Abstract
Introduction:
Pituitary adenomas are categorized into two groups: the secretory and non-secretory adenomas. The first group overproduce normal pituitary hormones, which cause Cushing’s disease (high ACTH- adernocorotitrophic hormone), Acromegaly (high growth hormon) and prolactinomas (high prolactin). They may diffuse into the sphenoid sinus, cavernous sinus and diaphragma sellae. Tumor invasion into the adjacent structures and incomplete tumor resection are the most common causes of hypercortisolism after the first surgery. The first explored radiation modality for persistent Cushing’s disease was fractionated radiation, with increased rates of hypopituitarism. Over time, several studies have demonstrated the power of the GKS (gamma knife surgery) for persistent Cushing’s disease.Case Presentation:
A 32-year-old female who was diagnosed with Cushing’s disease secondary to an ACTH secreting pituitary adenoma, underwent trans-sphenoidal resection of the adenoma. Surprisingly, no postoperative hormonal and clinical improvements were observed. The patient was treated with oral ketoconazole. Twenty months after surgery, she had complaints of sudden onset of headache with diplopia, ptosis and paralysis of extraocular muscles of the left eye. A repeated MRI revealed residual tumor with local invasion to the left cavernous sinus. Following the consultation with a neurosurgeon, the patient underwent GKS. Within two months, there was a significant improvement in peribulbar muscle paresis and laboratory results were satisfying.Conclusions:
The case presented here indicates that the most common indicators for radiosurgery are residual tumors, especially in the cavernous sinus, which are recurrent and/or resistant to medical treatment. The purpose is to call attention to the GKS as a successful adjunctive therapy in Cushing’s disease.Keywords
1. Introduction
Pituitary tumors are benign tumors, which cause 20% of all primary brain tumors. Pituitary adenomas are categorized into two groups: the secretory and non-secretory adenomas. The first group overproduce normal pituitary hormones, which cause Cushing’s disease (high adernocorotitrophic hormone), Acromegaly (high growth hormone) and prolactinomas (high prolactin). Cushing’s disease is an endocrine disorder, defined by excessive levels of cortisol, resulting from overproduction of adrenocorticotropic hormone by a pituitary adenoma. In order to normalize the hazardous effects of hypercortisolism, close cooperation between endocrinologists, neurosurgeons and radiation oncologists is required. Expansion of pituitary adenomas causes compression of the adjacent structures and sella turcica enlargement. They may diffuse into the sphenoid sinus, cavernous sinus and diaphragma sellae. Cavernous sinus invasion occurs in 6 to 10% of all pituitary adenomas. Treatment of secretory pituitary adenomas is based on surgery, fractionated radiotherapy and medication. TSS (Trans-spenoidal surgery) has remained the first choice of treatment with a 77 to 85% success rate. Tumor invasion into the adjacent structures and incomplete tumor resection are the most common causes of hypercortisolism after the first surgery. The first explored radiation treatment modality for persistent Cushing’s disease was fractionated radiation, with reported remission rates ranging from 56 to 83%. However, it is associated with increased rates of hypopituitarism (50-100%). Over time, recurrent or refractory Cushing’s disease has been treated by stereotactic radiosurgery. From 1991 to 2007, several published studies have demonstrated the power of the GKS (gamma knife surgery) for persistent Cushing’s disease (1-5).
2. Case Report
A 32-year-old female was referred to our specialized center with a recent history of depression weight gain, oligomenorrhea and hirsutism in 2009. She had noticed purple striae over the breasts, abdomen and thighs. She had two children. Past medical history and drug history were unremarkable. On examination, the patient had central obesity with moon face, pigmentation and atrophy of the skin. Neurological exam, including her visual field was intact. Psychiatric visit was arranged and 20 mg citalopram and 50 mg sertraline was prescribed. Her blood pressure, body mass index, body temperature and pulse rate were 150/100 mmHg, 30 kg/m2, 37.2 0c and 120/min respectively. Urinary 24-hour cortisol concentration was measured 105 µg/day (normal: 20 - 90). Plasma ACTH and cortisol (8 am) levels were 76 pg/mL (normal: 10 - 60 pg/mL) and 24 µg/dL (normal: 5 - 23 µg/dL) respectively. A High dose dexamethasone test (dexamethasone 2 mg orally every six hours for 48 hours) was able to suppress the cortisol levels although no inhibitory effect was observed with low-dose dexamethasone. The patient’s MRI (magnetic resonance imaging) at the time of admission showed a thin hyposignal linear area at the central aspect of pituitary gland, which was suggestive of adenoma. Lesion’s size was measured 10*12 mm. Parasellar spaces were normal (Figure 1).
Coronal T1 MR Image after Contrast Enhancement
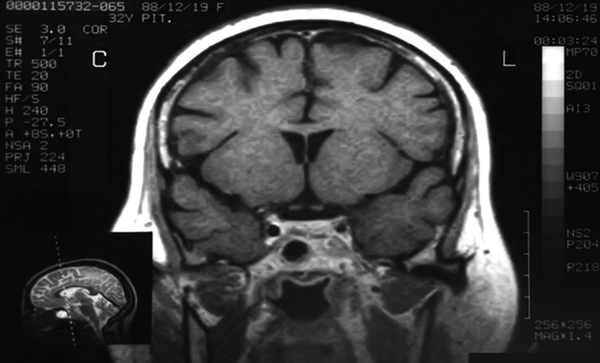
Initial diagnosis of Cushing’s disease secondary to an ACTH (adernocorotitrophic hormone) secreting pituitary adenoma was made and the patient underwent trans-sphenoidal resection of the pituitary adenoma. The definite postoperative histopathological diagnosis of the removed tumor was pituitary adenoma. Surprisingly, there was no postoperative decrease of ACTH and cortisol levels. Serum ACTH and urinary free cortisol levels were elevated to 117 pg/mL and 111 µg/d respectively. Serum cortisol (after midnight dexamethasone suppression test) level was measured 24 µg/dL (Table 1).
Laboratory results before and after TSS
| Parameter | Preop | 4-Month Post-Op |
|---|---|---|
| Urinary free cortisol, μg/24h | 105 | 111 |
| Serum cortisol after 1 mg dexamethasone, µg/dL | 28.5 | 21 |
| ACTH, pg/mL | 76 | 117 |
| Serum cortisol 8 am, µg/dL | 24 | 24 |
Following surgery signs and symptoms of hypercortisolism, i.e. weight gain with central fat distribution, hypertension, and hypokalemia persisted.
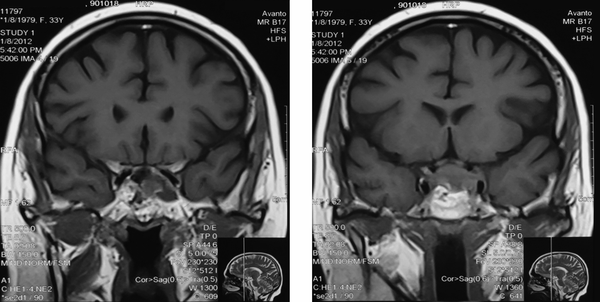
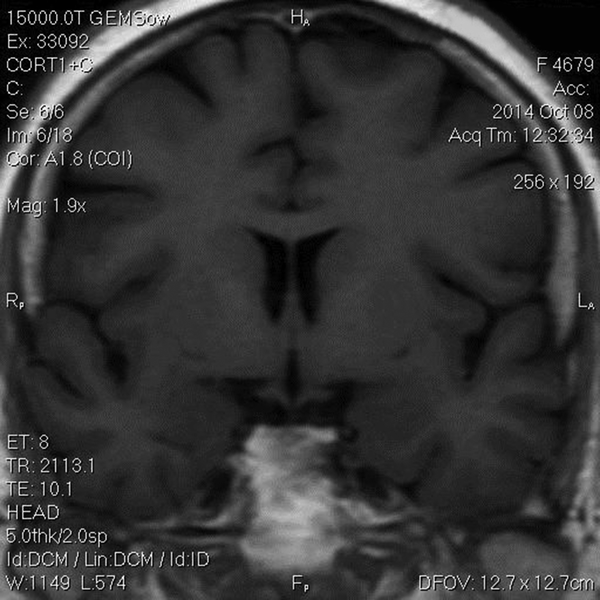
The Patient was treated with oral ketoconazole with modest effects. She also developed diabetes mellitus and treatment with insulin was initiated. Twenty months after trans-sphenoidal resection, she had complaints of sudden onset of headache with diplopia, ptosis and paralysis of extraocular muscles of the left eye. A repeated MRI revealed residual tumor (22*40 mm) with local invasion to the left cavernous sinus (Figure 2). The diagnosis of cavernous sinus invasion explained the persistently high cortisol levels. The adenoma causative for Cushing’s disease had not yet been removed completely. Urinary 24-hour cortisol level was raised at 201 µg/day. Following the consultation with a neurosurgeon and discussing the treatment options, the patient underwent GKS. Radiation treatment was completed and within two months, there was a significant improvement in ptosis and peribulbar muscle paresis (Figure 3). Short-term supplemental glucocorticoid therapy was prescribed for transient hypocortisolism, which was tapered off in 4 months. Following 6 months after surgery, she was weaned off from her psychotropic and antihypertensive medications, and her mental state and blood pressure were normal. Laboratory results post-GKS are summarized in Table 2.
Coronal T1 MR Image after Contrast Enhancement Showing Sinus Cavernous Invasion
Laboratory Results
| Parameter | 2-Mon Post-GK | 6-Mon Post-GK | 12-Mon Post-GK |
|---|---|---|---|
| ACTH, pg/mL | 47 | 52 | NL |
| Serum cortisol 8 am, µg/dL | 3.2 | 6 | 7.4 |
In the last follow-up, four years after GKS, she didn't take any medication and had normal pituitary function (Table 3).
Four years after GKS
| Parameter | Result |
|---|---|
| UFC (µg/day) | 11.9 |
| Cortisol 8 am (µg/dl) | 20.8 |
| ACTH (pg/ml) | 59 |
| LH | 2.43 |
| FSH | 5.08 |
| FT4 | 0.92 |
Coronal and Sagittal T1 Sections MR Post-GKS
3. Discussion
Since Cushing’s disease first defined by Harvey Cushing in 1912, it has been known as a main clinical dilemma to physicians. There are various treatment modalities to control hypercortisolism after failed pituitary surgery, including repeat pituitary surgery, bilateral adrenalectomy and radiotherapy, which have different response rates and complications (2). Occasionally, some secretory adenomas either cause compression of the optic pathway or invasion of the cavernous sinus. The most common indicators for radiosurgery are residual tumors especially in the cavernous sinus, which are recurrent and/or resistant to medical treatment (1). Joaquim et al. reported a high prevalence of invasion in macroadenomas (83.5%). Vieira et al. also mentioned that the grade of parasellar expansion was directly associated with the tumor’s size (6).
In 1968, the first patient with pituitary adenoma underwent the GKS by Leksell. Thereafter, stereotactic radiosurgery has been known as a primary modality in the neurosurgical treatment of secretory pituitary microadenomas or as a supplementary treatment option for secretory macroadenomas. GKS can facilitate adenoma growth control and long-term hormonal control. Furthermore, GKS does not affect the adjacent normal brain structures (1). In a study conducted by Jagannathan et al. 90 patients underwent GKS for Cushing’s disease. They demonstrated a 54% cure rate with a mean time to remission of 13 months, with a 22% rate of hypopituitarism, and a 5% rate of cranial nerve neuropathies (5).
Hayashi et al. reported tumor growth control rate between 93 and 94%, for pituitary adenoma after GKS. Many studies have demonstrated tumor growth control rate greater than 95% (1).
A mean tumor control rate of 96% (with a mean follow-up of 4 years or more) was reported by Sheehan et al. in 1621 patients with secretory pituitary adenoma, who underwent radiosurgery (7).
Castro et al. in their experiment on 28 patients with functioning pituitary adenomas treated with radiosurgery, showed 67% hormonal control (8).
Elshirbiny et al. reported 60% clinical improvement, tumor size control, and hormonal control in their results of GKS on 24 patients. None of their cases showed panhypopituitarism during the follow-up period (1). In our study after 4 years follow up no laboratory findings of pituitary insufficiency were observed.
Authors of several studies mentioned higher hormonal control rate in GKS group than in the conventional one. In the fractionated radiotherapy group, the risk of radiation-induced complications was higher than in the GKS group.
One of the major advantages of stereotactic radiosurgery is the low incidence of pituitary insufficiency. TSS has been chosen as the primary management modality due to the rapid control of hormonal hypersecretion in contrast to the radiosurgery. Shrinkage of tumor usually requires a long period of time, making this modality inapplicable for those patients with optic nerve compression symptoms (3).
Radiotherapy influences tumors gradually, and success rates for hormonal control are very different. This approach is indicated if the pituitary adenoma shows oncogenic characteristics. The best targets for stereotaxic radio surgery are apparent small adenoma residuals, which show evidence of expansion at repeated MRI and suspicious invasion to the cavernous sinus (9).
Acknowledgements
References
-
1.
Elshirbiny MF, Hafez RA, Ali N, Ezzeldien AA, Kassem MA. Role of gamma knife radiosurgery in the management of functioning pituitary adenomas. Benha Med J. 2015;32(1):6. https://doi.org/10.4103/1110-208x.170551.
-
2.
Capaldo CT, Farkas AE, Nusrat A. Epithelial adhesive junctions. F1000Prime Rep. 2014;6. https://doi.org/10.12703/p6-1.
-
3.
Kim M, Paeng S, Pyo S, Jeong Y, Lee S, Jung Y. Gamma Knife surgery for invasive pituitary macroadenoma. J Neurosurg. 2006;105 Suppl:26-30. [PubMed ID: 18503326]. https://doi.org/10.3171/sup.2006.105.7.26.
-
4.
Hafez RFA, Morgan MS, Fahmy OM. Gamma knife surgery in management of secretory pituitary adenoma preliminary evaluation of role, efficacy and safety. Neurosci Discovery. 2014;2(1):4. https://doi.org/10.7243/2052-6946-2-4.
-
5.
Aghi MK, Petit J, Chapman P, Loeffler J, Klibanski A, Biller BM, et al. Management of recurrent and refractory Cushing's disease with reoperation and/or proton beam radiosurgery. Clin Neurosurg. 2008;55:141-4. [PubMed ID: 19248680].
-
6.
Vieira Jr JO, Cukiert A, Liberman B. Magnetic resonance imaging of cavernous sinus invasion by pituitary adenoma diagnostic criteria and surgical findings. Arquivos de Neuro-Psiquiatria. 2004;62(2B):437-43.
-
7.
Sheehan JP, Niranjan A, Sheehan JM, Jane JJ, Laws ER, Kondziolka D, et al. Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J Neurosurg. 2005;102(4):678-91. [PubMed ID: 15871511]. https://doi.org/10.3171/jns.2005.102.4.0678.
-
8.
Castro DG, Cecilio SA, Canteras MM. Radiosurgery for pituitary adenomas: evaluation of its efficacy and safety. Radiat Oncol. 2010;5:109. [PubMed ID: 21083925]. https://doi.org/10.1186/1748-717X-5-109.
-
9.
Bertagna X, Guignat L. Approach to the Cushing's disease patient with persistent/recurrent hypercortisolism after pituitary surgery. J Clin Endocrinol Metab. 2013;98(4):1307-18. [PubMed ID: 23564942]. https://doi.org/10.1210/jc.2012-3200.