Abstract
Introduction:
We present a rare case of breast papillary carcinoma associated with intracystic component in a woman with a long history of autoimmune hypothyroidism and multiple sclerosis.Case Presentation:
The patient was a 59-year-old woman presented with complaints of pain and swelling in her right breast. She was a known case of autoimmune hypothyroidism since 20 years and secondary progressive multiple sclerosis since 13 years ago. She had received frequent interferon, corticosteroid and cytotoxic therapy for her multiple sclerosis. On imaging studies, there were multiple large cystic-solid lesions in the right breast and with presumptive diagnosis of infectious collection or phyllodes tumor, she finally underwent simple mastectomy. On gross pathological evaluation, a large multi-loculated cyst filled with blood clots and necrotic debris was identified. In serial sections, a 7 cm solid tumoral mass with papillary projections protruding into the cyst cavity was seen. Although the gross appearance of the lesion resembled an angiosarcoma accompanied by cystic hemorrhagic necrosis, after the microscopic evaluation of the specimen, papillary breast carcinoma associated with intracyctic component was finally diagnosed.Conclusions:
The aim of this study was to describe a rare variant of invasive breast cancer presenting with a large solid-cyctic mass in a woman with long standing autoimmune disorders.Keywords
1. Introduction
Papillary carcinoma of the breast is a very rare type of invasive ductal breast cancers with prevalence of approximately 0.5% (1, 2). The target group is postmenopausal females and males are rarely affected (3). Because of the central location of this tumor, symptoms usually include nipple discharge or bleeding as well as palpable mass (4). This type of carcinoma is slow growing and the prognosis is better than other types (2). In addition to well known risk factors for breast cancer including prior history of cancer, atypical hyperplasia, genetic predisposition, hormone use, alcohol consumption, obesity and nulliparity (5). A relationship between the effects of immune system, autoimmune and chronic inflammatory disease and malignancies, like breast cancer is suggested in some recent studies (6). Some studies show a relationship between hypothyroidism including autoimmune types with increased risk of breast cancer (7, 8). There are also some researches that indicate an increased risk of cancers such as breast cancer in patients with multiple sclerosis (9, 10).
We present a case of papillary breast cancer with multiple and uncommon risk factors which have recently come to attention.
2. Case Presentation
A 59-year-old married woman, a known case of autoimmune hypothyroidism since 20 years ago and secondary progressive multiple sclerosis since 13 years ago was admitted to our hospital because of a palpable mass in her right breast and asymmetry between the size of right and left breasts. One and half years earlier, she had noticed a breast mass but because of severe disability, mammogram procedure was impossible and she did not follow the problem. From three months before the recent admission, the mass became larger and the affected breast increased 2 - 3 times of its normal size. She had no complaint of nipple discharge, bleeding, nor alteration in overlying skin. In addition to the mentioned disease, in the past medical history she reported a uterine leiomyoma that had been surgically removed during her only pregnancy. She had received frequent interferon beta-1b injections and corticosteroid pulses and four episodes of mitoxantrone injections but since 6 years ago Gabapentin and Neuroaid (a traditional Chinese medicine) were the only medications she was using for her multiple sclerosis. For the hypothyroidism, she was taking levothyroxine daily. Family history revealed multiple sclerosis in her brother.
On physical examination, she was conscious and oriented, and her vital signs were in normal ranges. The scar of prior surgery was evident on the abdominal skin. A large cystic, well-circumscribed mass in the right breast with no nipple retraction or discharge was observed. No palpable axillary lymph nodes were detected. Neurologic assessment revealed markedly decreased muscle force of four extremities. No other abnormalities were evident in the examination.
In laboratory studies, blood cell counts and thyroid function tests were normal as a result of regular consumption of levothyroxin.
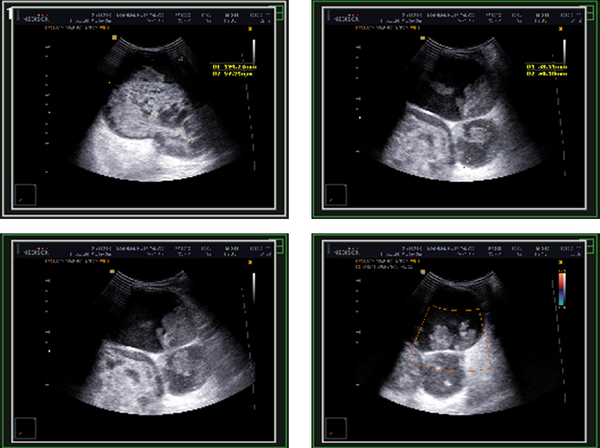
Ultrasound study revealed three cystic-solid lesions measuring 124 × 97 mm, 53 × 50 mm and 95 × 85 mm with vascular flow and thick walls interspersing them within the right breast (Figure 1). Additionally, a solid mass located superolateral to the abovementioned lesions had been reported which was 70 × 60 mm in size. The patient underwent simple mastectomy and the specimen was sent to our laboratory for pathologic evaluation. Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Ultrasonography of the Patient’s Right Breast Showing Cystic-Solid Lesions with Thick Walls Interspersing Them
3. Pathological Findings
3.1. Macroscopy
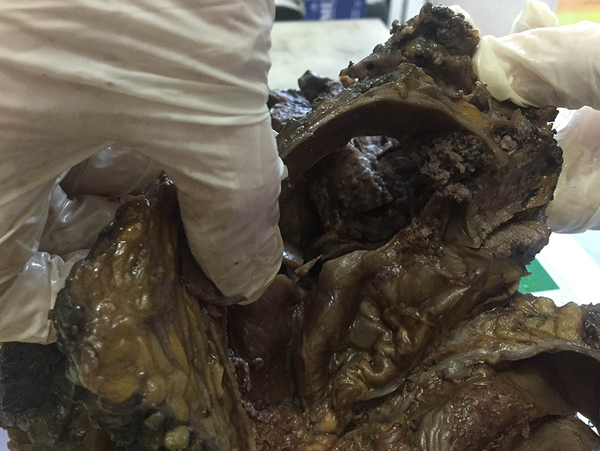
The specimen consisted of a breast tissue with 33 × 16 × 13 cm dimensions which was partially covered with skin and nipple with 2 cm diameter was observed on the skin. There was a cystic lesion in posterior side of the specimen which was filled with blood and resulted in two projections on lateral parts of the overlying skin. Muscular tissue with 5.5 × 5 × 0.5 cm dimensions was attached to the superolateral aspect of the posterior side. In serial sections, a multi-loculated cyst (14 × 12 × 10 cm) which was filled with blood clots and necrotic debris was identified. A solid and fragile tumoral mass (7 × 5 × 3 cm) with papillary projections protruding into the cyst cavity was seen on superior side of the cyst wall (Figure 2). Papillary projections were also identified in other parts of the cyst cavity. Non tumoral tissues were full of tan nodular lesions with 2 to 5 cm diameter and 4 lymph nodes were dissected from the lateral part of the specimen. Gross appearance of the lesion resembled an angiosarcoma which forms a friable, firm or spongy mass accompanied by cystic hemorrhagic necrosis. But after the microscopic evaluation of the specimen this differential diagnosis was excluded.
Gross Appearance of the Case Specimen Showing Cystic Spaces Associated with Solid Component and Papillary Projections
3.2. Microscopy
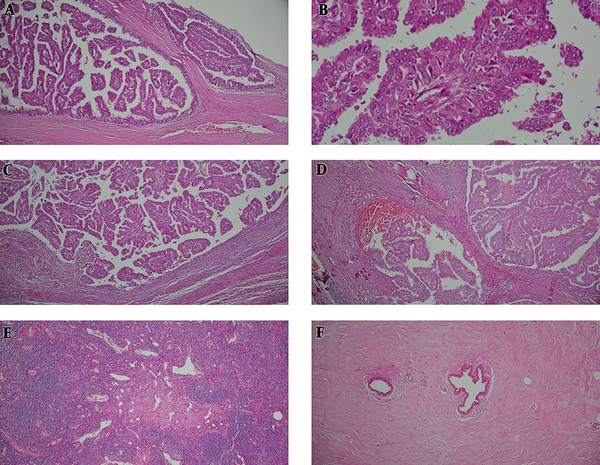
Histological examination revealed multi-loculated cystic spaces, some surrounded by fibrous wall with projection of arborizing papillary structures lined by malignant ductal epithelial cells devoid of a myoepithelial layer differentiation. Multifocal invasive components were also noted with extension beyond the fibrous wall and accompanied by stromal reaction. None of the tumoral breast tissue revealed complete disappearance of the acinar tissue. The only remaining epithelial elements consisted of the ductules which were surrounded by dense bundles of collagen. Four lymph nodes dissected from tumor periphery were tumor free but showed evidence of sinus vascular transformation (Figure 3).
Microscopic Examination, Hematoxylin and Eosin Staining
3.3. Further Treatment
After surgery, the patient was referred to the clinical oncologist and with a multidisciplinary approach, adjuvant chemotherapy and radiotherapy were planned.
4. Discussion
Papillary breast cancer is one of the rare subtypes of breast carcinomas which is often seen in postmenopausal women (1). The symptoms usually include bloody nipple discharge according to the most common site of the tumor which is central (3, 4). There are some microscopic variants including encysted type, encapsulated type, dimorphic type and solid type. On mammogram, papillary tumor is a multinodular lesion with increased density and either well defined or ill-defined borders. Sonography of invasive papillary carcinomas reveals well defined, solid or mixed solid and cystic, inhomogeneous and hypo-echogenic mass with posterior enhancement (4). The prognosis of this type of cancers is generally better than other types of ductal carcinomas because of the slow growing nature of the tumor (2). Due to the rarity of papillary breast cancer, it does not allow large or randomized studies to define an optimal approach (11). Reporting this case was interesting because of the rarity of these kinds of breast tumors and coexistences of multiple risk factors she has had. She was a menopaused woman with the chief complaint of breast mass and enlargement. She had no history of nipple discharge maybe because of the mildly eccentric location of the tumor. Appearance of solid cystic mass was reported in her sonography as a result of encysted nature of the tumor. Two differential diagnosis were suggested by the radiologist: infectious collections or phyllodes tumor which are acceptable with these radiologic findings (12). For a long time, encysted papillary breast carcinomas were classified as a variant of ductal carcinoma in situ but recent studies are debating the possibility of an invasive component (13, 14). Some studies have demonstrated the lack of myoepithelial cell markers at the periphery and also existence of tumor foci beyond the fibrous capsule of the cysts as evidences of invasion (14, 15). Even metastatic disease can occur and the most common site is lymph node (16, 17). This case was another evidence for confirmation of the theory that invasive component can exist in encysted papillary tumors. Another interesting point about this case is her past medical history which may have played a role in her newly diagnosed disease. As mentioned before, there is an increased risk of developing cancer in patients with autoimmune diseases. Some theories are suggested to explain this: immune mediators like cytokines, chemokines and free radicals in autoimmune conditions may cause tissue damage, chronic inflammation and increase risk of cancer consequently. On the other hand, ongoing stimulation and rapid proliferation of the immune cells may lead to malignant lymphoproliferation. Other factors, such as genetic mutations, environmental agents and immunomodulatory treatments, can also play roles (6). There are few authors who have directly indicated the increased risk of breast cancer in patients with autoimmune hypothyroidism and multiple sclerosis (18, 19). Interestingly this case can be another example to study more about these associations. Another point about this case is the history of Mitoxantrone injections which is a synthetic antineoplastic cytotoxic drug. The known side effects of this drug include increased risk of leukemia and colorectal cancers (20). Due to the immune system suppression mechanism of this agent, an association between this drug and increased risk of other types of cancers such as breast cancer is not unlikely. Further investigations may be needed to confirm these kinds of relationships.
There were two pathologic findings other than the main tumoral lesion in this case: one of them was the lesion called fibrous mastopathy, which is an uncommon form of breast stromal fibrosis and lymphocytic mastitis and typically occur in patients with long standing type-1 diabetes (21, 22). But it has been reported in non-diabetic patients, patients with autoimmune disease or even healthy people (21). Some authors suggest an autoimmune pathogenesis for these lesions in which a predominant B-cells and expressions of HLA-DR (Human Leukocyte Antigen - antigen D Related) involve the lobular epithelium (23). Additionally, recent studies support the theory of the important role of B-cells in addition to T-cells in pathogenesis of multiple sclerosis and autoimmune thyroid disease (24, 25). These three autoimmune based conditions have B-cell mediating pathogenesis in common. Investigations about the association of fibrous mastopathy with autoimmune diseases other than type-1 diabetes are limited and this patient can be an interesting case to study about these uncommon relationships. Another pathologic finding in lymph node microscopic examination was vascular transformation of sinuses in which the lymph node sinuses change into a complex network of anastomosing endothelial lined channels. This condition has been reported in retroperitoneal lymph nodes draining renal cell carcinomas and the secretion of angiogenic factor by the carcinoma cells is suggested as a result. Another etiology is also proposed and that is the proximal obstruction of the efferent vessels of the sinuses. Similarly, in this case the pressure of the blood filled cystic lesion on the lymph node was the probable cause of obstruction in the efferent vessels and resulted in the vascular transformation of the lymph node sinuses (26).
Acknowledgements
References
-
1.
Pal SK, Lau SK, Kruper L, Nwoye U, Garberoglio C, Gupta RK, et al. Papillary carcinoma of the breast: an overview. Breast Cancer Res Treat. 2010;122(3):637-45. [PubMed ID: 20524058]. https://doi.org/10.1007/s10549-010-0961-5.
-
2.
Soo MS, Williford ME, Walsh R, Bentley RC, Kornguth PJ. Papillary carcinoma of the breast: imaging findings. AJR Am J Roentgenol. 1995;164(2):321-6. [PubMed ID: 7839962]. https://doi.org/10.2214/ajr.164.2.7839962.
-
3.
Bhosale SJ, Kshirsagar AY, Sulhyan SR, Jagtap SV, Nikam YP. Invasive Papillary Breast Carcinoma. Case Rep Oncol. 2010;3(3):410-5. [PubMed ID: 21113352]. https://doi.org/10.1159/000321270.
-
4.
Koerner FC. Rosen's breast pathology. In: Hoda SA, Brogi E, Koerner FC, Rosen PP, editors. Papillary carcinoma. 4th ed. Philadelphia: Wolters Kluwer; 2014. p. 489-521.
-
5.
Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003;237(4):474-82. [PubMed ID: 12677142]. https://doi.org/10.1097/01.SLA.0000059969.64262.87.
-
6.
Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res. 2012;32(4):1119-36. [PubMed ID: 22493341].
-
7.
Kuijpens JL, Nyklictek I, Louwman MW, Weetman TA, Pop VJ, Coebergh JW. Hypothyroidism might be related to breast cancer in post-menopausal women. Thyroid. 2005;15(11):1253-9. [PubMed ID: 16356089]. https://doi.org/10.1089/thy.2005.15.1253.
-
8.
Itoh K, Maruchi N. Breast cancer in patients with Hashimoto's thyroiditis. Lancet. 1975;306(7945):1119-21.
-
9.
Nielsen NM, Rostgaard K, Rasmussen S, Koch-Henriksen N, Storm HH, Melbye M, et al. Cancer risk among patients with multiple sclerosis: a population-based register study. Int J Cancer. 2006;118(4):979-84. [PubMed ID: 16152598]. https://doi.org/10.1002/ijc.21437.
-
10.
Laplant J, Knobler R. Increased incidence of breast cancer in multiple sclerosis: The influence of vitamin d and estrogen in ms-breast cancer concordance (P6. 176). Neurology. 2014;82(10):P6-176.
-
11.
Yerushalmi R, Hayes MM, Gelmon KA. Breast carcinoma--rare types: review of the literature. Ann Oncol. 2009;20(11):1763-70. [PubMed ID: 19602565]. https://doi.org/10.1093/annonc/mdp245.
-
12.
Athanasiou A, Aubert E, Vincent Salomon A, Tardivon A. Complex cystic breast masses in ultrasound examination. Diagn Interv Imaging. 2014;95(2):169-79. [PubMed ID: 24433921]. https://doi.org/10.1016/j.diii.2013.12.008.
-
13.
Grabowski J, Salzstein SL, Sadler GR, Blair S. Intracystic papillary carcinoma: a review of 917 cases. Cancer. 2008;113(5):916-20. [PubMed ID: 18661510]. https://doi.org/10.1002/cncr.23723.
-
14.
Esposito NN, Dabbs DJ, Bhargava R. Are encapsulated papillary carcinomas of the breast in situ or invasive? A basement membrane study of 27 cases. Am J Clin Pathol. 2009;131(2):228-42. [PubMed ID: 19141383]. https://doi.org/10.1309/AJCP8A2UVLCYGTPU.
-
15.
Cadoo KA, McArdle O, O'Shea AM, Power CP, Hennessy BT. Management of unusual histological types of breast cancer. Oncologist. 2012;17(9):1135-45. [PubMed ID: 22826373]. https://doi.org/10.1634/theoncologist.2012-0134.
-
16.
Mogal H, Brown DR, Isom S, Griffith K, Howard-McNatt M. Intracystic papillary carcinoma of the breast: A SEER database analysis of implications for therapy. Breast. 2016;27:87-92. [PubMed ID: 27054753]. https://doi.org/10.1016/j.breast.2016.01.003.
-
17.
Mulligan AM, O'Malley FP. Metastatic potential of encapsulated (intracystic) papillary carcinoma of the breast: a report of 2 cases with axillary lymph node micrometastases. Int J Surg Pathol. 2007;15(2):143-7. [PubMed ID: 17478767]. https://doi.org/10.1177/1066896906299119.
-
18.
Smyth PP. The thyroid and breast cancer: a significant association? Ann Med. 1997;29(3):189-91. [PubMed ID: 9240623].
-
19.
Turken O, NarIn Y, DemIrbas S, Onde ME, Sayan O, KandemIr EG, et al. Breast cancer in association with thyroid disorders. Breast Cancer Res. 2003;5(5):R110-3. [PubMed ID: 12927040]. https://doi.org/10.1186/bcr609.
-
20.
Buttmann M, Seuffert L, Mader U, Toyka KV. Malignancies after mitoxantrone for multiple sclerosis: A retrospective cohort study. Neurology. 2016;86(23):2203-7. [PubMed ID: 27170571]. https://doi.org/10.1212/WNL.0000000000002745.
-
21.
Pereira MA, de Magalhaes AV, da Motta LD, Santos AM, Segura ME, Pereira CF, et al. Fibrous mastopathy: clinical, imaging, and histopathologic findings of 31 cases. J Obstet Gynaecol Res. 2010;36(2):326-35. [PubMed ID: 20492384]. https://doi.org/10.1111/j.1447-0756.2009.01146.x.
-
22.
Ely KA, Tse G, Simpson JF, Clarfeld R, Page DL. Diabetic mastopathy. A clinicopathologic review. Am J Clin Pathol. 2000;113(4):541-5. [PubMed ID: 10761456]. https://doi.org/10.1309/K5HM-9603-PQ5T-KY3F.
-
23.
Schwartz IS, Strauchen JA. Lymphocytic mastopathy. An autoimmune disease of the breast? Am J Clin Pathol. 1990;93(6):725-30. [PubMed ID: 1693260].
-
24.
Boyle EA, McGeer PL. Cellular immune response in multiple sclerosis plaques. Am J Pathol. 1990;137(3):575-84. [PubMed ID: 1698025].
-
25.
Weetman A, DeGroot LJ, et al. Autoimmunity to the Thyroid Gland. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, editors. Endotext. South Dartmouth (MA); 2000. [PubMed ID: 25905407].
-
26.
Steinmann G, Foldi E, Foldi M, Racz P, Lennert K. Morphologic findings in lymph nodes after occlusion of their efferent lymphatic vessels and veins. Lab Invest. 1982;47(1):43-50. [PubMed ID: 7087397].