Abstract
Background:
Breast cancer is the first leading cause of cancer-related deaths in women. Ki-67 is being used for evaluation of the prognosis of patients with breast cancer.Objectives:
The aim of the current study was to explore the association of the involvement of axillary lymph nodes status with the expression of Ki-67 in patients with breast cancer.Methods:
A total of 449 patients were enrolled followed by evaluation of the association of Ki67 levels with demographic, pathologic, and survival data of patients, using Chi-square, logistic regression models, student t test and Mann-Whitney.Results:
We observed a significant relationship between the expression level of Ki-67 and stage of tumor (P = 0.012), positive progesterone receptor (P = 0.003), and subtype pathologic features (P < 0.05). Also, a significant difference was detected between Her2 and expression level of Ki-67 (P = 0.015). Survival analysis showed the association for Ki-67 (P = 0.02), age (P = 0.005), stage of tumor (P < 0.05), lymph node involvement (P = 0.001), and the Her2 (P = 0.024) with clinical outcome (e.g., overall survival or disease free survival) of patients with breast cancer.Conclusions:
The results of this study demonstrated that the overexpression of Ki-67 was associated with large tumors, progesterone receptor expression, and stage of tumor, but it was not related with lymph node involvement.Keywords
1. Background
Breast cancer is the most common cancer and the first cause of death in women worldwide (1). In Iran, the 5 most common cancers (except skin cancer) are breast, esophagus, stomach, colon-rectum, and cervix uteri in females, while breast cancer ranks first (2, 3). Classified information of features of breast cancer is available from the developed countries, but clinicopathological aspects of this disease are rarely available in Iran (4-6). The prevalence of breast cancer is 120 in 100 000 and the incidence is 22 in 100 000 in Iran. Breast cancer in Iranian women occurs 1 decade earlier than the developed countries in the ages of 40 to 49 years old (7).
The expression of the biomarkers in breast cancer is important to identify prognosis. For example, the expression of human epidermal growth factor receptor 2 (HER2), as a member of the epidermal growth factor receptor family, would occur in 20% to 30% of breast cancer tumors (8). As a biomarker protein, Ki-67 is another cellular marker for proliferation and it is associated with the carcinomas of the prostate, brain and the breast, and nephroblastoma, which is known as one of the most powerful indicators of tumor behavior and a useful tool in determining the aggressiveness of malignant neoplasm in several studies (9-12).
The Ki-67 has roles, including ribosomal RNA transcription and inactivation of Ki-67, which leads to the inhibition of ribosomal RNA synthesis (13). High rate of proliferation of Ki-67 is considered to cell growth fraction and it predicts poor survival in prostate cancer, myeloma, breast cancer, and aggressive tumors (14, 15). In about 40% of patients with breast cancer, the malignancy is growing and spreading regionally to one axillary node, at least. In primary breast tumors, high level of Ki-67 expression is significantly related with the primary tumor stage (16).
The role and the prognostic value of Ki-67 in patients with breast cancer with positive axillary nodes are unknown. Because of the importance of breast cancer, as a mortal factor within women and lack of such study, we evaluated the involvement of axillary lymph nodes with increased expression of Ki-67marker in patients with breast cancer.
2. Methods
In this retrospective study, we assessed case files of 2 723 female patients with breast cancer, who were referred to radiotherapy and oncology centers of Mashhad University of Medical Sciences from 2002 to 2012 (Convenient Sampling). This project has been approved by the Ethics Committee of Mashhad University of Medical Sciences (Ethical Code: IR.MUMS.REC.1391.706). The inclusion criterion was non-metastatic breast cancer and the exclusion criteria were as follow: 1) T4 stage; 2) metastatic disease at diagnosis; 3) incomplete medical records; and 4) lack of access to patients’ information. We also excluded patients presenting with metastasis.
By considering the minimum power of 80% for Chi-squared test, the significance level and the effect size were obtained 5% and 0.2669, respectively. The sample size was considered 449 by NCSS & PASS software. According to the objectives and research questions, all obtained information was analyzed, using SPSS 11 after initial processing. All research variables were described by descriptive statistics methods, including frequency and agreement tables, diagrams of frequency distributions, and bar charts; statistical indicators were described. We used Chi-two, logistic regression analysis, t student for independent two groups or its equivalent, while Mann-Whitney was used for the nonparametric parameters. To assess the normality of quantitative data, the Kolmogorov–Smirnov was used.
3. Results
Among 2 723 cases of female breast cancer, 449 patients were included in this study. The mean age of the patients was 49.31 ± 11.6 and the median age was 48 years. A total of 105 (23.5%) cases were under 40 years and 341 (76.5%) were older than 40 years. Regarding the menopausal status, 241 (57.8%) patients were pre-menopause and 176 (42.2%) post-menopause. Invasive ductal carcinoma was seen in 416 (92.7%) and lobular carcinoma in 9 (2%) patients. Table 1 includes the characteristic features of patients. Also, 46 (13.3%) patients were in stage I; 162 (46.8%) had stage II, while 122 (35.3%), and 16 (4.6%) had stage III and IV, respectively (Table 1).
Characteristic Features of Patients
| Clinicopathologic Features | No. | % |
|---|---|---|
| Age | ||
| < 40 years | 105 | 23.5 |
| > 40 years | 341 | 76.5 |
| Menopausal status | ||
| Pre-menopausal | 241 | 57.8 |
| Post-menopausal | 176 | 42.2 |
| Histology of tumor | ||
| Invasive ductal carcinoma | 167 | 38 |
| Invasive lobular carcinoma | 262 | 59.7 |
| Other | 10 | 2.3 |
| Stage of tumor | ||
| I | 46 | 13.3 |
| II | 162 | 46.8 |
| III | 122 | 35.3 |
| IV | 16 | 4.6 |
| Size of tumor (T) | ||
| T1 | 142 | 35.4 |
| T2 | 202 | 50.4 |
| T3 | 40 | 10 |
| T4 | 17 | 4.2 |
| Lymph node involvement (N) | ||
| N0 | 133 | 37.9 |
| N1 | 103 | 29.3 |
| N2 | 81 | 23.1 |
| N3 | 34 | 9.7 |
| Distant metastasis (M) | ||
| M0 | 433 | 96.4 |
| M1 | 16 | 3.6 |
| Estrogen | ||
| + | 286 | 63.7 |
| - | 163 | 36.3 |
| Progesterone | ||
| + | 262 | 58.7 |
| - | 184 | 41.3 |
| HER-2 | ||
| Negative | 291 | 65.7 |
| 2+ | 74 | 16.7 |
| Positive | 78 | 17.6 |
| Subtype pathologic | ||
| A luminal | 90 | 22 |
| B luminal | 195 | 47.7 |
| HER-2+ | 38 | 9.3 |
| Triple negative | 86 | 21 |
| Disease recurrent | ||
| + | 370 | 17.6 |
| - | 79 | 82.4 |
The association of the expression level of Ki-67 with clinicopathological aspects of the patients was evaluated; there was a significant relation between the expression level of Ki-67 and HER2/neu status (P = 0.038) and progesterone receptor expression (P = 0.003), while there was no association between estrogen receptor (ER), distant metastasis, lymph node involvement, tumor size, tumor histology, menopausal status, stage of tumor pathological subtypes of tumor, and the age with the expression of Ki-67 (P > 0.05) (Table 2).
Relation of Expression Level of Ki-67 Marker and Patients’ Characteristics
| Characteristics | Low Ki-67 (KI-67 < 14%)a | High Ki-67 (KI-67 ≥ 14%)a | P-Value |
|---|---|---|---|
| Age | 0.094 | ||
| < 40 years | 28 (26.7) | 77 (73.3) | |
| > 40 years | 121 (35.5) | 220 (64.5) | |
| Menopausal status | 0.985 | ||
| Pre-menopausal | 81 (33.6) | 160 (66.4) | |
| Post-menopausal | 59 (33.5) | 117 (66.5) | |
| Histology of tumor | 0.929 | ||
| Invasive ductal carcinoma | 54 (32.3) | 113 (67.7) | |
| Invasive lobular carcinoma | 89 (34) | 173 (66) | |
| Other | 7 (35) | 13 (65) | |
| Size of tumor (T) | 0.645 | ||
| T1 | 50 (35.2) | 92 (64.8) | |
| T2 | 61 (30.2) | 141 (69.8) | |
| T3 | 12 (30) | 28 (70) | |
| T4 | 7 (41.2) | 10 (58.8) | |
| Lymph node involvement (N) | 0.623 | ||
| N0 | 45 (33.8) | 73 (70.9) | |
| N1 | 30 (29.1) | 50 (61.7) | |
| N2 | 31 (38.3) | 22 (64.7) | |
| N3 | 12 (35.3) | 121 (20) | |
| Stage of tumor | 0.012 | ||
| I | 22 (47.8) | 24 (52.2) | |
| II | 41 (25.3) | 121 (74.7) | |
| III | 48 (39.3) | 74 (60.7) | |
| IV | 6 (37.5) | 10 (62.5) | |
| Distant metastasis (M) | 0.724 | ||
| M0 | 144 (33.3) | 289 (66.7) | |
| M1 | 6 (37.5) | 10 (62.5) | |
| Estrogen | 0.179 | ||
| + | 48 (29.4) | 115 (70.6) | |
| - | 102 (35.7) | 184 (64.3) | |
| Progesterone | 0.003 | ||
| + | 47 (25.5) | 137 (74.5) | |
| - | 102 (39.8) | 160 (61.1) | |
| HER-2 | 0.015 | ||
| Positive | 21 (26.9) | 57 (73.1) | |
| 2+ | 17 (23) | 57 (77) | |
| Negative | 112 (38.5) | 179 (61.5) | |
| Subtype pathologic | 0.000 | ||
| A luminal | 88 (97.8) | 2 (2.2) | |
| B luminal | 8 (8) | 187 (95.9) | |
| HER-2+ | 21 (21) | 65 (75.6) | |
| Triple negative | 16 (16) | 22 (57.9) |
3.1. Survival Analysis
We followed up the survival in median of 120 months (4 - 124) by Kaplan-Mayer. Patients less than 14% of Ki67 (low Ki67) showed longer survival than other cases. The assessment of the studied variables on survival analysis showed significant association for Ki-67 (P = 0.02), age (P = 0.005), stage of tumor (P < 0.05), lymph node involvement (P = 0.001), and the Her2 (P = 0.024) (Table 3 and Figure 1). Because of the significance of Ki-67 in total survival, we used the Cox regression for clinical variables; age (P = 0.013), stage of tumor (P < 0.05), and Ki-67 (P = 0.048) were found significant in multi variant (Table 4; Figure 2).
Evaluation of Effect of Studied Variables on Survival (Kaplan-Mayer)
| Studied Variables | Disease-Free Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|
| Mean | Chi-Square | P Value | Mean | Chi-Square | P-Value | |
| KI-67 | 0.02 | |||||
| < 14 | 98.48 | 2.112 | 0.146 | 102.95 | 5.394 | |
| ≥ 14 | 78.07 | 78.7 | ||||
| Age | 0.005 | |||||
| < 40 years | 60.79 | 4.255 | 0.039 | 63.099 | 8.017 | |
| > 40 years | 97.48 | 99.061 | ||||
| Menopausal status | 0.433 | |||||
| Pre-menopausal | 93.002 | 0.033 | 0.855 | 90.438 | 0.615 | |
| Post-menopausal | 80.348 | 84.014 | ||||
| Histology of tumor | 0.582 | |||||
| Invasive ductal carcinoma | 78.86 | 0.806 | 0.688 | 80.436 | 1.083 | |
| Invasive lobular carcinoma | 92.75 | 86.164 | ||||
| Other | 72.3 | 71.714 | ||||
| Stage of tumor | 0.000 | |||||
| I | 84.544 | 33.027 | 0.000 | 89.429 | 52.39 | |
| II | 85.505 | 88.418 | ||||
| III | 60.457 | 62.861 | ||||
| IV | 32 | 46.155 | ||||
| Size of tumor (T) | 0.065 | |||||
| T1 | 85.309 | 3.686 | 0.297 | 82.893 | 7.212 | |
| T2 | 78.903 | 81.896 | ||||
| T3 | 61.62 | 66.679 | ||||
| T4 | 57.39 | 53.521 | ||||
| Lymph node involvement | 0.001 | |||||
| N0 | 87.66 | 8.07 | 0.045 | 93.192 | 16.688 | |
| N1 | 82.85 | 82.153 | ||||
| N2 | 60.74 | 64.246 | ||||
| N3 | 52.51 | 55.691 | ||||
| ER | 0.184 | |||||
| + | 89.23 | 0.898 | 0.343 | 89.215 | 1.761 | |
| - | 77.78 | 80.271 | ||||
| PR | 0.07 | |||||
| + | 85.73 | 2.605 | 0.107 | 85.75 | 3.288 | |
| - | 79.73 | 82.234 | ||||
| HER-2 | 0.024 | |||||
| Positive | 85.4 | 6.524 | 0.038 | 81.45 | 7.441 | |
| 2+ | 69.6 | 76.9 | ||||
| Negative | 82.1 | 85.982 | ||||
| Subtype pathologic | 0.201 | |||||
| A luminal | 82.128 | 3.034 | 0.386 | 83.06 | 4.634 | |
| B luminal | 72.71 | 75.2 | ||||
| HER-2+ | 71.86 | 72.05 | ||||
| Triple negative | 98.2 | 96.17 | ||||
Association of Ki67 with OS

Association of Ki-67 with Clinical and Histopathologic Parameters
| Variables | Overall Survival | ||
|---|---|---|---|
| HR | Wald | P-Value | |
| KI-67 | 2.337 | 3.898 | 0.048 |
| Age No. (%) | 0.43 | 6.123 | 0.013 |
| Stage of tumor | 4.959 | 20.446 | 0.000 |
| Lymph node involvement | 0.772 | 1.291 | 0.256 |
| HER-2 | 1.315 | 1.875 | 0.171 |
Association of Ki67 with DFS
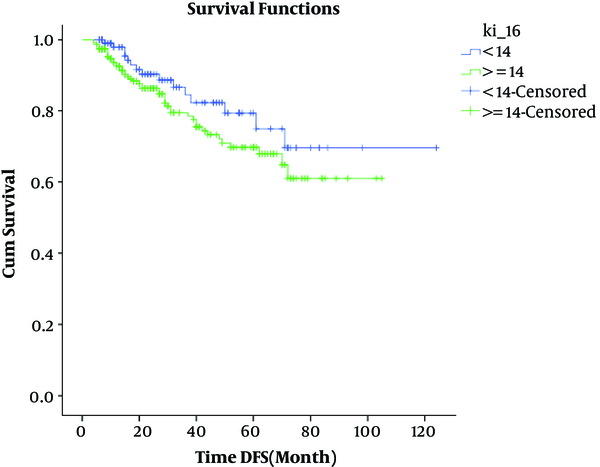
4. Discussion
The Ki-67 is a monoclonal anti-body and its expression is reported in proliferating cells during the active phase of the cell cycle (G1, S, and G2). Several studies have suggested its role as a prognostic biomarker (17). It has been shown that Ki-67 might be related with axillary lymph node status in primary breast tumors. It is reported to be associated with progesterone and estrogen receptor status (18, 19). The current study showed a significant difference between PR with Ki-67 expression. Also, the findings showed an association between Her2 and gene expression of Ki-67, which is in line the data by Suthipintawong et al. (20).
Nishimura et al. (21) studied the Ki-67 as a prognostic marker according to the breast cancer subtype and a predictor of recurrence time in primary breast cancer. They concluded that it is important to consider the Ki-67 in the treatment and follow-up of patients with breast cancer. In their study, the relation of invasive lobular carcinoma was higher than other types of carcinoma types. Also, the follow-up of patients by Kaplan-Mayer assessed the studied variables on survival analysis. The results of this research showed a significant association for Ki-67, age, stage of tumor, and the Her2/neu status.
Inwald et al. (22) also reported the significant association of Ki-67 with the age of patients. Correspondingly, Aysegul investigated the tumor proliferative activity determined by Ki-67 as an independent prognostic parameter, which reflects histopathologic features (23).
Similar to the findings of this study, Campani et al. (24) and Marchetti et al. (25) showed that progesterone receptor was inversely associated with the proliferating activity of Ki-67. Correspondingly, they showed that androgen receptor, progesterone receptor expression negatively correlates with Ki-67 expression.
We showed that there was a significant association between HER2, subtype pathologic, and Ki-67. Tamaki et al. (26) analyzed clinically relevant values of Ki-67 labeling index in Japanese patients with breast cancer. Correspondingly, they showed significant positive correlation between Ki-67 labeling index and HER2 status in their study. They suggested optimal cutoff point of Ki-67 as a labeling index.
4.1. Conclusions
In conclusion, we evaluated ALN involvement in breast cancer patients. We showed that high Ki-67 expression was associated with the age, large tumors, progesterone, and stage of tumor; however, it was related with lymph node involvement. The Ki-67 detection represents a valuable and prognostic tool that should be analyzed further in combination with other clinical, pathologic, and biologic parameters in breast cancer treatment.
Acknowledgements
References
-
1.
Siegel R, Jemal A. Cancer facts & figures. Atlanta: American Cancer Society, Inc; 2013.
-
2.
Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. Five common cancers in Iran. Arch Iran Med. 2010;13(2):143-6. [PubMed ID: 20187669].
-
3.
Sadjadi A, Nouraie M, Mohagheghi MA, Mousavi-Jarrahi A, Malekezadeh R, Parkin DM. Cancer occurrence in Iran in 2002, an international perspective. Asian Pac J Cancer Prev. 2005;6(3):359-63. [PubMed ID: 16236000].
-
4.
Harirchi I, Ebrahimi M, Zamani N, Jarvandi S, Montazeri A. Breast cancer in Iran: a review of 903 case records. Public Health. 2000;114(2):143-5. [PubMed ID: 10800155]. https://doi.org/10.1038/sj.ph.1900623.
-
5.
Harirchi I, Karbakhsh M, Kashefi A, Momtahen AJ. Breast cancer in Iran: results of a multi-center study. Asian Pac J Cancer Prev. 2004;5(1):24-7. [PubMed ID: 15075000].
-
6.
Rezaianzadeh A, Peacock J, Reidpath D, Talei A, Hosseini SV, Mehrabani D. Survival analysis of 1148 women diagnosed with breast cancer in Southern Iran. BMC Cancer. 2009;9:168. [PubMed ID: 19497131]. [PubMed Central ID: PMC2699348]. https://doi.org/10.1186/1471-2407-9-168.
-
7.
Mousavi SM, Montazeri A, Mohagheghi MA, Jarrahi AM, Harirchi I, Najafi M, et al. Breast cancer in Iran: an epidemiological review. breast j. 2007;13(4):383-91.
-
8.
Cole KD, He HJ, Wang L. Breast cancer biomarker measurements and standards. Clin Appl. 2013;7(1-2):17-29.
-
9.
Apostolou G, Apostolou N, Biteli M, Kavantzas N, Patsouris E, Athanassiadou P. Utility of Ki-67, p53, Bcl-2, and Cox-2 biomarkers for low-grade endometrial cancer and disordered proliferative/benign hyperplastic endometrium by imprint cytology. Diagn Cytopathol. 2014;42(2):134-42. https://doi.org/10.1002/dc.23010.
-
10.
Apostolou G, Apostolou N, Nikolaidou C, Kavantzas N, Patsouris E, Athanassiadou P. Cytodiagnosis of endometrial carcinoma and hyperplasia on imprint smears with additional immunocytochemistry using Ki-67 and p53 biomarkers. Cytopathol. 2014;25(2):86-94. https://doi.org/10.1111/cyt.12095.
-
11.
Luukkaa H, Klemi P, Leivo I, Vahlberg T, Grenman R. Prognostic significance of Ki-67 and p53 as tumor markers in salivary gland malignancies in Finland: an evaluation of 212 cases. Acta Oncol. 2006;45(6):669-75. [PubMed ID: 16938809]. https://doi.org/10.1080/02841860500543208.
-
12.
Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311-22. [PubMed ID: 10653597]. https://doi.org/10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9.
-
13.
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133(4):1710-5. [PubMed ID: 6206131].
-
14.
Stuart-Harris R, Caldas C, Pinder SE, Pharoah P. Proliferation markers and survival in early breast cancer: A systematic review and meta-analysis of 85 studies in 32,825 patients. Breast. 2008;17(4):323-34. https://doi.org/10.1016/j.breast.2008.02.002.
-
15.
Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23(28):7212-20. [PubMed ID: 16192605]. https://doi.org/10.1200/JCO.2005.07.501.
-
16.
Koda M, Jarzabek K, Kanczugakoda L, Przystupa W, Tomaszewski J, Sulkowska M, et al. [Comparative studies of K1-67 expression between the primary tumor and breast cancer metastases to regional lymph nodes]. Ginekol Pol. 2003;74(9):754-60. Polish. [PubMed ID: 14674120].
-
17.
Wintzer HO, Zipfel I, Schulte‐Monting J, Hellerich U, von Kleist S. Ki‐67 immunostaining in human breast tumors and its relationship to prognosis. Cancer. 1991;67(2):421-8.
-
18.
Buxant F, Anaf V, Simon P, Fayt I, Noel JC. Ki-67 immunostaining activity is higher in positive axillary lymph nodes than in the primary breast tumor. Breast Cancer Res Treat. 2002;75(1):1-3. [PubMed ID: 12500929].
-
19.
Koda M, Sulkowski S, Kanczuga-Koda L, Surmacz E, Sulkowska M. Expression of ERα, ERβ and Ki-67 in primary tumors and lymph node metastases in breast cancer. Oncol Rep. 2004. https://doi.org/10.3892/or.11.4.753.
-
20.
Suthipintawong C, Wejaranayang C, Vipupinyo C. Prognostic significance of ER, PR, Ki67, c-erbB-2, and p53 in endometrial carcinoma. J Med Assoc Thai. 2008;91(12):1779-84. [PubMed ID: 19133508].
-
21.
Nishimura R, Osako T, Okumura Y, Hayashi M, Toyozumi Y, Arima N. Ki-67 as a prognostic marker according to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp Ther Med. 2010;1(5):747-54. https://doi.org/10.3892/etm.2010.133.
-
22.
Inwald EC, Klinkhammer-Schalke M, Hofstadter F, Zeman F, Koller M, Gerstenhauer M, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139(2):539-52. [PubMed ID: 23674192]. [PubMed Central ID: PMC3669503]. https://doi.org/10.1007/s10549-013-2560-8.
-
23.
Sahin AA, Ro JY, El‐Naggar AK, Ordonez NG, Ayala AG, Ro J, et al. Ki‐67 immunostaining in node‐negative stage I/II breast carcinoma. Significant correlation with prognosis. Cancer. 1991;68(3):549-57.
-
24.
Campani D, De Negri F, Fabbri R, Martini L, Giani C, Squartini F, et al. Estrogen, progesterone receptors and proliferating activity evaluated by immunocytochemistry in breast cancer. Int J Biol Markers. 1991;6(3):144-50. [PubMed ID: 1791308].
-
25.
Marchetti E, Querzoli P, Marzola A, Bagni A, Ferretti S, Fabris G, et al. Assessment of proliferative rate of breast cancer by Ki-67 monoclonal antibody. Mod Pathol. 1990;3(1):31-5. [PubMed ID: 2308919].
-
26.
Tamaki K, Ishida T, Tamaki N, Kamada Y, Uehara K, Miyashita M, et al. Analysis of clinically relevant values of Ki-67 labeling index in Japanese breast cancer patients. Breast Cancer. 2014;21(3):325-33. [PubMed ID: 22782361]. https://doi.org/10.1007/s12282-012-0387-5.