Abstract
Background:
Cancer is one of the most significant causes of death. Plants with anti-cancer effects have shown to eliminate tumor cells by the induction of apoptosis.Objectives:
The present study aimed at investigating the apoptotic effects of 3 Euphorbia native species (E. microciadia Boiss, E. osyridea Boiss, and E. heteradenia Jaub. & Sp.).Methods:
Cell lines with the strongest sensitivity to the extracts including HeLa for E. microciadia, K562 for E. heteradenia, and Fen for E. osyridea were investigated for the effects of the plants’ hexane extracts on the induction of apoptosis, according to annexin V/propidium iodide staining by flow cytometry. We used real-time PCR to evaluate the changes in expressions of genes related to the intrinsic and extrinsic apoptosis pathways.Results:
Flow cytometry results indicated that 50 μg/mL of all the extracts induced apoptosis in more than 60% of treated cells. Administration of the extracts resulted in increased caspase-3 activity for all treated cell lines. Real-time PCR results showed decreased B-cell lymphoma-2 (Bcl-2) and increased Bax and Fas mRNA levels in cells treated with the extracts. The Bax/Bcl-2 ratio in cells treated with 50μg/mL of E. microciadia was 29.25 ± 12.0 (P < 0.05) and for 100 μg/mL of E. heteradenia. and E. osyridea was 30.2 ± 13.0 (P < 0.05) and 22.2 ± 2.4 (P < 0.001), respectively.Conclusions:
The studied plants have shown remarkable apoptosis-inducing effects on tumor cell lines by affecting both the extrinsic and intrinsic apoptosis pathways. Additional studies in terms of their beneficial effects as natural sources of potential anti-cancer agents will be necessary.Keywords
1. Background
Cancer, which is the result of uncontrollable proliferation of cells from body tissues and their spread throughout the body, is a primary threat to human health worldwide and is one of the most significant causes of death (1). Current cancer treatments include chemotherapy, radiotherapy, immunotherapy, or surgery. In chemotherapy, chemically-derived medicines are used that kill tumor cells by their effects on DNA and different proteins, but they may also harm normal cells, such as bone marrow precursor cells and epithelial cells of different organs. These chemotherapeutic agents also cause numerous side effects, such as bleeding, constipation, diarrhea, and infection. In addition, some cancers are resistant to such treatments (2, 3).
Researchers have sought to locate new medicines from various sources, one of which is medicinal plants. Various anti-cancer agents have been derived from plants. For example, astragalus and taxol are medicines used as cancer treatments that have plant origins (4). Currently, most individuals are aware of the importance of plants in the treatment of diseases due to their lesser side effects compared to chemically-derived medications. Numerous pharmacologists and researchers have attempted to identify the pharmacological substances in medicinal plants for cancer treatments (5). Researchers have investigated various plant extracts and isolated components for their cytotoxic potential on various leukemia or solid tumor-derived cell lines (6).
Euphorbia is the largest genus of the Euphorbiaceae plant family. There are over 1000 species distributed broadly in both temperate and tropical regions. In Iran, 17 out of 70 reported species are endemic, with traditional medical uses against skin infections, gonorrhea, migraines, intestinal parasites, and warts (7, 8). Several plants from this family have also been traditionally used as treatments for cancers and viral diseases (5). Some of these plants with anti-cancer effects have shown to eliminate tumor cells by the induction of apoptosis (9, 10).
Apoptosis, originally described as a mechanism of controlled cell death, is characterized by a variety of cellular changes that include loss of membrane phospholipid asymmetry, chromatin condensation, mitochondrial swelling, and DNA cleavage. The end result of these changes is a form of programmed cell death (11). In general, apoptosis induction occurs via 2 classic pathways, extrinsic, and intrinsic. The extrinsic pathway is initiated by the ligation of death receptors (i.e., Fas) in the cell membrane, which results in the conversion of procaspase-8 to its activated form, after which procaspase-3 is activated either directly or by cleavage of Bid (12). In the intrinsic pathway, the 2 main molecules involved in the regulation of apoptosis are Bax that have a pro-apoptotic effect and B-cell lymphoma-2 (Bcl-2), with anti-apoptotic activity (13). The activation of Bax results in the release of cytochrome c from the mitochondria followed by the activation of caspase-9 and the subsequent activation of caspase-3. Bcl-2 prevents this process. Caspase-3 is a key enzyme, which is activated during the cascade of events that leads to apoptosis. This cysteine protease cleaves a variety of cellular substrates with the amino acid motif DEVD, such as poly ADP-ribose polymerase (PARP) (14).
Various studies investigated the anti-tumor effects of Euphorbia species on different cell lines. For example, E. kansui Liou ex S. B. Ho Fl. has shown growth inhibitory effects accompanied by cell cycle changes in SGC-7901 human gastric cancer cell line (15). A number of isolated compounds from the methanolic extract of E. liagascae Spreng. had cytotoxic effects on a human cervical adenocarcinoma cells (16). Various other Ephorbia species that included E. hirta Linn, E. helioscopia L, E. lathyris L., and E. cheridenia Boiss showed growth inhibitory effects on different tumor cell lines (10, 17, 18).
In our previous study on the Euphorbia species, we reported that the hexane extract from 3 native Euphorbia species (E. microciadia Boiss, E. heteradenia Jaub. & Sp., and E. osyridea Boiss) had the strongest cytotoxic effects among different extracts of the plants on various cell lines (9). The cell lines with the strongest sensitivity to the hexane extracts included HeLa cervix epitheloid carcinoma cell line for E. microciadia, K562 myelogenous leukemia cell line for E. heteradenia, and Fen bladder carcinoma cell line for E. osyridea.
2. Objectives
The induction of apoptosis is an important mechanism for the eradication of cancer cells, therefore, in this study we have investigated whether the hexane extracts of E. microciadia Boiss, E. heteradenia Jaub. & Sp., and E. osyridea Boiss possess the ability to induce apoptosis in the selected cancer-derived cell lines. In addition, we have assessed the contributions of extrinsic and/or intrinsic apoptosis pathways by measuring the gene expressions of key molecules involved in these pathways.
3. Methods
3.1. Preparation of the Extracts
E. microciadia, E. heteradenia, and E. osyridea plants were collected in May from Fars Province, Iran and authenticated by Dr. Khosravi from the department of biology, Shiraz University, Shiraz, Iran. Voucher specimens were deposited in the Shiraz University Herbarium. Extraction was performed as previously described (9). Briefly, aerial parts of the plants were subjected to maceration in methanol at room temperature for 48 hours. The methanol extracts were filtered and concentrated under reduced pressure, and were subsequently suspended in 500 mL of water and re-extracted 3 times by hexane. The fractions were concentrated under reduced pressure, using a rotary evaporator and, then, freeze-dried. For the experiments, samples were dissolved in dimethyl sulfoxide (DMSO) and, then, diluted in RPMI 1640 culture medium (Sigma, St. Louis, MO). In all the experiments, DMSO, as the solvent, was added to the negative control at the highest concentration used in the test wells.
3.2. Cell lines and Cell Cultures
Cell lines included HeLa cervix epitheloid carcinoma, K562 myelogenous leukemia, and Fen bladder carcinoma were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS, Gibco-BRL, Germany) in culture flasks at 37°C in a 5% humidified CO2 incubator. Adherent cell lines (HeLa and Fen) were expanded by trypsinization and subcultured at lower numbers in new culture flasks. The viability of cells was determined by the trypan blue dye exclusion test and was routinely greater than 90% (19).
3.3. Flow Cytometry Analysis
Pre-determined concentrations of cell lines [15 × 103/100 μL/well (K562) and 7.5 × 103/100 μL/well (HeLa and Fen)] were added onto flat-bottomed 96-well culture plates in the presence of various concentrations of the extracts (in triplicate). The cells were incubated at 37°C in a 5% humidified CO2 incubator for 24 hours. The induction of apoptosis in tumor cell lines was quantified by flow cytometry, using FITC-conjugated annexin V/propidium iodide (PI) staining kit (Q product, Netherland) according to the manufacturer’s protocol. Specific binding of annexin V was achieved by incubating the cells with annexin V for 20 minutes at 4°C in the dark. To discriminate between apoptosis and necrosis, cells were simultaneously stained with annexin V and PI before analysis. The binding of annexin V-FITC and PI to the cells was measured by flow cytometry (FACSCalibur, Becton Dickinson (BD) Biosciences, San Jose, CA) and data were analyzed, using FlowJo7.6 software (Tree Star, Ashland, OR). As positive control, cells were treated with 50 µg/mL cisplatin and as negative control they were treated only with DMSO at the highest concentration used in test wells (e.g., 0.05%). At least, 10000 cells were counted in each sample. Experiments were performed and the total of early (annexin V + /PI-) and late (annexin V + /PI+) apoptotic cells was considered the percentage of apoptosis.
3.4. Caspase 3 Colorimetric Assay
The K562 cell line was seeded in 24-well plates and treated with 50 and 100 µg/mL of E. heteradenia. The HeLa and Fen adherent cells were seeded in 6-well plates and respectively treated with 10 and 50 µg/mL of E. microciadia extract and 50 and 100 µg/mL of E. osirydea extract for 24 hours. Cells treated with 50 µg/mL cisplatin were considered the positive control and cells treated only with DMSO (0.05%) were considered the negative control. The enzymatic activity of caspase 3 was determined by caspase colorimetric assay kit (R&D Systems Inc., Minneapolis, MN) according to the manufacturer’s instructions. In brief, cells were lysed in ice-cold lysis buffer for 10 minutes. After the removal of cellular debris by centrifugation, protein levels in the lysates (cytosolic extract) were measured by Bradford method (Bio-Rad, Hercules, CA) and equalized accordingly to obtain 2 mg/mL to 4 mg/mL of cytosolic extract per sample. Samples were incubated at 37°C for 2 hours with 5 μL caspase 3 substrate DEVD-p-nitroanilide (pNA). Upon cleavage of the substrate by caspase 3, free pNA light emission was quantified, using a BioTek microplate reader at 405 nm. In this method, the level of caspase 3 activity in the cell lysate is directly proportional to the color reaction. The absorbance of pNA from treated cells were compared with the negative control and the fold changes in caspase 3 activity relative to the value of negative control (RFC) were determined.
3.5. RNA Isolation and Real-Time Polymerase Chain Reaction (PCR)
The cell lines were cultured and treated as mentioned for caspase 3 colorimetric assay. After cell harvesting, total RNA was extracted, using the total RNA extraction Kit (Parstous Biotechnology, Tehran) according to the manufacturer’s instructions. Total RNA quality was evaluated, using agarose gel electrophoresis and was it quantified via a Nanodrop 1000 spectrophotometry (Thermo Scientific, Wilmington, DE). Extracted RNA (1 µg) was reverse transcribed with random primer to double-strand cDNA, using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed, using Takara SYBR Premix Ex taq II (Bio Inc., Japan) in duplicate. The mRNA expression levels of Bcl-2 (β isoform), Bax, Fas (membrane bound isoforms), and β-actin, as a housekeeping gene, were detected by Step One real-time PCR (Applied Biosystems). The sequences of primers designed by AlleleID 6 software (Biosoft, San Diego, CA) and blasted in NCBI BLAST are as follow as previously described (20). β-actin, F: GGC GGC ACC ACC ATG TAC CC, R: GGA GGG GCC GGA CTC GTC AT; Fas, F: CAC ACT CAC CAG CAA CAC, R: TCC TTT CTC TTC ACC CAA AC; Bax F: ATG CGT CCA CCA AGA AGC, R: GGC GGC AAT CAT CCT CTG; Bcl-2, F: AAG ATT GAT GGG ATC GTT GC, R: GCG GAA CAC TTG ATT CTG GT. The PCR conditions for the amplification of cDNA were 95°C for 30 seconds followed by 40 cycles of denaturation (95°C for 5 seconds), annealing [57°C for 15 seconds (β-actin, Fas) and 54°C for 15 seconds (Bax and Bcl-2)], and extension at 72°C for 30 seconds. Reaction with water instead of cDNA template was considered a non-template control. The amplified PCR product was electrophoresed through 1.5% agarose gel. Data were analyzed by the 2-ΔΔCt method. The results of target mRNA levels were normalized against β-actin mRNA in each sample. All target genes results were shown as relative fold change (RFC) to negative control.
3.6. Statistical Analysis
Data were presented as mean ± standard deviation, and assessment between groups was analyzed by one-way ANOVA in GraphPad, Prism 5 software (San Diego, CA). P value < 0.05 was considered significant. All experiments were repeated 3 times.
4. Results
4.1. Effects of the Extracts on Apoptosis Induction
In our previous study, the effects of the hexane extracts from the studied Euphorbia species on the growth and viability of the cell lines were shown by MTT colorimetric assay (9). According to these results, we chose appropriate concentrations and studied the effect of the hexane extracts from the plants on the induction of apoptosis by conducting flow cytometry and annexin V/PI staining.
E. microciadia, as seen in Figure 1A, at all extract concentrations significantly induced apoptosis in HeLa cancer cells compared to the negative control (P < 0.05). The maximum amount of apoptosis was observed at 50 µg/mL, which closely approximated the effect of the positive control. At this concentration, apoptosis was induced in more than 68% of HeLa cells.
The Apoptosis-Inducing Effect of the Extract of E. microciadia on HeLa Cells (A), E. heteradenia on K562 Cells (B) and E. osyridea on Fen Cells (C); Cells were treated with various concentrations of the extracts for 24 hours and, then, annexin V/propidium iodide (PI) staining using flow cytometry was performed. Cisplatin at concentration of 50 µg/mL was used as positive control (C+). Wells with no extract containing DMSO (0.05%) was used as negative control (C-). The total of early and late apoptosis was considered the percentage of apoptosis. Data represent mean ± the standard deviation of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 show significant difference with the negative control.
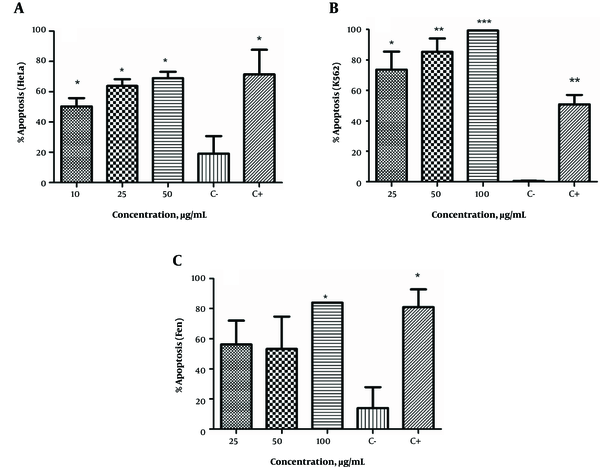
The apoptosis-inducing effect of E. heteradenia was examined on K562 cells. As shown in Figure 1B, this extract induced a significant increase in the number of apoptotic cells at all concentrations compared to the negative control. This effect was dose-dependent, as the percentage of apoptosis increased with increased extract concentration. At 100 µg/mL, almost 99% of cells had undergone apoptosis, which was more than observed for the cisplatin (P < 0.001).
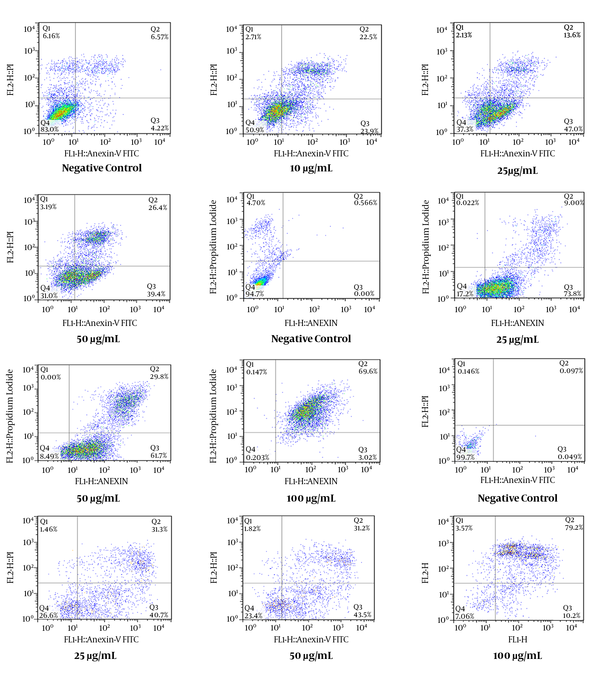
As shown in Figure 1C, all concentrations of E. osyridea extract increased the percentage of apoptotic Fen cells; however, this increase was only significant at 100 µg/mL (86.65 ± 3.8%) compared to the negative control (13.9 ± 13.5%) (P < 0.05). Examples of the flow cytometry results of the effects of the extracts on the cell lines, shown as dot plots, are presented in Figure 2.
Flow Cytometry Dot Plots Representing the Apoptosis-Inducing Effect of E. microciadia on HeLa Cells (A), E. heteradenia on K562 Cells (B), and E. osyridea on Fen Cells (C); Cells were treated with various concentrations of the extract for 24 hours and, then, annexin V/propidium iodide (PI) staining using flow cytometry was performed. Cisplatin was used as positive control and DMSO as negative control. The total of early (lower right) and late (upper right) apoptosis was considered the percentage of apoptosis.
4.2. Effects of the Extracts on Caspase-3 Activity
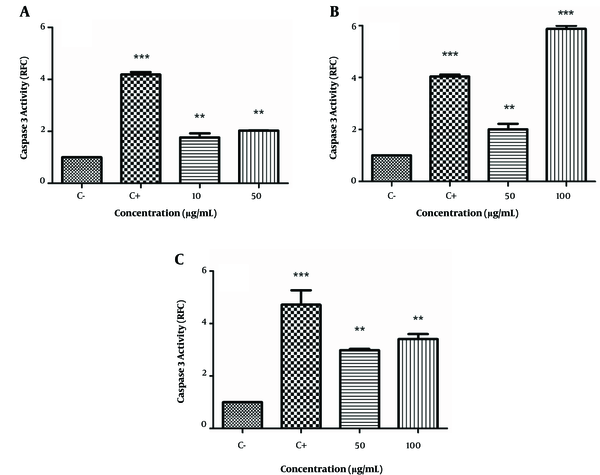
We examined the effects of these extracts on caspase-3 activation by colorimetric assay that measured the cleavage of its substrate DEVD-pNA. As seen in Figure 3A - 3C, the positive control, cisplatin, increased caspase-3 activity in all 3 cell lines (4.04 - 4.2 RFC, P < 0.001). There was increased caspase-3 activation in HeLa cells at the 10 μg/mL (1.76 ± 0.16 RFC) and 50 μg/mL (2.03 ± 0.01 RFC) concentrations of E. microciadia extract (P < 0.01; Figure 3A). The caspase-3 level of activity in K562 cells treated with E. heteradenia was 2.01 ± 0.21 RFC at the 50 μg/mL (P < 0.01) concentration and 5.87 ± 0.12 RFC at the 100 μg/mL (P < 0.001) concentration of this extract (Figure 3B). We observed increased caspase-3 activity in Fen cells with the E. osyridea extract at 50 μg/mL (2.98 ± 0.05 RFC) and at 100 μg/mL (3.41 ± 0.19 RFC; P< 0.01; Figure 3C).
Caspase 3 Activation by the Extract of E. microciadia on HeLa (A), E. heteradenia on K562 (B), and E. osyridea on Fen Cells (C); Cells were treated with various concentrations of the extracts for 24 hours and, then, the caspase 3 activation was measured by colorimetric assay. Wells with no extract containing DMSO (0.05%) was used as negative control (C-) and cisplatin was used as positive control (C+). Data represent fold changes in caspase 3 activity relative to negative control (RFC). Error bars are standard deviation of three experiments. **P < 0.01, ***P < 0.001.
4.3. Effects of the Extracts on the Expressions of Apoptosis-Related Genes
We used real-time PCR to investigate the expressions of apoptosis-related genes (Fas, Bax, and Bcl-2) in the extract-treated cells. As shown in Figure 4A, treatment of the cell lines with cisplatin increased Fas gene expression to 1.86 to 2.58 fold of the negative control (P < 0.05). E. microciadia extract increased Fas mRNA at 10 μg/mL (1.98 ± 0.22 RFC, P < 0.05) and 50 μg/mL (5.70 ± 0.43 RFC, P < 0.001) concentrations of the extract. Similarly, E. heteradenia extract caused Fas gene upregulation to 2.35 ± 0.07 RFC at 50 μg/mL and 8.78 ± 0.07 RFC at 100 μg/mL (P < 0.001; Figure 4B). E. osyridea extract at 50 μg/mL caused Fas gene upregulation to 3.28 ± 0.21 RFC (P < 0.01) and at 100 μg/mL to 3.79 ± 0.08 RFC (P < 0.001; Figure 4C).
Real-time PCR for the Fas (A, B, C), Bax (D, E, F), and Bcl-2 (G, H, I) gene expressions after treatment of HeLa cells with E. microciadia, K562 cells with E. heteradenia and Fen cells with E. osyridea extracts; Cells were treated with various concentrations of the extracts for 24 hours and, then, RNA extracted. Wells with no extract containing DMSO (0.05%) was used as negative control (C-) and cisplatin was used as positive control (C+). Data are presented as relative fold change (RFC) to negative control. Error bars represent standard deviation of three experiments. *P < 0.05, **P < 0.01 *** P < 0.001.

We observed upregulation in Bax mRNA for all the extracts. The relative gene expression of Bax in HeLa cells treated with E. microciadia extract was 1.81 ± 0.02 RFC at 10 μg/mL (P < 0.01) and 4.17 ± 0.20 RFC at 50 μg/mL (P < 0.001; Figure 4D). E. heteradenia extract at 50 μg/mL resulted in upregulation to 5.45 ± 0.64 (P < 0.01) and at 100 μg/mL, the relative gene expression was 6.50 ± 0.57 (P < 0.001; Figure 4E). There was significant upregulation of Bax in Fen cells treated with 100 μg/mL of E. osyridea extract (2.13 ± 0.23 RFC; P < 0.01; Figure 4F).
In contrast to Bax, Bcl-2 expression downregulated in HeLa cells at the 10 μg/mL (0.74 ± 0.08 RFC; P < 0.05) and 50 μg/mL (0.16 ± 0.07 RFC; P< 0.001) concentrations of E. microciadia extract (Figure 4G). Bcl-2 expression in cells treated with E. heteradenia decreased to 0.45 ± 0.7 RFC (P< 0.01) at 50 μg/mL and 0.25 ± 0.07RFC (P < 0.001) at 100 μg/mL (Figure 4H). Corresponding data for E. osyridea extract were 0.27 ± 0.03 RFC for the 50 μg/mL and 0.06 ± 0.06 RFC for the 100 μg/mL (P < 0.001) concentrations (Figure 4I).
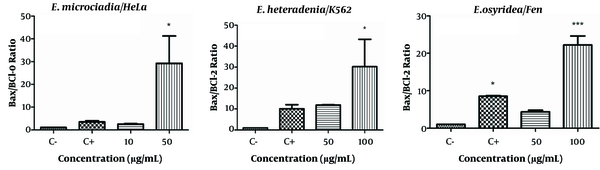
Changes in Bax and Bcl-2 expressions resulted in an increased Bax/Bcl-2 ratio for all extracts (Figure 5). The Bax/Bcl-2 ratio in cells treated with the highest concentrations of the extracts were 29.25 ± 12.0 (P < 0.05) for 50 μg/mL of E. microciadia; 30.2 ± 13.01 (P < 0.05) for 100 μg/mL of E. heteradenia, and 22.2 ± 2.4 (P < 0.001) for 100 μg/mL of E. osyridea. The Bax/Bcl-2 ratio for the positive control ranged from 3.46 to 10.1 in different cell lines.
Bax/Bcl-2 Expression Ratio; The gene expression levels of Bax and Bcl-2 after 24 hours treatment of HeLa cells with E. microciadia, K562 cells with E. heteradenia, and Fen cells with E. osyridea extracts were determined by real-time PCR and the ratio was calculated. Wells with no extract containing DMSO (0.05%) was used as negative control (C-) and cisplatin was used as positive control (C+). Error bars represent standard deviation of three experiments. *P < 0.05, *** P < 0.001 show significant differences with the negative control.
5. Discussion
The study of different biologic activities of plants for various conditions, in particular their anti-cancer effects, can provide additional information regarding possible therapeutic applications. The primary goal of this study was to find the extracts of plants that had the capability to induce apoptosis in tumor cells. By finding such extracts and performing bioassay-guided fractionation, the compounds responsible for apoptosis induction could be identified. By performing additional studies, it is possible that their usefulness in cancer treatment be determined. In our previous study, we studied the effects of several native Euphorbia species extracts on various tumor cell lines. It was shown that the hexane extracts of E. microciadia and E. osyridea on HeLa and Fen cancer cells, respectively and E. heteradenia on K562 leukemia cells had the strongest growth inhibitory effects. The variation between the plants in terms of the type of affected cells probably was due to the variations in tumor cell biology and the presence of different compounds in these plants. In this study, we analyzed these extracts for their possible anti-apoptotic effects on the selected cancer cell lines initially by annexin V/PI staining. As the results showed, E. microciadia, at a concentration of 50 µg/mL, induced apoptosis in 68% of HeLa cells. E. heteradenia at the concentration of 100 µg/mL induced apoptosis in nearly 99% of K562 cells. The same concentration of E. osyridea had the maximum apoptosis-inducing effect (more than 86%) on Fen cells. These results showed the strong apoptotic effect of the extracts on tumor cell lines, which were in agreement with the results of our previous cytotoxicity assay (9), in which the extracts had strong growth inhibitory effects. A comparison of the effects of the extracts at 50 µg/mL on apoptosis induction, regardless of cell type, showed that E. heteradenia had the strongest apoptotic effect, followed by E. microciadia and E. osyridea. This difference in the activity of the plants possibly can be due to the presence of compounds with the different modes of action in these plants. The concentrations of the compounds presented in the extracts could also be effective on the results obtained.
We have examined the effects of the extracts on caspase-3 activation. Caspase-3 is a frequently activated death protease that catalyzes the specific cleavage of numerous key cellular proteins. This enzyme is crucial for apoptotic chromatin condensation and DNA fragmentation in all cell types. It is required for the execution of apoptosis (21). As the data have shown, all 3 extracts enhanced the activity of this molecule. We observed the maximum activity in K562 cells treated with 100 μg/mL E. heteradenia extract (≈ 5.8 RFC). These data supported the ability of the extracts to induce apoptosis in the selected cell lines.
The studied plants have the ability to induce apoptosis and decrease the growth of different tumor cell lines. Previously, Ghanadian et al. reported the anti-proliferative effect of a jatrophane diterpene isolated from E. osyridea through the induction of apoptosis in the Caov-4 ovarian cancer cell line (22). Our previous study on another species of the Euphorbia genus found the same ability to induce apoptosis. We demonstrated that E. cheiradenia had strong cytotoxic activity and induction of apoptosis on the K562 and Jurkat leukemia cell lines (9).
Apoptosis is an active process that results in DNA damage, followed by cell death in the absence of inflammation (23, 24). Therefore, medicines and compounds that have the capability to promote apoptosis are valuable candidates for cancer therapy (25). Apoptosis is mainly regulated by the action of the Bcl-2 family members (intrinsic/mitochondrial pathway) and/or death receptors (extrinsic pathway). We have used real-time PCR to determine the effects of these extracts on the expressions of primary genes involved in the intrinsic and extrinsic apoptosis pathways. Treatment of cell lines by the hexane extract of the studied Euphorbia species resulted in increased Fas gene expression, which might show the involvement of the extrinsic pathway. Changes to Bax and Bcl-2 gene expressions following treatment by the extracts demonstrated the possible involvement of the intrinsic pathway, as well. We observed the upregulation of Bax pro-apoptotic and downregulation of Bcl-2 anti-apoptotic gene expressions in the treated cell lines. Consequently, determination of the proportion of Bax expression to Bcl-2 showed an increased Bax/Bcl-2 ratio, which supported the effects of the extracts through the mitochondrial pathway of apoptosis.
Previous studies on other species of Euphorbia investigated the mechanism of apoptosis induction. Wang et al. reported that jolkinolide B, a compound from E. fischeriana Steud., induced apoptosis in U937 cell lines by the activation of caspases-3 and -9 (26). Lin et al. identified that euphol extracted from E. Tirucalli L. had a strong apoptotic effect on the CS12 gastric cancer cell line by increasing the Bax expression and decreasing Bcl-2 expression (27). Hsieh et al. in a study on the latex of E. antiquorum L., observed increased Bax expression and decreased Bcl2 expression that resulted in the loss of mitochondrial membrane potential and release of cytochrome c. This plant also increased the expressions of Fas and FasL on various cell lines (28).
At this time, we did not identify the compounds responsible for the induction of apoptosis by the extracts. A few studies have reported the major constituents of some of the studied plants. Several flavonoids, saponins, tannins, alkanes, sterols, and cycloartane triterpenoids were isolated from E. microciadia (29). Some biologically active diterpenoids with myrsinane skeletons that have anti-tumor activity have been isolated from E. heteradenia (30). Which of these compounds are involved in the induction of apoptosis needs further investigation.
6. Conclusions
The results of the current study have indicated that the hexane extracts from all studied plants had the ability to induce apoptosis by both the intrinsic and extrinsic pathways in the treated cell lines. Apoptosis is an important mechanism in tumor immunity; hence, the plants we have researched should be additionally investigated in terms of their anti-cancer activities and active components involved in the induction of apoptosis by its intrinsic and/or extrinsic pathways.
Acknowledgements
References
-
1.
Short SE, Yang YC, Jenkins TM. Sex, gender, genetics, and health. Am J Public Health. 2013;103 Suppl 1:S93-101. [PubMed ID: 23927517]. https://doi.org/10.2105/AJPH.2013.301229.
-
2.
Yekta ZP, Ebrahimi SM, Hosseini M, Nasrabadi AN, Sedighi S, Surmaghi MH, et al. Ginger as a miracle against chemotherapy-induced vomiting. Iran J Nurs Midwifery Res. 2012;17(5):325-9. [PubMed ID: 23853643].
-
3.
Agustoni F, Platania M, Vitali M, Zilembo N, Haspinger E, Sinno V, et al. Emerging toxicities in the treatment of non-small cell lung cancer: ocular disorders. Cancer Treat Rev. 2014;40(1):197-203. [PubMed ID: 23850197]. https://doi.org/10.1016/j.ctrv.2013.05.005.
-
4.
Kaur R, Kapoor K, Kaur H. Plants as a source of anticancer agents. J Nat Prod Plant Resour. 2011;1(1):119-24.
-
5.
Amirghofran Z, Bahmani M, Azadmehr A, Javidnia K. Anticancer effects of various Iranian native medicinal plants on human tumor cell lines. Neoplasma. 2006;53(5):428-33. [PubMed ID: 17013538].
-
6.
Rossi E, Vita A, Baccetti S, Di Stefano M, Voller F, Zanobini A. Complementary and alternative medicine for cancer patients: results of the EPAAC survey on integrative oncology centres in Europe. Support Care Cancer. 2015;23(6):1795-806. [PubMed ID: 25471177]. https://doi.org/10.1007/s00520-014-2517-4.
-
7.
Jassbi AR. Structure elucidation and chemistry of novel diterpenoids from Euphorbia plants of Iran. Iran J Pharm Res. 2010:87.
-
8.
Amirghofran Z. Medicinal plants as immunosuppressive agents in traditional Iranian medicine. Iran J Immunol. 2010;7(2):65-73. [PubMed ID: 20574119].
-
9.
Amirghofran Z, Malek-hosseini S, Gholmoghaddam H, Kalalinia F. Inhibition of tumor cells growth and stimulation of lymphocytes by Euphorbia species. Immunopharmacol Immunotoxicol. 2011;33(1):34-42. [PubMed ID: 20331330]. https://doi.org/10.3109/08923971003699018.
-
10.
Amirghofran Z, Bahmani M, Azadmehr A, Javidnia K. Induction of apoptosis in leukemia cell lines by Linum persicum and Euphorbia cheiradenia. J Cancer Res Clin Oncol. 2006;132(7):427-32. [PubMed ID: 16477442]. https://doi.org/10.1007/s00432-006-0084-x.
-
11.
Renault TT, Floros KV, Elkholi R, Corrigan KA, Kushnareva Y, Wieder SY, et al. Mitochondrial shape governs BAX-induced membrane permeabilization and apoptosis. Mol Cell. 2015;57(1):69-82. [PubMed ID: 25482509]. https://doi.org/10.1016/j.molcel.2014.10.028.
-
12.
Arasteh JM, Sarvestani EK, Aflaki E, Amirghofran Z. Fas gene polymorphisms in systemic lupus erythematosus and serum levels of some apoptosis-related molecules. Immunol Invest. 2010;39(1):27-38. [PubMed ID: 20064083]. https://doi.org/10.3109/08820130903401736.
-
13.
Leverson JD, Phillips DC, Mitten MJ, Boghaert ER, Diaz D, Tahir SK, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med. 2015;7(279):279ra40. [PubMed ID: 25787766]. https://doi.org/10.1126/scitranslmed.aaa4642.
-
14.
D'Amours D, Sallmann FR, Dixit VM, Poirier GG. Gain-of-function of poly (ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: implications for apoptosis. Cell Sci. 2001;114(20):3771-8.
-
15.
Yu FR, Lian XZ, Guo HY, McGuire PM, Li RD, Wang R, et al. Isolation and characterization of methyl esters and derivatives from Euphorbia kansui (Euphorbiaceae) and their inhibitory effects on the human SGC-7901 cells. J Pharm Pharm Sci. 2005;8(3):528-35. [PubMed ID: 16401398].
-
16.
Duarte N, Ramalhete C, Varga A, Molnar J, Ferreira MJ. Multidrug resistance modulation and apoptosis induction of cancer cells by terpenic compounds isolated from Euphorbia species. Anticancer Res. 2009;29(11):4467-72. [PubMed ID: 20032393].
-
17.
Patil SB, Magdum CS. Phytochemical investigation and antitumour activity of Euphorbia hirta Linn. Eur J Exp Biol. 2011;1:51-6.
-
18.
Wang ZY, Liu HP, Zhang YC, Guo LQ, Li ZX, Shi XF. Anticancer potential of Euphorbia helioscopia L extracts against human cancer cells. Anat Rec (Hoboken). 2012;295(2):223-33. [PubMed ID: 22190452]. https://doi.org/10.1002/ar.21517.
-
19.
Esmaeilbeig M, Kouhpayeh SA, Amirghofran Z. An Investigation of the Growth Inhibitory Capacity of Several Medicinal Plants From Iran on Tumor Cell Lines. Iran J Cancer Prev. 2015;8(5). e4032. [PubMed ID: 26634114]. https://doi.org/10.17795/ijcp-4032.
-
20.
Ebrahimnezhad Darzi S, Amirghofran Z. Dichloromethane fraction of Melissa officinalis induces apoptosis by activation of intrinsic and extrinsic pathways in human leukemia cell lines. Immunopharmacol Immunotoxicol. 2013;35(3):313-20. [PubMed ID: 23432355]. https://doi.org/10.3109/08923973.2013.768268.
-
21.
McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5(4). a008656. [PubMed ID: 23545416]. https://doi.org/10.1101/cshperspect.a008656.
-
22.
Ghanadian SM, Ayatollahi AM, Afsharypour S, Hareem S, Abdalla OM, Jules Kezetas Bankeu J. Flavonol Glycosides from Euphorbia microsciadia Bioss. with their Immunomodulatory Activities. Iran J Pharm Res. 2012;11(3):925-30. [PubMed ID: 24250520].
-
23.
Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015;14:48. [PubMed ID: 25743109]. https://doi.org/10.1186/s12943-015-0321-5.
-
24.
Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21(3):485-95. https://doi.org/10.1093/carcin/21.3.485.
-
25.
Kontos CK, Christodoulou MI, Scorilas A. Apoptosis-related BCL2-family members: Key players in chemotherapy. Anticancer Agents Med Chem. 2014;14(3):353-74. [PubMed ID: 23848200].
-
26.
Wang JH, Zhou YJ, Bai X, He P. Jolkinolide B from Euphorbia fischeriana Steud induces apoptosis in human leukemic U937 cells through PI3K/Akt and XIAP pathways. Mol Cells. 2011;32(5):451-7. [PubMed ID: 22083305]. https://doi.org/10.1007/s10059-011-0137-0.
-
27.
Lin MW, Lin AS, Wu DC, Wang SS, Chang FR, Wu YC, et al. Euphol from Euphorbia tirucalli selectively inhibits human gastric cancer cell growth through the induction of ERK1/2-mediated apoptosis. Food Chem Toxicol. 2012;50(12):4333-9. [PubMed ID: 22634261]. https://doi.org/10.1016/j.fct.2012.05.029.
-
28.
Hsieh WT, Lin HY, Chen JH, Kuo YH, Fan MJ, Wu RS, et al. Latex of Euphorbia antiquorum induces apoptosis in human cervical cancer cells via c-jun n-terminal kinase activation and reactive oxygen species production. Nutr Cancer. 2011;63(8):1339-47. [PubMed ID: 22044063]. https://doi.org/10.1080/01635581.2011.608481.
-
29.
Ghanadian M, Saeidi H, Aghaei M, Rahiminejad MR, Ahmadi E, Ayatollahi SM, et al. New jatrophane diterpenes from Euphorbia osyridea with proapoptotic effects on ovarian cancer cells. Phytochem Lett. 2015;12:302-7. https://doi.org/10.1016/j.phytol.2015.04.011.
-
30.
Jassbi AR. Chemistry and biological activity of secondary metabolites in Euphorbia from Iran. Phytochemistry. 2006;67(18):1977-84. [PubMed ID: 16889806]. https://doi.org/10.1016/j.phytochem.2006.06.030.