leading cause of death after cardiovascular diseases and accidents. It is estimated that more than 70,000 new cases of cancer occur every year in the country (8). In 2009, the total number of cancer cases reported were 74,067 people, 55.58% in males and 44.42% in females. In the meantime, the age-standardized incidence rate of Lymphoma cancer is 4.57 among men in Fars province (9).
Due to the increase in life expectancy and lifestyle choices related to cancer, the burden of cancer is increasing in developing countries (10). The study of Khajedaluee et al. in Mashhad, Iranindicated that the most disability adjusted life years (DALY) were attributed to gastric, leukemia and lung cancers and the maximum DALY was related to breast cancer in women aged 30 - 44 years old (11). Murray et al. in a study concluded that stomach cancer, other neoplasms, liver cancer, and trachea, bronchus, and lung cancers caused more than 15 million DALYs (12). In one study in Australia in 2001, years life lost (YLL) constituted 78% of the overall DALYs associated with cancer (13).
Because cancer is considered a chronic disease, it impacts the lives of many people in all ages (14). National Institute for America’s Health, in 2010, estimated overall costs of cancer to be about 263.8 billion that includes direct costs ($102.2 billion), indirect costs due to illness, or loss of productivity due to illness ($20.9 billion) and indirect costs due to mortality or reduced productivity due to premature death ($140) (15). Therefore, one can say that after the damage, death and destruction of human, the most important aspect of cancer is the economic aspects and costs that are imposed on the lives of individuals and communities (16). Therefore, it is important to measure the quality of life and cost-effectiveness, and costs as well as quality should be measured in order to make decisions about utilization of resources (17).
Many patients with lymphatic cancer received chemotherapy as part of their treatment. Chemotherapy for cancer of the lymph nodes is often composed of several drugs that are used in a treatment program. Although lymphomas are potentially curable with standard chemotherapy, many patients either relapse or never achieve remission unless they use high dose chemotherapy (18). One of these methods is ESHAP regimen, a combination of the chemotherapeutic drugs etoposide, methylprednisolone, high-dose cytarabine, and cisplatin (19), and it has been shown to be active against refractory or relapsed lymphoma (20). Another method is IEV, a combination of three drugs Ifosfamide, epirubicin, and etoposide (21).
2. Objectives
Due to the various medical expenses in the use of these two methods and, consequently, different financial and economic burden on the health system and also the limited knowledge about the costs and their effectiveness, the researcher aimed to do a study to determine the cost-utility IEV versus ESHAP, which is used commonly in treatment of lymphoma in Iran.
3. Patients and Methods
This study was a cost- utility analysis done as a cross-sectional study in Amir Hospital in Shiraz, in the south of Iran. The study was conducted on patients who were admitted in Amir Hospital for chemotherapy in 2013. According to hospital statistics, in this period, approximately lymphoma patients were about 65 people, 27 of whom had received IEV and 38 patients ESHAP. Therefore, sampling was not done and all patients were included in the study. All of them underwent one of the two methods: IEV and ESHAP. When using these two methods, courses were repeated every 21 days about 3 - 4 times (21). To determine the cost-utility of IEV versus ESHAP, the decision tree was used. These patients responded to chemotherapy in three forms: complete response, partial response, non-response, complete response (CR) was defined as the complete disappearance of signs and symptoms due to lymphoma and maintained for at least 6 weeks; partial response (PR) was defined as a reduction of at least 50% in the product of the two largest perpendicular diameters of all measurable lesions for a duration of at least 6 weeks. Progression of disease (PD) or non- response was used where there was unequivocal evidence of advancing disease, despite continuation of the treatment (22).
First, using this model, the expected costs and QALY were calculated for both methods and the incremental cost-effectiveness ratio was measured; it was defined as the ratio of the difference between the expected cost and expected QALY. In this study, a decision tree model was used to examine the cost and effectiveness of IEV and ESHAP. The decision tree model is a graphic representation of the pathways of diagnosis and treatment of different diseases in which the probabilities, costs and consequences of pathways are shown. The decision tree was shown in Figure 1.
Decision Tree of IEV Versus ESHAP
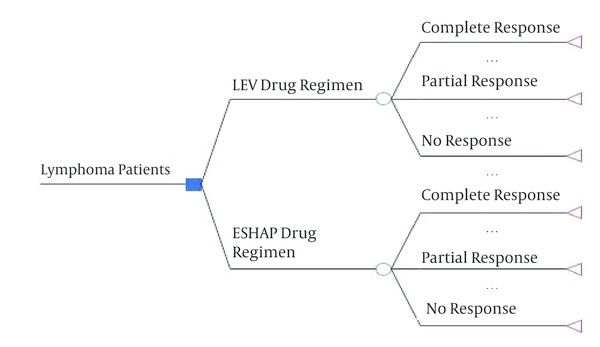
Data for this study was divided into two parts: utility and costs. The costs were identified and measured from social perspective. These items include medical and non- medical direct costs and indirect costs. In this study, a data collection form was used to collect data. It consisted of two parts. The first part included demographic data of the patients and the second section contained information about the therapy and pharmaceutical costs, diagnostic and laboratory (lab, radiology, MRI, etc.), the cost of accommodation and traveling and related expenses during chemotherapy. It should be noted that indirect costs are calculated using the human capital approach and because the study period was one year, we did not use the discount rate. To assess the utility of two regimens, the European organization for research and treatment of cancer quality of life questionnaire-core30 (EORTC QLQ-C30) was used (23). This questionnaire consisted of performance scale (physical, role, emotional, cognitive and social), symptom scale (dyspnea, insomnia, appetite loss, constipation, diarrhea and financial difficulties, fatigue, nausea and vomiting, pain), and an overall health-status scale. In the questionnaire, raw scores are considered from 0 to 100 and the highest score represents a high level of functioning, excluding symptom scales in which high scores represent a high level of symptoms. In general, this questionnaire reports separate scores for each dimension. First, using the weighted scores of quality of life, we calculated the utility (between zero and one) finally; the QALY was measured by multiplying the amount of utility in the treatment period (6 months or half a year).
The data was collected in this part of the study by interviewing the patient one month after the last chemotherapy because according to oncology experts, drug effect emerges one month after chemotherapy. We also used the contact number of patients, available in patient records, designated time and place of the interview. At the beginning of each interview, the overall goal of the project and interview was explained to each patient separately. The patients who were interested in participation answered questions fully informed and voluntarily. Next, the researcher collected cost data through data collection forms and data on utilities by using a questionnaire (EORTC QLQ-C30). In this section, these data (cost and utility) were simultaneously obtained from the patient. However, during the interview, the interviewer was unaware of the treatment protocol because the study was designed as a double blind study. This increases the accuracy of the interview. At this stage, only the patient record numbers were recorded on a demographic questionnaire. Then, the researchers recorded treatment protocol by referring to the patient records. To perform this analysis, Treeage 2011 and SPSS 16.0 specific software were used as well as descriptive statistical analysis. Also, the Mann-Whitney test was used to determine the significant differences in costs between the two groups. Also, 95% confidence intervals (CIs) were calculated using non-parametric bootstrapping approach for cost and QALY. By the decision tree, the expected costs and QALY were calculated and to increase the accuracy of the study, one-way deterministic sensitivity analysis (Tornado Diagram) and probabilistic sensitivity analysis were performed.
4. Results
Based on the results of the present study, of the 65 patients studied, 66.1% were male, 67.7% were married, 60% were aged less than 40 years and all patients had insurance.
Table 1 shows the mean QALY in patients, using two different regimens of IEV and ESHAP. According to the results, in the IEV, the QALY’s mean was 0.3676 (in Hodgkin lymphoma, 0.3712 and in non-Hodgkin lymphoma 0.362) and in the ESHAP; the QALY’s mean was 0.3029 (in Hodgkin lymphoma, 0.3065 and in non-Hodgkin lymphoma, 0.295).
QALY Value Based on the Type of Lymphoma
| Type of Lymphoma | Type of Treatment Protocol | Number of Patients | QALY |
|---|---|---|---|
| IEV | 17 | 0.3712 ± 0.0754 | |
| ESHAP | 26 | 0.3065 ± 0.0651 | |
| IEV | 10 | 0.362 ± 0.0885 | |
| ESHAP | 12 | 0.295 ± 0.0602 | |
| IEV | 27 | 0.3676 ± 0.0789 | |
| ESHAP | 38 | 0.3029 ± 0.063 |
According to Table 2, in both IEV and ESHAP, the mean of medical direct costs was the highest (1191.1 and 1819.57 dollars, respectively) and that of non-medical direct costs was minimum (237.06 and 208.39 dollars, respectively). As seen in Table 2, the mean of medical direct costs was significant in both IEV and ESHAP (P = 0.0001). But the means of non-medical direct costs, indirect costs and total cost difference were not significant statistically. As seen in Table 3, the mean cost of chemotherapy in the IEV was 488.94 dollars that is the highest type of medical direct costs. Also, in the ESHAP, the cost of chemotherapy was 866.92 dollars and it was the highest medical direct costs. Travel costs and indirect costs of the patients were respectively the highest type of non-medical direct costs and indirect costs in both (in the IEV, 149.52 and 562.2 dollars and in the ESHAP 104.75 and 351.65 dollars, respectively).
The Cost of Cancer Patients With Lymph Node Based on the Type of Costs
| Type of Treatment ProtocolCosts Mean | IEV | ESHAP | P-Value |
|---|---|---|---|
| 1191.1 ± 610.74 | 1819.57 ± 789.73 | 0.0001 | |
| 237.06 ± 207.42 | 208.39 ± 179.7 | 0.545 | |
| 598.24 ± 584.25 | 456.83 ± 666.09 | 0.249 | |
| 2026.42 ± 916.91 | 2484.79 ±1060.27 | 0.123 |
| Strategy | IEV | ESHAP |
|---|---|---|
| 1191.1 ± 610.74 | 1819.57 ± 789.73 | |
| Medication | 488.94 ± 338.57 | 866.92 ± 354.91 |
| Hospitalization | 127.84 ± 30.25 | 261.35 ± 94.32 |
| Sonography | 20.63 ± 35.34 | 20.39 ± 34.09 |
| Radiology | 53.37 ± 42.38 | 72.4 ± 54.05 |
| MRI | 10.46 ± 25.12 | 16.36 ± 34.88 |
| Surgical cost | 160.73 ± 399.53 | 113.14 ± 164.96 |
| Laboratory tests | 143.09 ± 76.89 | 231.6 ± 210.90 |
| CT-sCAN | 71.47 ± 81.39 | 77.76 ± 80.56 |
| Visits | 22.57 ± 24.23 | 26.28 ± 21.61 |
| Other | 91.95 ± 389.42 | 133.33 ± 420.12 |
| 237.06 ± 207.42 | 208.39 ± 179.7 | |
| Traveling | 149.52 ± 142.95 | 104.75 ± 98.56 |
| Lodging | 48.89 ± 77.24 | 55.56 ± 102.37 |
| Phone | 25.19 ± 23.78 | 23.42 ± 14.32 |
| Auxiliary equipment | 4.78 ± 23.30 | 1.8 ± 7.31 |
| Special diet | 8.67 ± 14.50 | 22.84 ± 34.52 |
| 598.24 ± 584.25 | 456.83 ± 666.09 | |
| Time spent by the patient | 562.2 ± 606.49 | 351.65 ± 655.41 |
| Time spent by the patient’s accompany | 36.03 ± 52.98 | 105.17 ± 280.48 |
| 2026.42 ± 916.91 | 2484.79 ±1060.27 |
The results of decision tree in Figure 1 showed that in the IEV arm, the expected cost was 20952.93 dollars and the expected QALYs were 3.89 and in the ESHAP arm, the expected cost was 31691.74 dollars and the expected QALYs were 3.86. Therefore, IEV is dominant compared to ESHAP (less costly and more effective).
4.1. Sensitivity Analysis
Given that any economic evaluation study accompanies uncertainty, in this study the effects of uncertainty were examined using one-way and probabilistic sensitivity analysis. In the one-way sensitivity analysis, the value of each variable increased by 20%; in order to create the tornado diagram (24). According to the tornado diagram in Figure 2, changes in most of the input parameters had a few effects on the outcome. The incremental cost-effectiveness ratio (ICER) had high sensitivity to QALY of patients who did not respond to ESHAP and had low sensitivity to the cost of patients who did not respond to IEV.
Results of One-Way Sensitivity Analysis (Tornado Diagram)
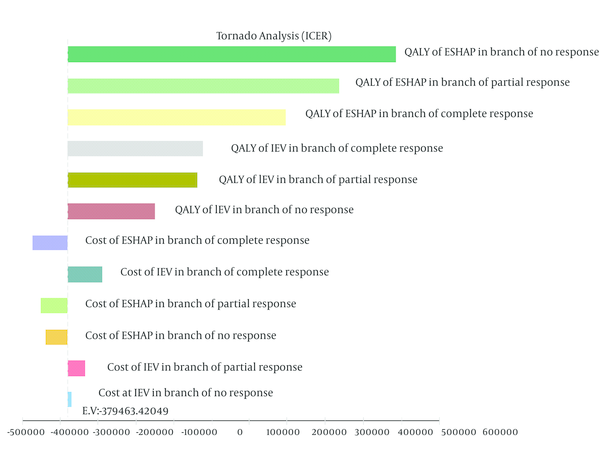
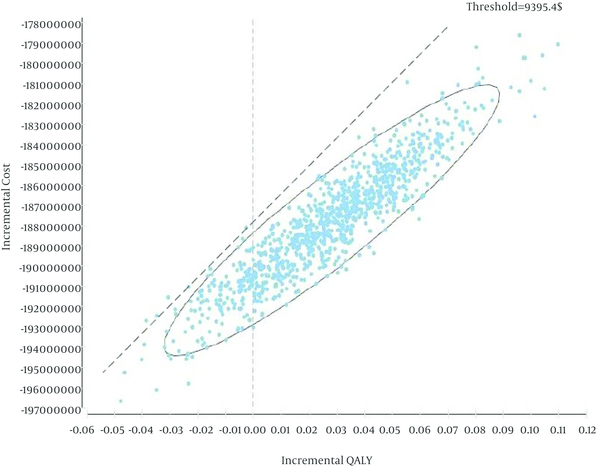
Figure 3 indicated the results of probabilistic sensitivity analysis using Monte Carlo simulation of incremental costs and QALYs IEV versus ESHAP. For each one of the 10,000 iterations, values for parameters were randomly selected from their probability distributions and results showed in 97% of the iterations, IEV was a dominant strategy. In other words, IEV was estimated to have a lower cost and greater QALYs than ESHAP. In addition, maximum willingness to pay (threshold) was calculated based on WHO method (three times of Gross Domestic Product per capita, $9395.4) (25).
Results of Probabilistic Sensitivity Analysis (Each Point Indicates the Differences in Costs and QALYs for IEV vs. ESHAP)
5. Discussion
The costs and consequences of interventions and programs are compared in economic evaluation for the optimal use of scarce resources. Therefore, the aim of this study was to perform a cost-utility analysis of IEV versus ESHAP in patients with lymphoma. To our knowledge, this is the first full economic evaluation study in patients with lymphoma in Iran. Because lymphoma is among the ten most common cancers and also cancer is the third leading cause of death in Iran; besides, IEV and ESHAP medication regimens are common drugs in the treatment of lymphoma, the discussion about the effectiveness and costs associated with them is of great importance.
The findings of this study showed that the average direct cost of treatment in IEV and ESHAP arms were 119,1.1 and 181,9.57 dollars respectively and the difference was significant (P value = 0.0001). However, a significant difference was not observed between the mean of non-medical direct costs and indirect costs in two arms. Also, the mean cost of chemotherapy was 866.92 dollars in the ESHAP arm; it is 47.7% of medical direct costs. However, the mean cost of chemotherapy was 866.92 dollars in the IEV arm, which is 41.04% of direct costs. In addition, the mean length of stay for each period of chemotherapy was 3 days in the IEV arm and 5 days in the ESHAP arm. These results could be due to the higher cost of chemotherapy in the ESHAP and probably a lengthier stay would lead to more paraclinical costs. Hackshaw et al. (26), Woronoff-Lemsi et al. (27), Johnston et al. (28), Leese et al. (29), Ray et al. (30) and Kuderer et al. (31) in their study concluded that the main cost drivers were medical direct costs especially chemotherapy drugs and medical direct costs were higher because of the higher cost of chemotherapy. Also, earlier discharge of patients with lymphoma would lead to reducing the length of stay and the medical direct costs.
Based on the results of this study, the means of QALY’s were 0.3676 and 0.3029 in the IEV and ESHAP arms, respectively. In the meantime, the mean of QALY in patients with Hodgkin’s lymphoma was 0.3712 and 0.3065, and in patients with non-Hodgkin’s lymphoma it was 0.362 and 0.295 for the IEV and ESHAP arms, respectively. Based on the findings of this study, it can be stated that quality of life of patients who had received ESHAP is lower than those who had received IEV. This could be because of the lengthier stay for these patients, having a more negative impact on quality of life or may be due to the appropriate dose in the IEV than ESHAP. Hjermstad et al. (32) in their study concluded that the length of stay has much impact on activities including work, family and social and daily activities, affecting their quality of life. So they will have a better quality in functional and emotional dimensions if their length of stay is shorter. Also, Hasanpoor et al. indicated that there was a significant relationship between quality of life and type of cancer (33). Also, Mols et al. (34), Webster and Cella (35) and van Dis et al. (36) indicated that patients who received chemotherapy reported lower overall health-related quality of life scores compared with patients who did not receive chemotherapy.
Based on the results of the study, ESHAP not cost-effective as compared to IEV and it is dominated because the expected cost was 20952.93 dollars and the expected QALY was 3.89 in the IEV arm whereas the expected cost was 31691.74 dollars and the expected QALY was 3.86 in the ESHAP arm. It can be stated that patients who had used the ESHAP had higher medical direct costs because of expensive chemotherapy and the higher mean of length of stay (5 days versus. 3 days) than IEV. Based on the results of the sensitivity analysis, the ICER was highly sensitive to QALY of patients who did not respond to ESHAP and less sensitive to the cost of patients who did not respond to IEV. Overall, the results showed that IEV versus ESHAP was dominant in the treatment of patients with lymphoma. Also, ICER was -379463.42 dollars (using IEV saves 379,463.42 dollars per each additional QALY). Therefore, it is recommended that oncologists should use IEV instead of ESHAP in the treatment of these patients. In addition, the results of the one-way and probabilistic sensitivity analysis powerfully support the conclusion that IEV regimen is a cost-effective option to ESHAP regimen.
As to the generalizability of these findings, we can generalize these results to other Iranian hospitals, because of using IEV and ESHAP in the treatment of lymphoma therein. However, we cannot generalize these results to other countries certainly due to differences in the costs covered by insurance organizations, the patients’ ability to pay, the incidence and prevalence of disease, difference in clinical guidelines, relative prices , payment system and ceiling ratio.
This study had several limitations: first, due to time limits all patients were studied during a course of treatment. Probably, different results would have been obtained if it had been done in a longer time period. Second, due to lack of studies in this field, using Markov models was not possible. Third, drug prices varied during the study so in relation with this case, the average prices were used. Fourth, due to the sensitivity of cancer patients about their disease, using the gentle way to communicate with them is essential. In addition, in this study we used the weighted scores of European quality of life questionnaire for measuring the utility of two regimens (IEV and ESHAP); therefore, the limitation of this questionnaire for Iranian setting must be considered and in future studies, it is suggested that researchers estimate these weighted scores for Iran.
Given the result of this study, it is suggested that IEV drugs be covered by insurance because of high costs of cancer patients or IEV drug costs be paid in health sectors. When developing clinical guidelines for the treatment of lymphoma, the government and the Ministry of Health should consider using IEV method by oncologists to reduce costs.
Acknowledgements
References
-
1.
Stewart BW, Wild CP. World Cancer Report 2014. Lyon, France: International Agency for Research on Cancer World Health Organization; 2014. Available from: http://www.iarc.fr/en/publications/books/wcr/.
-
2.
Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31(1):100-10. [PubMed ID: 19934210]. https://doi.org/10.1093/carcin/bgp263.
-
3.
Canadian Cancer Statistics. Toronto: Canadian Cancer Society; 2008. Available from: http://www.cancer.ca/en/cancer-information/cancer-101/canadian-cancer-statistics-publication/?region=on.
-
4.
Altekruse S, Kosary C, Krapcho M, Neyman N, Aminou R, Waldron W. SEER Cancer Statistics Review, 1975-2007. Bethesda: National Cancer Institute; 2010. Available from: http://seer.cancer.gov/archive/csr/1975_2007/.
-
5.
Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790-801. https://doi.org/10.1016/s1470-2045(12)70211-5.
-
6.
Jaffe E, Harris N, Stein H, Vardiman J. WHO classification: Pathology and genetics of tumors of haematopoietic and lymphoid tissues. Ann Oncol. 2001;13(3):490-1. https://doi.org/10.1093/annonc/mdf146.
-
7.
Townsend W, Linch D. Hodgkin's lymphoma in adults. Lancet. 2012;380(9844):836-47. https://doi.org/10.1016/s0140-6736(12)60035-x.
-
8.
Mousavi S. Executive Guideline for Registering and Reporting Cancer Cases. Tehran: Cancer Office and the Research fellowship of Cancer Research Center of Cancer Institute; 2007. 22 p.
-
9.
Iranian National Cancer. Iranian national cancer registration report, 2010. Tehran; 2010.
-
10.
The GlobalBurden of Disease. 2008. Available from: http://www.who.int/healthinfo/ global_burden_disease/GBD_report_2004update_full.pdf.
-
11.
Khajedaluee M, Dadgarmoghaddam M, Saeedi R, Izadi-Mood Z, Abrishami M, Zamani M. Mortality, Morbidity, Survival, and Burden of Top 9 Cancers in a Developing Country. Razavi Int J Med. 2014;2(3).
-
12.
Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197-223. https://doi.org/10.1016/s0140-6736(12)61689-4.
-
13.
Department of Human Services. Victorian burden of disease study, Mortality and morbidity in 2001. Melbourne: The Public Health Group, Rural and Regional Health and Aged Care Services Division; 2005. 228 p.
-
14.
Mousavi Jarrahi Y, Mousavi Jarrahi A, Mohagheghi MA. The indirect cost of cancer in Tehran, preliminary report. Cancer Bullet. 2010;2:60-4.
-
15.
Atlanta G. Cancer Facts & Figures 2010. American Cancer Society; 2010. Available from: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2010/index.
-
16.
Davari M, Yazdanpanah F, Aslani A, Hosseini M, Nazari AR, Mokarian F. The Direct Medical Costs of Breast Cancer in Iran: Analyzing the Patient's Level Data from a Cancer Specific Hospital in Isfahan. Int J Prev Med. 2013;4(7):748-54. [PubMed ID: 24049592].
-
17.
Wright MJ, Galea V, Barr RD. Proficiency of balance in children and youth who have had acute lymphoblastic leukemia. Phys Ther. 2005;85(8):782-90. [PubMed ID: 16048425].
-
18.
Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327(21):1478-84. [PubMed ID: 1383821]. https://doi.org/10.1056/NEJM199211193272102.
-
19.
Choi CW, Paek CW, Seo JH, Kim BS, Shin SW, Kim YH, et al. ESHAP salvage therapy for relapsed or refractory non-Hodgkin's lymphoma. J Korean Med Sci. 2002;17(5):621-4. [PubMed ID: 12378012].
-
20.
Velasquez WS, McLaughlin P, Tucker S, Hagemeister FB, Swan F, Rodriguez MA, et al. ESHAP--an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994;12(6):1169-76. [PubMed ID: 8201379].
-
21.
Zinzani PL, Tani M, Molinari AL, Stefoni V, Zuffa E, Alinari L, et al. Ifosfamide, epirubicin and etoposide regimen as salvage and mobilizing therapy for relapsed/refractory lymphoma patients. Haematologica. 2002;87(8):816-21.
-
22.
Zinzani PL, Barbieri E, Visani G, Gherlinzoni F, Perini F, Neri S, et al. Ifosfamide, epirubicin and etoposide (IEV) therapy in relapsed and refractory high-grade non-Hodgkin's lymphoma and Hodgkin's disease. Haematologica. 1994;79(6):508-12. [PubMed ID: 7534744].
-
23.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-76. [PubMed ID: 8433390].
-
24.
Taylor M. What is sensitivity analysis?. Heslington: University of York; 2009.
-
25.
Hutubessy R, Chisholm D, Edejer TT. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003;1(1):8. [PubMed ID: 14687420]. https://doi.org/10.1186/1478-7547-1-8.
-
26.
Hackshaw A, Sweetenham J, Knight A. Are prophylactic haematopoietic growth factors of value in the management of patients with aggressive non-Hodgkin's lymphoma? Br J Cancer. 2004;90(7):1302-5. [PubMed ID: 15054445]. https://doi.org/10.1038/sj.bjc.6601708.
-
27.
Woronoff-Lemsi MC, Arveux P, Limat S, Deconinck E, Morel P, Cahn JY. Cost comparative study of autologous peripheral blood progenitor cells (PBPC) and bone marrow (ABM) transplantations for non-Hodgkin's lymphoma patients. Bone Marrow Transplant. 1997;20(11):975-82. [PubMed ID: 9422478]. https://doi.org/10.1038/sj.bmt.1700998.
-
28.
Johnston KM, Marra CA, Connors JM, Najafzadeh M, Sehn L, Peacock SJ. Cost-effectiveness of the addition of rituximab to CHOP chemotherapy in first-line treatment for diffuse large B-cell lymphoma in a population-based observational cohort in British Columbia, Canada. Value Health. 2010;13(6):703-11. [PubMed ID: 20561333]. https://doi.org/10.1111/j.1524-4733.2010.00737.x.
-
29.
Leese B, Collin R, Clark DJ. The costs of treating febrile neutropenia in patients with malignant blood disorders. Pharmacoeconomics. 1994;6(3):233-9. [PubMed ID: 10155266].
-
30.
Ray JA, Carr E, Lewis G, Marcus R. An evaluation of the cost-effectiveness of rituximab in combination with chemotherapy for the first-line treatment of follicular non-Hodgkin's lymphoma in the UK. Value Health. 2010;13(4):346-57. [PubMed ID: 20070643]. https://doi.org/10.1111/j.1524-4733.2009.00676.x.
-
31.
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-66. [PubMed ID: 16575919]. https://doi.org/10.1002/cncr.21847.
-
32.
Hjermstad MJ, Oldervoll L, Fossa SD, Holte H, Jacobsen AB, Loge JH. Quality of life in long-term Hodgkin's disease survivors with chronic fatigue. Eur J Cancer. 2006;42(3):327-33. [PubMed ID: 16377179]. https://doi.org/10.1016/j.ejca.2005.09.028.
-
33.
Hasanpour Dehkordi A, Shaaban M. Relationship between cancer characteristics and quality of life in the cancer patients under chemotherapy referred to selected clinic of Tehran university of medical sciences. J Shahrekord Univ Med Sci. 2005;6(4):63-71.
-
34.
Mols F, Aaronson NK, Vingerhoets AJ, Coebergh JW, Vreugdenhil G, Lybeert ML, et al. Quality of life among long-term non-Hodgkin lymphoma survivors: a population-based study. Cancer. 2007;109(8):1659-67. [PubMed ID: 17330853]. https://doi.org/10.1002/cncr.22581.
-
35.
Webster K, Cella D. Quality of life in patients with low-grade non-Hodgkin's lymphoma. Oncology (Williston Park, NY). 1998;12(5):697-714.
-
36.
van Dis FW, Mols F, Vingerhoets AJ, Ferrell B, van de Poll-Franse LV. A validation study of the Dutch version of the Quality of Life-Cancer Survivor (QOL-CS) questionnaire in a group of prostate cancer survivors. Qual Life Res. 2006;15(10):1607-12. [PubMed ID: 16826441]. https://doi.org/10.1007/s11136-006-0015-y.