Abstract
Background:
The model of obesity-induced insulin resistance has long been used to explain the development of type 2 diabetes mellitus (T2DM) in obese individuals (body mass index (BMI) > 25 kg/m2), but this model failed to explain the development of the disease in lean individuals (BMI < 18.5 kg/m2). Defects in the insulin signaling pathway have been postulated to play a role in these patients, particularly in suppressors of cytokine signaling (SOCS) proteins, which are involved in the downregulation of insulin transduction. The expression of SOCS is also known to be induced by cytokines such as interferon gamma (IFN-γ). It is still not clear whether these pathways operate differently in lean versus obese patients with T2DM. Therefore, this pilot study was designed to study the expression of SOCS1, SOCS3, and IFN-γ in lean and obese patients with T2DM.Objectives:
The levels of IFN-γ in serum and the messenger RNA (mRNA) expression of SOCS (SOCS1 and SOCS3) and IFN-γ genes in whole blood in lean and obese patients with T2DM.Methods:
Sixty newly diagnosed T2DM patients (not on any pharmacotherapy) were enrolled and divided into 2 groups of lean (BMI < 18.5 kg/m2) and obese (BMI > 25 kg/m2) patients (n = 30 per group). Serum IFN-γ was measured by enzyme-linked immunosorbent assay (ELISA), and mRNA expression of IFN-γ, SOCS1, and SOCS3 was measured by real-time polymerase chain reaction (PCR) using the ∆∆ Ct method.Results:
Serum IFN-γ levels were 10.83 ± 5.81 pg/mL in the lean group and 9.35 ± 5.14 pg/mL in the obese group (P = 0.02). Fasting serum insulin levels were 16.07 ± 8.39 µIU/mL in the lean group and 27.11 ± 4 .91 µIU/mL in the obese group (P = 0.001). There was a 3.16-fold increase in mRNA expression of IFN-γ and a 1.3-fold increase in mRNA expression of SOCS1 in the lean group compared to the obese group. mRNA expression of SOCS3 was similar in both groups.Conclusions:
The level of IFN-γ increased at both transcriptional and translational levels, and mRNA expression of SOCS1 was higher in the lean group than in the obese group. The SOCS protein is a known negative regulator in insulin signaling pathways. Thus, our findings and available scientific literature suggest that IFN-γ might impair the insulin signaling pathway to a greater extent in lean patients than in obese patients via induction of SOCS1. This signaling pathway could be a major contributing factor to hyperglycemia in lean patients with T2DM compared with obese counterparts. This suggests that different therapeutic approaches to these groups might be of greater benefit in the treatment of T2DM.Keywords
1. Background
Diabetes mellitus (DM) is defined as a group of metabolic diseases characterized by hyperglycemia, resulting from defects in the secretion and/or action of insulin (1). Type 2 DM (T2DM) is largely associated with obesity and insulin resistance. However, a certain proportion of the population suffering from this disease are lean (body mass index (BMI) < 18.5 kg/m2) (2).
The exact etiopathogenesis of T2DM in the lean population is still unclear. However, many factors have been implicated, such as dysfunction in the signaling pathway, oxidative stress, mitochondrial defects, and imbalance in chemical mediators (including interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), lipocalins, etc.) (3).
The suppressors of the cytokine signaling (SOCS) family comprise molecules involved in the negative regulation of cytokine-triggered signaling. They contribute to the downregulation of insulin transduction pathways. SOCS1 and SOCS3 target molecules in the insulin signaling pathway [insulin receptor substrate (IRS) 1 and 2] for proteasomal degradation. Therefore, increased levels of SOCS1 and SOCS3 could lead to insulin signaling defects, resulting in hyperglycemia (4).
IFN-γ is a proinflammatory cytokine and induces the expression of SOCS (5). Many theories have been put forward to elaborate the role of IFN-γ in the pathogenesis of T2DM. One study indicated that IFN-γ increased the expression of major histocompatibility complex (MHC) I and II antigens and adhesion molecules on the pancreatic beta cells (6). Another proposed theory is the role of IFN-γ in potentiating the destructive effects of macrophages and CD8+ lymphocytes on pancreatic beta cells, thus decreasing insulin levels (7). In vitro studies have demonstrated the cytotoxic effects of IFN-γ on β cells. Exposure of human islets to IFN-γ, along with interleukin 1 beta (IL-1 β) or TNF-α, induced β cell death (8). Another study showed that IFN-γ led to the downregulation of the insulin signaling pathway via the induction of SOCS (9). Thus, this study was designed to assess the role of SOCS and IFN-γ in newly diagnosed and untreated lean and obese patients with T2DM to gain an insight into the differences between these 2 groups regarding the pathogenesis of T2DM.
2. Objectives
To compare the levels of IFN γ in serum and the messenger RNA (mRNA) expression of of SOCS (SOCS1 and SOCS3) and IFN-γ genes in whole blood in lean and obese patients with T2DM.
3. Methods
This descriptive study was designed and conducted in the Department of Biochemistry and Department of Endocrinology of the University College of Medical Sciences and Guru Teg Bahadur Hospital GTB Hospital, Delhi, India. Approval of the Institutional Ethics Committee/Human Research (IEC-HR/2019/41/25) was obtained. Patients were recruited after taking written informed consent. A detailed literature search did not yield any studies comparing these parameters; therefore, this study can be considered as a pilot study. Lean patients with T2DM were recruited first, followed by obese patients who were matched for age and sex. As per the study design, the duration of the study was 18 months, given an average of 2 newly diagnosed, lean patients with T2DM that attended the Out Patient Department (OPD) in a month. Therefore, a convenience sample of 60 patients (30 lean and 30 obese) was recruited.
The diagnosis of T2DM was made as per World Health Organization (WHO) criteria (10). The patients were divided into 2 groups of lean (BMI < 18.5 kg/m2) and obese (BMI > 25 kg/m2) based on BMI as per WHO Asia-Pacific Guidelines (11). Newly diagnosed age /sex matched patients of T2DM (not on pharmacotherapy for T2DM) aged 20-65 years were recruited. Patients with renal and hepatic disorders, severe comorbid diseases, thyroid disorders, and chronic alcoholism, as well as pregnant and lactating women, were excluded from the study. A detailed history was taken from the patients recruited in the study, followed by a clinical examination and anthropometric measurements taken as per standard guidelines (12).
Routine biochemical investigations (including plasma glucose) were conducted using the RANDOX RX Imola Autoanalyzer (RANDOX, UK) as per the manufacturer’s instructions. HbA1C levels were estimated in whole blood using the BIORAD D-10 Autoanalyzer (BIO-RAD, USA) as per the manufacturer’s instructions.
3.1. Enzyme-Linked Immunosorbent Assay
IFN-γ was estimated in serum using a sandwich enzyme-linked immunosorbent assay (ELISA) (Diaclone, France; sensitivity: 5 pg/mL; precision: intra-assay 4.9% and inter-assay 10%), following the protocol provided by the manufacturer. Fasting serum insulin was estimated by sandwich ELISA (DRG International, USA), following the protocol provided by the manufacturer (sensitivity: 1.76 µIU/mL; precision: intra-assay 2.8% and inter-assay 5.99%).
3.2. RNA Isolation, Complementary DNA Synthesis, Real-Time Polymerase Chain Reaction
RNA was extracted using RiboZol RNA extraction reagent (Amresco, USA) according to manufacturer’s instructions. First-strand complementary DNA (cDNA) was synthesized using a commercially available cDNA synthesis kit (RevertAid First Strand cDNA synthesis kit, Thermo SCIENTIFIC, USA). Non-reverse-transcriptase controls (RT controls) were prepared without adding the enzyme.
Expression profiling by quantitative polymerase chain reaction (PCR, dye based chemistry) was performed using 20 µL (microlitre) reactions in duplicates. Each reaction tube consisted of cDNA, Maxima Hot Start PCR Master Mix (Thermo SCIENTIFIC, USA), SYTO9 fluorescent nucleic acid dye (Invitrogen, USA), and the forward and reverse primers of the gene of interest. The primer sequences used are shown in Table 1. The following were the thermal conditions for the PCR: 95°C for 4 minutes (enzyme activation), followed by 40 cycles at 95°C for 20 seconds (denaturation), at annealing temperature for 30 seconds, and at 72°C for 30 seconds (extension). Fluorescence acquisition was performed at the end of each step of extension. The annealing temperature for each primer set was determined via gradient PCR. Negative controls (non-template water instead of cDNA) were also included to ensure that the reagents were free from contamination with non-specific DNA. mRNA expression of SOCS1, SOCS3, and IFN-γ (target gene) was determined by real-time PCR using CFX Connect Real-Time System (BIO-RAD, USA) (13). GAPDH, 18s, and β2M were used as housekeeping genes. The average Ct of 18s, GAPDH, and β2M was taken as normalizer Ct. The ∆Ct value was calculated as Ct (normalizer) - Ct (target gene). Average ∆Ct was then calculated for each group. The ∆∆ Ct value was calculated from the average ∆Ct values as ∆Ct (group 1, i.e., lean) - ∆Ct (group 2, i.e., obese).
Determination of fold change (FC) was based on FC = 2ΔΔCt. Since FC was > 1, it was considered a true FC.
Statistical analysis was performed using SPSS version 26 (SPSS Inc., Chicago, Ill, USA). As described above, the relative expression mRNA of SOCS1, SOCS3, and IFN-γ was expressed as FC (13). The serum levels of IFN-γ, fasting serum insulin, fasting plasma glucose, 2-hour plasma glucose, and HbA1c were compared between the groups by the unpaired Student t test or Mann-Whitney U test. A correlation analysis was done using the Spearman rho correlation test. P values less than 0.05 were considered significant.
Sequence of Primers
| Primer | 5' to 3' Sequence |
|---|---|
| SOCS1, F | TTGGAGGGAGCGGATGGGTGTAG |
| SOCS1, R | AGAGGTAGGAGGTGCGAGTTCAGGTC |
| SOCS3, F | ATACTATACCTTCCTGTACCTGGGTGGATGGAGCG |
| SOCS3, R | TGAGTATGTGGCTTTCCTATGCTGGGTCCCTCT |
| IFNγ, F | TGACCAGAGCATCCAAAAGA |
| IFNγ, R | CTCTTCGACCTCGAAACAGC |
| 18s, F | GTAACCCGTTGAACCCCATT |
| 18s, R | CCATCCAATCGGTAGTAGCG |
| GAPDH, F | TGACTTCAACAGCGACACCCA |
| GAPDH, R | CACCCTGTTGCTGTAGCCAAA |
| β2M, F | TAGCTGTGCTCGCGCTACT |
| β2M, R | TCTCTGCTGGATGACGTGAG |
4. Results
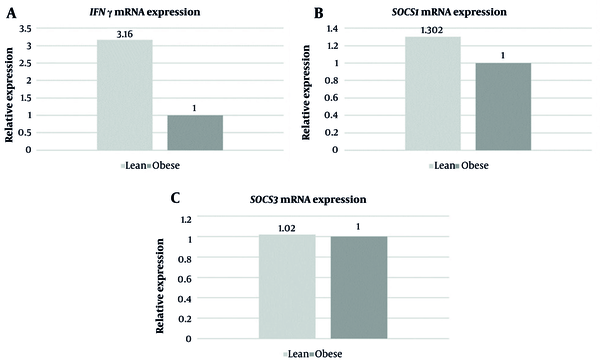
Each group consisted of 30 participants. Out of the 30 patients in each group, 8 (26.6 %) were female, and 22 (73.3%) were male. The mean age of patients was 52.10 ± 10.67 years in the lean group and 51.5 ± 10.40 years in the obese group. The values of other parameters, such as fasting and post prandial levels of glucose, levels of glycated hemoglobin, fasting serum insulin, and serum IFN-γ levels, are presented in Table 2. The mean level of serum IFN-γ was 10.83 ± 5.81 pg/mL in the lean group and 9.35 ± 5.14 pg/mL in the obese group (P = 0.02). The mean level of fasting serum insulin was 16.07 ± 8.39 µIU/mL in the lean group and 27.11 ± 4.91 µIU/mL in the obese group (P = 0.001). FCs in the expression of IFN-γ, SOCS1, and SOCS3 are depicted in Figure 1A - C, respectively. There was a 3.16-fold increase in mRNA expression of IFN-γ and a 1.3-fold increase in mRNA expression of SOCS1 in the lean group compared with the obese group. There was no difference in mRNA expression of SOCS3 between the 2 groups.
| Variables | Lean (n = 30) | Obese (n = 30) | P Value |
|---|---|---|---|
| BMI (kg/m2) | 17.88 ± 0.86 | 27.24 ± 2.73 | - |
| Interferon gamma (IFN-γ) (pg/mL) | 10.83 ± 5.81 | 9.35 ± 5.14 | 0.02 |
| Fasting plasma glucose (mg/dL) | 254.25 ± 63.13 | 207.28 ± 73.85 | 0.01 |
| 2-hour post prandial plasma glucose (mg/dL) | 361.24 ± 76.65 | 329.16 ± 88.05 | 0.131 |
| HbA1C (%) | 11.5 ± 2.65 | 9.42 ± 2.11 | 0.001 |
| Fasting serum insulin (µIU/mL) | 16.07 ± 8.39 | 27.11 ± 4.91 | 0.001 |
A, mRNA expression of interferon gamma (IFN-γ) (fold change); B, mRNA expression of suppressors of cytokine signaling (SOCS1) (fold change); C, mRNA expression of SOCS3 (fold change) in lean and obese patients with T2DM.
A correlation analysis was performed between serum levels of IFN-γ and the ∆Ct value [Ct (normalizer) - Ct (target gene)] of SOCS1 and SOCS3. The correlation coefficient (r) was 0.023 (P = 0.860) for serum IFN-γ vs mRNA expression of SOCS1 in all subjects. The correlation coefficient (r) was -0.065 (P = 0.624) for serum levels of IFN-γ vs mRNA expression of SOCS3 in all subjects. Both were not found to be statistically significant.
5. Discussion
In order to function, chemical mediators should bind to their receptors and activate the downstream signal transduction pathway. Regulators exist to prevent the over-activation of these downstream pathways, and these can terminate the incoming signal after an appropriate level of response is generated. In turn, these regulators are controlled by inducers and repressor molecules. The insulin transduction pathway is no exception, and SOCS proteins have been known to terminate this signaling pathway once the desired effect is produced. Therefore, factors that lead to the overexpression of SOCS can result in hyperglycemia.
In this study, we studied the mRNA expression of SOCS1, SOCS3, and IFN-γ (an inflammatory marker). SOCS has been known to terminate insulin signaling by binding to IRS1 and IRS2 and targeting them for proteasomal degradation. IRS1 and IRS2 play an important role in downstream insulin signaling. Many studies (5, 14) have reported increased mRNA expression of SOCS1 and SOCS3 in obese patients with T2DM; however, there are no studies on SOCS expression in lean patients with T2DM.
In this study, the mRNA expression of SOCS1 slightly increased in the lean group compared with the obese group, while the mRNA expression of SOCS3 was similar in both groups. Factors that could have interfered with our results, such as chronic infection, cancers, autoimmune disorders, etc, were excluded from the study based on predefined exclusion criteria.
IFN-γ is an inflammatory cytokine and a known inducer of SOCS. In this study, we found that the level of IFN-γ was higher at both transcriptional and translational levels in the lean group than in the obese group. Although there is no previous study comparing IFN-γ levels in lean and obese patients with T2DM, earlier studies have reported a lower level of serum IFN-γ in obese patients with T2DM than in healthy control subjects (9, 15).
Based on our findings, we postulate that increased levels of IFN-γ induce the expression of SOCS1, thereby disrupting the insulin signaling pathway (5) by targeting IRS1 and IRS2 for proteasomal degradation. This could lead to the premature termination of insulin signaling, potentially resulting in hyperglycemia. The above mechanism possibly plays a more prominent role in lean patients than in obese patients with T2DM.
Another probable mechanism involved in the pathogenesis of T2DM in lean patients is a decreased mass of beta cells through IFN-γ. This is supported by studies that have reported the role of IFN-γ in the cytotoxic death of beta cells (7, 8). Based on our findings, we propose that increased levels of IFN-γ could lead to premature destruction of beta cells, thereby decreasing the basal insulin output and resulting in hyperglycemia in lean patients with T2DM. This theory is supported by our finding of decreased serum insulin levels in lean patients compared with obese patients with T2DM (Table 2).
Another perspective about increased IFN-γ in lean patients is also possible. The glycemic parameters were found to be higher in lean patients than in obese patients, as depicted in Table 2. Therefore, it is also possible that a greater degree of hyperglycemia in the lean group could result in cytotoxic end-organ damage (cellular damage due to prolonged exposure of cells and tissues to high glucose levels). This could result in increased levels of IFN-γ in lean patients. Therefore, tissue-specific expression studies are needed to further clarify the exact mechanism of increased IFN-γ expression in lean patients.
5.1. Conclusions
The level of IFN-γ increased at both transcriptional and translational levels, and mRNA expression of SOCS1 was higher in the lean group than in the obese group. The SOCS protein is a known negative regulator in insulin signaling pathways. Thus, our findings and available scientific literature suggest that IFN-γ might impair the insulin signaling pathway to a greater extent in lean patients than in obese patients via the induction of SOCS1.
This signaling pathway could be a major contributing factor toward hyperglycemia in lean patients with T2DM compared with their obese counterparts. This suggests that different therapeutic approaches to both these groups might be of greater benefit in the treatment of T2DM.
Hyperglycemia-induced cytotoxic organ damage can also lead to increased levels of IFN-γ in lean patients with T2DM, which needs to be studied further.
5.2. Limitations and Future Prospects
The present study was performed with a limited sample size due to constraints of time and resources. We plan to extend this study to include a larger sample size to further confirm the above-mentioned findings for better statistical accuracy. Other cytokines (such as TNF-α, interleukins, etc) have also been known to affect SOCS1 and SOCS3 expression. Thus, a comprehensive study of these cytokines and their interactions with SOCS in lean and obese patients with T2DM will help to elucidate their role in the pathogenesis of this disease.
5.3. Clinical Implications
This is the first study to compare various molecular parameters in lean (BMI < 18.5 kg/m2) and obese (BMI > 25 kg/m2) patients with T2DM. Thus, it described important baseline details and highlighted the difference in the disease process, which might warrant different management strategies in these 2 groups.
Acknowledgements
References
-
1.
American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15-33. [PubMed ID: 33298413]. https://doi.org/10.2337/dc21-S002.
-
2.
Hoet JJ, Tripathy BB. Consensus statement from the International Workshop on Types of Diabetes Peculiar to the Tropics, 17-19 October 1995, in Cuttack, India. Acta Diabetol. 1996;33(1):62-4. [PubMed ID: 8777288]. https://doi.org/10.1007/BF00571945.
-
3.
Zhou T, Hu Z, Yang S, Sun L, Yu Z, Wang G. Role of Adaptive and Innate Immunity in Type 2 Diabetes Mellitus. J Diabetes Res. 2018;2018:7457269. [PubMed ID: 30533447]. [PubMed Central ID: PMC6250017]. https://doi.org/10.1155/2018/7457269.
-
4.
Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004;24(12):5434-46. [PubMed ID: 15169905]. [PubMed Central ID: PMC419873]. https://doi.org/10.1128/MCB.24.12.5434-5446.2004.
-
5.
Feng X, Tang H, Leng J, Jiang Q. Suppressors of cytokine signaling (SOCS) and type 2 diabetes. Mol Biol Rep. 2014;41(4):2265-74. https://doi.org/10.1007/s11033-014-3079-8.
-
6.
Tsiavou A, Hatziagelaki E, Chaidaroglou A, Koniavitou K, Degiannis D, Raptis SA. Correlation between intracellular interferon-gamma (IFN-gamma) production by CD4+ and CD8+ lymphocytes and IFN-gamma gene polymorphism in patients with type 2 diabetes mellitus and latent autoimmune diabetes of adults (LADA). Cytokine. 2005;31(2):135-41. [PubMed ID: 15935691]. https://doi.org/10.1016/j.cyto.2005.02.011.
-
7.
Tsiavou A, Degiannis D, Hatziagelaki E, Koniavitou K, Raptis SA. Intracellular IFN-gamma production and IL-12 serum levels in latent autoimmune diabetes of adults (LADA) and in type 2 diabetes. J Interferon Cytokine Res. 2004;24(7):381-7. [PubMed ID: 15296648]. https://doi.org/10.1089/1079990041535665.
-
8.
Yi Z, Li L, Garland A, He Q, Wang H, Katz JD, et al. IFN-gamma receptor deficiency prevents diabetes induction by diabetogenic CD4+, but not CD8+, T cells. Eur J Immunol. 2012;42(8):2010-8. [PubMed ID: 22865049]. [PubMed Central ID: PMC3883988]. https://doi.org/10.1002/eji.201142374.
-
9.
Bosek I, Kuczerowski R, Miłek T, Sulich A, Kaleta B, Kniotek M, et al. Evaluation of interferon-gamma in patients with type 2 diabetes and colorectal cancer. J Diabetes Metab. 2015;7(1):1000639. https://doi.org/10.4172/2155-6156.1000639.
-
10.
World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia : report of a WHO/IDF consultation. Geneva, Switzerland: World Health Organization; 2006.
-
11.
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157-63. [PubMed ID: 14726171]. https://doi.org/10.1016/S0140-6736(03)15268-3.
-
12.
World Health Organization. WHO STEPwise Approach to Chronic Disease Risk-Factor Surveillance (Part 3: Training and Practical Guides). 1 ed. Geneva, Switzerland: World Health Organization; 2003. p. 121-204.
-
13.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402-8. [PubMed ID: 11846609]. https://doi.org/10.1006/meth.2001.1262.
-
14.
Rieusset J, Bouzakri K, Chevillotte E, Ricard N, Jacquet D, Bastard JP, et al. Suppressor of cytokine signaling 3 expression and insulin resistance in skeletal muscle of obese and type 2 diabetic patients. Diabetes. 2004;53(9):2232-41. [PubMed ID: 15331532]. https://doi.org/10.2337/diabetes.53.9.2232.
-
15.
Kartika R, Purnamasari D, Pradipta S, Larasati RA, Wibowo H. Impact of Low Interferon-gamma and IL-10 Levels on TNF-alpha and IL-6 Production by PHA-Induced PBMCs in Type 2 Diabetes Mellitus. J Inflamm Res. 2020;13:187-93. [PubMed ID: 32425577]. [PubMed Central ID: PMC7190380]. https://doi.org/10.2147/JIR.S245064.