Abstract
Background:
The health related quality-of-life (HRQoL) among females with polycystic ovary syndrome (PCOS) is reduced due to emotional, psychosocial, infertility, marital, and hirsutism problems.Objectives:
The current study aimed at analyzing exploratory and confirmatory factor structures of HRQoL questionnaire for polycystic ovary syndrome (PCOSQ-50) in females with PCOS in order to verify the validity of the developed instrument.Methods:
The current cross validation study was conducted on females with PCOS using the PCOSQ-50. The PCOSQ-50 was developed based on a qualitative study. The exploratory factor analysis (EFA) and the confirmatory factor analysis (CFA) were conducted to examine the factor structure of PCOSQ-50. After the CFA, the reliability of the new instrument was also evaluated using Cronbach’s alpha coefficient.Results:
Totally, 350 females with PCOS were entered into the study. The mean age of the subjects was 26.9 ± 5.1 years. Based on the results of CFA, data were fit to the 43-item model: the comparative fit index (CFI) = 0.91; the normal fit index (NFI) = 0.90; the goodness of fit index (GFI) = 0.60; incremental fit index (IFI) = 0.91; the root mean square error of approximation (RMSEA) = 0.09; standardized root mean square residual (SRMR) = 0.09 and the relative chi-square (x2/df) = 2.20, P < 0.05. The intraclass correlation coefficient ranged 0.91 to 0.94 indicating a satisfactory finding.Conclusion:
The 43-item PCOSQ showed appropriate validity and reliability and its psychometric quality was superior to that of the original version. However, further longitudinal studies should be conducted to evaluate its predictive efficacy.Keywords
Polycystic Ovary Syndrome Quality-of-Life Factor Analysis Reproductive Health
1. Background
Poly cystic ovary syndrome (PCOS), as a common hormone disorder, is estimated to involve 2.2% - 26% of females (1, 2). It is a chronic condition recognized by clinical and/or biochemical signs of androgen excess, ovulatory dysfunction, and polycystic ovaries (3). The prevalence of PCOS in Iran was reported 7.1% (95% confidence interval (CI): 5.4 - 8.8) according to the national institutes of health (NIH) definition, while it was11.7% (95% CI: 9.5 - 13.7) and 14.6% (95% CI: 12.3 - 16.9) based on the AES and Rotterdam criteria, respectively (4). Typically, PCOS is identified during the early years of reproductive age (5). Although the etiology of PCOS is poorly understood, some forms of hormonal dysfunction including hyperandrogenism and diminished reproductive function are suggested (6). PCOS is a complex condition with diverse multidisciplinary approaches; females with PCOS show a wide range of symptoms such as anovulation, amenorrhea, oligomenorrhea, menorrhea, hirsutism, subfertility or infertility, weight gain or obesity, acne vulgaris, androgenic alopecia, excess androgen production, and insulin resistance (7, 8). Also, PCOS is identified as a risk factor for endometrial cancer (1, 9) and metabolic disturbances (e g, hypertension, diabetes, cardiovascular diseases, and dyslipidemia) (1, 9-11). Several studies showed a diminished health-related quality-of-life (HRQoL) among females with PCOS due to emotional, psychosocial, infertility, marital, and hirsutism problems (12-15). The general QoL questionnaires are not valid tools to assess QoL in females with PCOS, because it is a heterogeneous disease affecting the QoL. Therefore, based on a qualitative design, a new instrument was developed to measure QoL particularly in females with PCOS. The development process of this questionnaire was described in details in authors’ previous study (16).
2. Objectives
The current study aimed at analyzing the exploratory and confirmatory factor structures of HRQoL in females with PCOS using the HRQoL questionnaire for polycystic ovary syndrome (PCOSQ-50.3).
3. Methods
3.1. Participants and Data Collection
The current cross sectional study was conducted from March to September 2013. Study samples included females with PCOS referring to the gynecology, endocrinology, and dermatology clinic of Babol University of Medical Sciences, Babol, Iran. The females were selected using a convenience sampling method. The number of samples was determined based on the adequacy of sample size to evaluate a questionnaire as follows: 300 = good, 500 = very good, 1000 or more = excellent and recommended for factor analysis (17). Therefore, a total of 350 females with PCOS, diagnosed by gynecologists or endocrinologists, were scheduled for a routine follow-up appointment. The objectives and method of the study were explained to the subjects, and written informed consent forms were signed by the ones who willingly agreed to take part in the current study. Inclusion criteria were: age 18 - 40 years and the diagnosis of PCOS based on the Rotterdam criteria (11). Coexistence of illnesses such as pituitary adenoma, hypothyroidism, hyperprolactinemia, chronic kidney diseases, endometriosis, inability to read and speak Farsi, and having conditions similar to PCOS such as congenital adrenal hyperplasia were considered as the exclusion criteria.
3.2. Measurements
PCOSQ-50 is a valid and reliable instrument to assess the QoL among females with PCOS. However, the Cronbach’s alpha coefficient and the intraclass correlation coefficient (ICC) for the coping subscale were 0.61 and 0.5, respectively, which may be a limitation to the PCOSQ-50 (16). Therefore, this questionnaire should be modified in terms of psychometric properties.
Demographic information as well as reproductive and gynecological history, including the menstrual cycle, hyperandrogenic symptoms (acne, hirsutism, and alopecia), and infertility were collected through face-to-face interviews and physical examinations using a standard questionnaire (4). This questionnaire consisted of 33 questions regarding demographic characteristics (such as height, weight, waist circumference, and hip circumference), the menstrual status, status of physical appearance (hirsutism (18), acne (19) and obesity), chief complaint, medications, and family history. The sampling process was conducted by a trained midwife who also conducted the physical examinations. The severity of acne was assessed using the Hayashi acne severity scale (19). Also, hirsutism was assessed based on the Ferriman-Gallwey scale, in which the females’ hair growth on nine different body parts was scored from 0 (no terminal hair) to 4 (maximal growth) with a maximum score of 36. A score of ≥ 8 indicated hirsutism (18).
3.3. Ethics Statement
The institutional ethical review board of Shahid Beheshti University of Medical Sciences, Iran, approved the research protocol (Grant number: 8033). The informed consent forms were signed by the subjects who willingly took part in the current study.
3.4. Statistical Analysis
The rate of missing data in the current study was checked before conducting factor analysis that was less than 5% and indicated the eligibility criteria. Also, the adequacy of sample size was checked using the Kaiser-Meyer-Olkin (KMO) and Bartlett test of sphericity (KMO = 0.80); the approximate chi-square values of Bartletttest of sphericity were χ2 = 3807.82, df = 154, P < 0.001, indicating the adequacy of sample size and data to conduct factor analysis. A confirmatory factor analysis (CFA) was conducted to examine the factor structure of the PCOSQ-50 using covariance matrices and the maximum-likelihood estimation method. The goodness-of-fit of the model was assessed using the chi-square, relative chi-square (χ2/df), the goodness-of-fit index (GFI), the comparative fit index (CFI), the normed fit index (NFI), the root mean square error of approximation (RMSEA), and the standardized root mean square residual (SRMR). The χ2 statistic was the most common method to evaluate the goodness-of-fit, but it was highly sensitive to the sample size. An alternate evaluation of the χ2 statistic was to examine the relative chi-square (χ2/df) for the model (20). A χ2/df ≤ 2 indicated a good fit model (21). For other goodness-of-fit indexes, values indicating good fit were GFI, CFI, and NFI > 0.95, RMSEA < 0.06, and SRMR < 0.08 (22).
Internal consistency and test-retest methods helped to assess the reliability of the questionnaire. The internal consistency of the PCOSQ-50 was examined using the Cronbach’s alpha coefficient and item-total correlations. The stability of the questionnaire was assessed using the test-retest method with 20 females with PCOS who filled out the questionnaire twice within a 2-week interval. Descriptive statistics for continuous variables included mean ± SD and for categorical variables were numbers and percentages. The statistical analysis was performed using the SPSS version 23.0 and the CFA was performed using the Lisrel 8.80. All statistical tests were 2-tailed and P < 0.05 was considered statistically significant.
4. Results
4.1. Participants
A total of 350 females with PCOS were included in the current study. The mean age of the subjects was 26.9 ± 5.1 years, ranged 18 to 40. The sociodemographic and clinical characteristics of the subjects are shown in Table 1.
Demographic Characteristics and Medical History of the Study Participants
| Variable | Mean ± SD |
|---|---|
| Age, y | 26.9 ± 5.1 |
| BMI, kg/m2 | 28.1 ± 9.7 |
| Period of education, y | 13.1 ± 3.0 |
| Income, $US per day | 19.33 ± 14.54 |
| Waist circumference, cm | 83.2 ± 11.8 |
| Hip circumference, cm | 107.3 ± 11.2 |
| Waist-to-hip ratio | 0.77 ± 0.59 |
| Interval of menstruation, d | 30.4 ± 12.1 |
| The number of menstruation in a year | 8.4 ± 3.3 |
| FG scoring | 10.8 ± 4.7 |
| No. (%) | |
| Married | 242 (69.1) |
| BMI (26 - 29), kg/m2 | 92 (26.3) |
| BMI (≥ 30), kg/m2 | 121 (34.6) |
| Menstrual disorder in the past 6 months | 242 (69.1) |
| History of menstrual disorders | 307 (87.7) |
| Onset of menstrual disorders of puberty | 292 (83.4) |
| Pregnancy experience | 126 (36) |
| Abortion experience | 30 (8.6) |
| History of pregnancy complications | 43 (12.3) |
| Infertility | 96 (27.4) |
| Acne | 138(39.4) |
| Hirsutism | 227 (64.9) |
4.2. Factor Analysis
The EFA and CFA were performed to examine the factor structure of the PCOSQ-50. Before testing the concepts of factor analysis, the skewness and kurtosis of the variable were in the normal range. This indicated that the variable distribution was normal. Also, to improve model fit, 7 low loading items found within the ‘coping’ factor were dropped, through excluding the items, which led to the development of a 43-item questionnaire. The decision regarding the number of factors to be retained for rotation was made based on the Kaiser criteria (eigenvalues > 1). EFA was repeated through limiting the analysis to 6 factors with varimax rotation. Items with a coefficient of at least 0.40 were retained.
As a result, 43 items accounting for 50.83% of the observed variance were obtained. They were grouped into 6 factors as follows: ‘psychosocial and emotional’ (9 items), ‘self-body image’ (4) ‘fertility’ (9 items), ‘sexual function’ (7 items), ‘obesity and menstrual disorder’ (8 items), and ‘hirsutism’ (6 items). Also, the self-body image factor was added to the structure and the items of coping factor were incorporated into other factors.
The factor loadings of the observed variables on latent constructs were estimated using the CFA (Table 2). The results of the confirmatory factor analysis are shown in Figure 1.
Factor Loadings and Variances for the PCOSQ-43
| Variable: In the Past 4 Weeks Have You Ever Experienced/Suffered From/Felt | Factor Loading | Variance (%) |
|---|---|---|
| Psychosocial and emotional domain | 17.85 | |
| Bad mood due to PCOS? | 0.71 | |
| Impatience due to PCOS? | 0.70 | |
| Blamed your-self for having PCOS? | 0.77 | |
| Trouble dealing with others? | 0.61 | |
| Low self-esteem due to PCOS? | 0.77 | |
| Aggressiveness due to PCOS? | 0.78 | |
| Pessimistic about the treatment? | 0.55 | |
| Lack of control of emotions? | 0.74 | |
| Easily tired? | 0.61 | |
| Self-body image domain | 13.60 | |
| Embarrassment due to your appearance? | 0.65 | |
| Different to normal females? | 0.88 | |
| Lack of satisfaction with your role as a wife? | 0.80 | |
| Lack of satisfaction with being a woman? | 0.58 | |
| Fertility domain | 10.89 | |
| Trouble getting pregnant? | 0.85 | |
| Sad seeing children? | 0.93 | |
| Sad seeing pregnant women? | 0.93 | |
| Felt you will accept all other PCOS manifestations if assured of pregnancy? | 0.91 | |
| Fear of abortion? | 0.91 | |
| Concerned about infertility in the future? | 0.85 | |
| Fear of divorce or separation? | 0.80 | |
| Uselessness of sexual intercourse due to infertility? | 0.88 | |
| Concerned about the long term effects of PCOS medication? | 0.84 | |
| Unsatisfied with sex? | 0.83 | |
| Lack of sexual stimulation? | 0.77 | |
| Sexual function domain | 10.14 | |
| Lack of sexual desire? | 0.81 | |
| Lack of lubrication during sexual intercourse? | 0.66 | |
| Lack of orgasm? | 0.83 | |
| Ashamed of sexual coldness/unresponsiveness? | 0.75 | |
| Loss of libido because of PCOS? | 0.75 | |
| Obesity and menstrual disorder domain | 8.50 | |
| Did you feel being obese? | 0.87 | |
| Concerned about being overweight? | 0.96 | |
| Concerned about a fast return to your previous weight after any weight loss? | 0.72 | |
| The need to decrease your weight to control PCOS status? | 0.79 | |
| Concerned about the complete cessation of menstruation? | 0.59 | |
| Concerned about menstruation at long intervals? | 0.49 | |
| Fear of diseases such as diabetes, hypertension and heart disease? | 0.49 | |
| Fear of cancer? | 0.44 | |
| Hirsutism domain | 5.26 | |
| Sad to see hair in the mirror? | 0.87 | |
| Concerned about the progression of excess hair? | 0.82 | |
| Embarrassed because of excess facial hair? | 0.84 | |
| Concerned about having excess body hair? | 0.63 | |
| Concerned about rapid re-growth of unwanted hair after its removal? | 0.70 | |
| The need to cover your body and face because of excess hair? | 0.70 | |
| Total | 66.29 |
Confirmatory Factor Analysis of PCOSQ-43
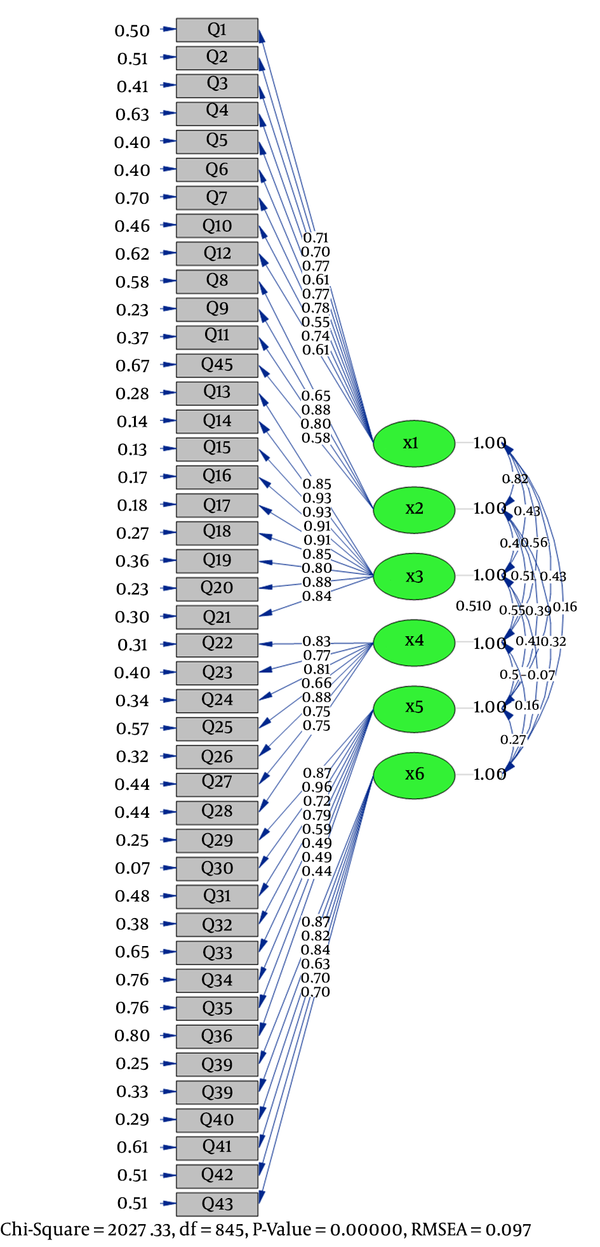
The result of CFA for the 6 factors was a 43-item model with an acceptable fit for the proposed model as root mean square error of approximation (RMSEA) = 0.09, normal fit index (NFI) = 0.90, comparative fit index (CFI) = 0.91, incremental fit index (IFI) = 0.91, goodness-of-fit index (GFI) = 0.60, and standardized root mean square residual (SRMR) = 0.09 (Table 3).
The Correlation Matrix of Subscales and Items of PCOSQ-43
| Subscale | Psychosocial and Emotional | Self- Image | Fertility | Sexual Function | Obesity and Menstrual Disorder | Hirsutism |
|---|---|---|---|---|---|---|
| Psychosocial and emotional | 1 | 0.82 | 0.43 | 056 | 0.43 | 0.16 |
| Self-body image | 1 | 0.40 | 0.53 | 0.39 | 0.32 | |
| Fertility | 1 | 0.54 | 0.47 | 0.07 | ||
| Sexual function | 1 | 0.52 | 0.16 | |||
| Obesity and menstrual disorder | 1 | 0.27 | ||||
| Hirsutism | 1 |
The subjects were asked to score each item over the past 4 weeks based on a 5-option Likert scale (always, often, sometimes, rarely, never) ranging 1: always (worst condition) to 5: never (best condition). The final score ranged 43 to 215 demonstrating the lowest to highest QoL. The range for various subscales depended on its number of items. The average score for each domain was calculated by dividing the sum scores of answered items to the total number of the answered items. The missing items were not included to calculate the average score of each domain. The subjects were requested to fill out the Farsi version of the questionnaire.
In addition, the results of correlation analysis showed that the item scores were correlated with own scale scores rather than other scales lending support to its appropriate convergent validity (Table 4).
The Fit Indicators of the Revised Model (PCOSQ-43)
| Indicator | Level |
|---|---|
| Chi-squared P value (χ2) | > 0.05 |
| RMSEA | 0.09 |
| NFI | 0.90 |
| CFI | 0.91 |
| GFI | 0.60 |
| SRMR | 0.09 |
4.3. Reliability
After the exploratory factor analysis, the internal consistency of the instrument measured by the Cronbach’s alpha and the ICC was evaluated. The stability was assessed through the test-retest analysis; the Spearman-Brown coefficient was 0.70. The ICC for the PCOSQ-43 subscales ranged 0.91 to 0.94. The Cronbach’s alpha coefficient for the whole scale was 0.92 indicating appropriate reliability (Table 5). After applying the modifications, the coping subscale of the PCOSQ-50 was replaced by the self-body image subscale.
Descriptive Statistics and Reliability Measures of the PCOSQ-43
| Subscale | Item, No. | Mean ± SD | Cronbach’s Alpha | ICC (95% CI) | Spearman -Brown Coefficient | P Value |
|---|---|---|---|---|---|---|
| Psychosocial and emotional | 9 | 3.35 ± 0.90 | 0.89 | 0.89 (0.86 - 0.91) | 0.85 | < 0.0001 |
| Self-body image | 4 | 3.10 ± 0.70 | 0.82 | 0.81(0.76 - 0.86) | 0.78 | |
| Fertility | 9 | 3.28 ± 1.51 | 0.96 | 0.96 (0.95 - 0.97) | 0.86 | < 0.0001 |
| Sexual function | 7 | 3.70 ± 0.85 | 0.90 | 0.90 (0.87 - 0.92) | 0.92 | < 0.0001 |
| Obesity and menstrual disorders | 8 | 2.69 ± 1.10 | 0.87 | 0.87 (0.84 - 0.90) | 0.70 | < 0.0001 |
| Hirsutism | 6 | 2.04 ± 0.96 | 0.89 | 0.89 (0.86 - 0.94) | 0.83 | < 0.0001 |
| Total | 43 | 2.94 ± 0.64 | 0.92 | 0.93 (0.91 - 0.94) | 0.70 | < 0.0001 |
5. Discussion
Results of the current study demonstrated that the 43-item PCOSQ can precisely assess the QoL of females with PCO. The newly developed questionnaire had appropriate validity and reliability and its psychometric quality was superior to that of the original version.
The PCOSQ-50 was designed based on a previous article including 6 factors to measure HRQoL in females with PCOS (16). It enabled the authors to assess some aspects of this syndrome overlooked by previous HRQoL questionnaires. Further studies on this instrument are suggested to establish its validity and reliability.
The Cronbach’s alpha and ICC for the coping subscale of the PCOSQ-50 were reported as 0.61 and 0.5, respectively, which may be considered a limitation to the PCOSQ-50. The current study used EFA and CFA to assess the validity of the factor structure of the PCOSQ-50 with 350 Iranian females with PCOS. The CFA led to dropping 7 items with low factor loadings.
The EFA demonstrated that the subscale of the coping factor was omitted and replaced by the body image factor. The correlation between the questions in each area and the total questions was assessed by the calculation of the Cronbach’s alpha coefficient. Cronbach alpha was a measure of the internal consistency of the scale expressed as a number from 0 to 1. Internal consistency described the extent to which all items measured the same concept or construct and was connected to the inter-relatedness of the items within the test (23). For the PCOSQ-43, ICC for the 6-factor solution ranged 0.91 to 0.94 with 0.93 for the whole scale. Compared to the 50-item model, the 43-item model showed a marked improvement in reliability. The PCOSQ-43 had an acceptable internal consistency and reliability.
It was evident that females with PCOS more likely had psychosocial and emotional disorders, compared with the ones with a similar body mass index (BMI) and without PCOS. Obesity, hirsutism, acne, hyperandrogenism, insulin resistance, irregularity of menstrual cycle, and infertility had psychological impacts on the study subjects (24-30). Mansson M., concluded that PCOS was associated with extensive psychological morbidity, and depression was the most common psychological condition associated with PCOS (31).
The PCOSQ-43 included the self-body image subscale ignored by previous questionnaires. Several studies reported that females with PCOS had a worse body image than the ones without PCOS (32-37).
Hyperandrogenism is manifested by hirsutism, acne, and male pattern balding. In rare cases, increased muscle mass, deepening the voice or clitoromegaly may occur, especially if the disease has a rapid onset, which requires the assessment of adrenal or ovarian androgen-secreting tumors (38). Clinical hyperandrogenism such as hirsutism, acne, and alopecia were associated with poorer satisfaction from the body (39).
Some aspects of PCOS can potentially cause considerable sexual dysfunction. A poor body image has a documented adverse consequence on females` sexual satisfaction (40). Females with PCOS may exhibit high levels of body image dissatisfaction contributing to self-consciousness (41) and depression (33).
Infertility is associated with a significant psychosocial change including personal distress, depression, anxiety, and reduced self-esteem. Therefore, it can have a negative impact on sexual function (42). Changes in physical appearance are associated with PCOS and may lead to decreased sexual satisfaction (19).
PCOS is identified as a chronic illness with a number of potential negative effects on the females’ lives (43). Given the chronic process of PCOS, females may experience marital distress compared with the ones without PCOS. The increased rates of martial distress and physical factors (e g, pelvic pain) may further impact females’ sexual relationships (43). Hahn et al., found that females with PCOS expressed less sexual satisfaction compared with the ones without PCOS. Specifically, they reported a greater impact of hirsutism on sexuality and the feeling of being less sexually attractive compared with the ones without PCOS (14). Overweight or obesity is generally the most important cause of distress in females with PCOS (14, 43, 44). Although obesity and menstrual disorders appear to be different, the EFA puts them as a single item, which might partly be explained by the strong link between menstrual problems and obesity (18).
A reliable tool is required to increase the study power and detect differences and significant relationships in studies (45). As the strengths of the current study, the sample size was greater than most relevant studies on this topic (46). Also, subjects of the current study were selected from females with PCO and different socioeconomic levels in order to provide a reliable and comprehensive evaluation of such females using PCOSQ-43.
As the limitation of the current study, a convenience sample was selected out of the gynecology, endocrinology, and dermatology clinic clients and the results cannot be generalized to the total population of females with PCO. Also, it was not possible to measure serum androgen among the subjects. Finally, the 43-item PCOSQ was psychometrically superior to the original version. However, its predictive efficacy should be examined in longitudinal studies. The PCOSQ-43 is useful for research, but additional measurements are required to determine the extent to which this instrument is suitable for use in diverse populations and in clinical settings.
Acknowledgements
References
-
1.
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745-9. [PubMed ID: 15181052]. https://doi.org/10.1210/jc.2003-032046.
-
2.
March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544-51. [PubMed ID: 19910321]. https://doi.org/10.1093/humrep/dep399.
-
3.
Azziz R. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome: the Rotterdam criteria are premature. J Clin Endocrinol Metab. 2006;91(3):781-5. [PubMed ID: 16418211]. https://doi.org/10.1210/jc.2005-2153.
-
4.
Tehrani FR, Simbar M, Tohidi M, Hosseinpanah F, Azizi F. The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Reprod Biol Endocrinol. 2011;9:39. [PubMed ID: 21435276]. https://doi.org/10.1186/1477-7827-9-39.
-
5.
Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28-38 e25. [PubMed ID: 22153789]. https://doi.org/10.1016/j.fertnstert.2011.09.024.
-
6.
DuRant EM, Leslie NS, Critch EA. Managing polycystic ovary syndrome: a cognitive behavioral strategy. Nurs Womens Health. 2009;13(4):292-300. [PubMed ID: 19686552]. https://doi.org/10.1111/j.1751-486X.2009.01439.x.
-
7.
Kitzinger C, Willmott J. 'The thief of womanhood': women's experience of polycystic ovarian syndrome. Soc Sci Med. 2002;54(3):349-61. [PubMed ID: 11824912]. https://doi.org/10.1016/S0277-9536(01)00034-X.
-
8.
Snyder BS. The lived experience of women diagnosed with polycystic ovary syndrome. J Obstet Gynecol Neonatal Nurs. 2006;35(3):385-92. [PubMed ID: 16700688]. https://doi.org/10.1111/j.1552-6909.2006.00047.x.
-
9.
Solomon CG. The epidemiology of polycystic ovary syndrome. Prevalence and associated disease risks. Endocrinol Metab Clin North Am. 1999;28(2):247-63. [PubMed ID: 10352918]. https://doi.org/10.1016/S0889-8529(05)70069-4.
-
10.
Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab. 1999;84(6):1897-9. [PubMed ID: 10372683]. https://doi.org/10.1210/jcem.84.6.5803.
-
11.
Rotterdam EAPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19-25. [PubMed ID: 14711538]. https://doi.org/10.1016/j.fertnstert.2003.10.004.
-
12.
Ching HL, Burke V, Stuckey BG. Quality of life and psychological morbidity in women with polycystic ovary syndrome: body mass index, age and the provision of patient information are significant modifiers. Clin Endocrinol (Oxf). 2007;66(3):373-9. [PubMed ID: 17302871]. https://doi.org/10.1111/j.1365-2265.2007.02742.x.
-
13.
Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622-9. [PubMed ID: 8452328]. https://doi.org/10.7326/0003-4819-118-8-199304150-00009.
-
14.
Hahn S, Janssen OE, Tan S, Pleger K, Mann K, Schedlowski M, et al. Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. Eur J Endocrinol. 2005;153(6):853-60. [PubMed ID: 16322391]. https://doi.org/10.1530/eje.1.02024.
-
15.
Nasiri Amiri F, Ramezani Tehrani F, Simbar M, Montazeri A, Mohammadpour Thamtan RA. The experience of women affected by polycystic ovary syndrome: a qualitative study from iran. Int J Endocrinol Metab. 2014;12(2). e13612. [PubMed ID: 24829583]. https://doi.org/10.5812/ijem.13612.
-
16.
Nasiri-Amiri F, Ramezani Tehrani F, Simbar M, Montazeri A, Mohammadpour RA. Health-related quality of life questionnaire for polycystic ovary syndrome (PCOSQ-50): development and psychometric properties. Qual Life Res. 2016;25(7):1791-801. [PubMed ID: 26814480]. https://doi.org/10.1007/s11136-016-1232-7.
-
17.
Comrey AL, Lee HB. A first course in factor analysis. 2 ed. Hillsdale, N.J: L. Erlbaum Associates; 1992.
-
18.
Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440-7. [PubMed ID: 13892577]. https://doi.org/10.1210/jcem-21-11-1440.
-
19.
Hayashi N, Akamatsu H, Kawashima M, Acne Study G. Establishment of grading criteria for acne severity. J Dermatol. 2008;35(5):255-60. [PubMed ID: 18477223]. https://doi.org/10.1111/j.1346-8138.2008.00462.x.
-
20.
Bollen KA. Structural equation models. Encyclopedia of biostatistics. 1998.
-
21.
Fidell LS, Tabachnick BG. Using multivariate statistics. New York Harper and Row; 2006.
-
22.
Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1-55. https://doi.org/10.1080/10705519909540118.
-
23.
Tavakol M, Dennick R. Making sense of Cronbach's alpha. Int J Med Educ. 2011;2:53-5. [PubMed ID: 28029643]. https://doi.org/10.5116/ijme.4dfb.8dfd.
-
24.
Thomson RL, Buckley JD, Lim SS, Noakes M, Clifton PM, Norman RJ, et al. Lifestyle management improves quality of life and depression in overweight and obese women with polycystic ovary syndrome. Fertil Steril. 2010;94(5):1812-6. [PubMed ID: 20004371]. https://doi.org/10.1016/j.fertnstert.2009.11.001.
-
25.
Deeks AA, Gibson-Helm ME, Teede HJ. Anxiety and depression in polycystic ovary syndrome: a comprehensive investigation. Fertil Steril. 2010;93(7):2421-3. [PubMed ID: 20117778]. https://doi.org/10.1016/j.fertnstert.2009.09.018.
-
26.
Bhattacharya SM, Jha A. Prevalence and risk of depressive disorders in women with polycystic ovary syndrome (PCOS). Fertil Steril. 2010;94(1):357-9. [PubMed ID: 19896652]. https://doi.org/10.1016/j.fertnstert.2009.09.025.
-
27.
Rassi A, Veras AB, dos Reis M, Pastore DL, Bruno LM, Bruno RV, et al. Prevalence of psychiatric disorders in patients with polycystic ovary syndrome. Compr Psychiatry. 2010;51(6):599-602. [PubMed ID: 20965306]. https://doi.org/10.1016/j.comppsych.2010.02.009.
-
28.
Barry JA, Kuczmierczyk AR, Hardiman PJ. Anxiety and depression in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2011;26(9):2442-51. [PubMed ID: 21725075]. https://doi.org/10.1093/humrep/der197.
-
29.
Dokras A, Clifton S, Futterweit W, Wild R. Increased prevalence of anxiety symptoms in women with polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2012;97(1):225-30 e2. [PubMed ID: 22127370]. https://doi.org/10.1016/j.fertnstert.2011.10.022.
-
30.
Dokras A, Clifton S, Futterweit W, Wild R. Increased risk for abnormal depression scores in women with polycystic ovary syndrome: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(1):145-52. [PubMed ID: 21173657]. https://doi.org/10.1097/AOG.0b013e318202b0a4.
-
31.
Mansson M, Holte J, Landin-Wilhelmsen K, Dahlgren E, Johansson A, Landen M. Women with polycystic ovary syndrome are often depressed or anxious--a case control study. Psychoneuroendocrinology. 2008;33(8):1132-8. [PubMed ID: 18672334]. https://doi.org/10.1016/j.psyneuen.2008.06.003.
-
32.
Weiner CL, Primeau M, Ehrmann DA. Androgens and mood dysfunction in women: comparison of women with polycystic ovarian syndrome to healthy controls. Psychosom Med. 2004;66(3):356-62. [PubMed ID: 15184695].
-
33.
Himelein MJ, Thatcher SS. Depression and body image among women with polycystic ovary syndrome. J Health Psychol. 2006;11(4):613-25. [PubMed ID: 16769740]. https://doi.org/10.1177/1359105306065021.
-
34.
Keegan A, Liao LM, Boyle M. 'Hirsutism': a psychological analysis. J Health Psychol. 2003;8(3):327-45. [PubMed ID: 14670212]. https://doi.org/10.1177/13591053030083004.
-
35.
McCook JG, Reame NE, Thatcher SS. Health-related quality of life issues in women with polycystic ovary syndrome. J Obstet Gynecol Neonatal Nurs. 2005;34(1):12-20. [PubMed ID: 15673641]. https://doi.org/10.1177/0884217504272945.
-
36.
Bazarganipour F, Ziaei S, Montazeri A, Foroozanfard F, Kazemnejad A, Faghihzadeh S. Body image satisfaction and self-esteem status among the patients with polycystic ovary syndrome. Iran J Reprod Med. 2013;11(10):829-36. [PubMed ID: 24639704].
-
37.
Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3(3):141-6. [PubMed ID: 12164465]. https://doi.org/10.1046/j.1467-789X.2002.00065.x.
-
38.
Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. [PubMed ID: 20591140]. https://doi.org/10.1186/1741-7015-8-41.
-
39.
de Niet JE, de Koning CM, Pastoor H, Duivenvoorden HJ, Valkenburg O, Ramakers MJ, et al. Psychological well-being and sexarche in women with polycystic ovary syndrome. Hum Reprod. 2010;25(6):1497-503. [PubMed ID: 20356900]. https://doi.org/10.1093/humrep/deq068.
-
40.
Nobre PJ, Pinto-Gouveia J. Cognitive and emotional predictors of female sexual dysfunctions: preliminary findings. J Sex Marital Ther. 2008;34(4):325-42. [PubMed ID: 18576234]. https://doi.org/10.1080/00926230802096358.
-
41.
Liao LM, Nesic J, Chadwick PM, Brooke-Wavell K, Prelevic GM. Exercise and body image distress in overweight and obese women with polycystic ovary syndrome: a pilot investigation. Gynecol Endocrinol. 2008;24(10):555-61. [PubMed ID: 19012098]. https://doi.org/10.1080/09513590802288226.
-
42.
Millheiser LS, Helmer AE, Quintero RB, Westphal LM, Milki AA, Lathi RB. Is infertility a risk factor for female sexual dysfunction? A case-control study. Fertil Steril. 2010;94(6):2022-5. [PubMed ID: 20206929]. https://doi.org/10.1016/j.fertnstert.2010.01.037.
-
43.
Coffey S, Bano G, Mason HD. Health-related quality of life in women with polycystic ovary syndrome: a comparison with the general population using the Polycystic Ovary Syndrome Questionnaire (PCOSQ) and the Short Form-36 (SF-36). Gynecol Endocrinol. 2006;22(2):80-6. [PubMed ID: 16603432]. https://doi.org/10.1080/09513590600604541.
-
44.
Dokras A, Sarwer DB, Allison KC, Milman L, Kris-Etherton PM, Kunselman AR, et al. Weight Loss and Lowering Androgens Predict Improvements in Health-Related Quality of Life in Women With PCOS. J Clin Endocrinol Metab. 2016;101(8):2966-74. [PubMed ID: 27253669]. https://doi.org/10.1210/jc.2016-1896.
-
45.
Burns N, Grove SK. The Practice of Nursing Research; Conduct, Critique and Utilization. 5thed. Philadelphia; 2005.
-
46.
Elsenbruch S, Hahn S, Kowalsky D, Offner AH, Schedlowski M, Mann K, et al. Quality of life, psychosocial well-being, and sexual satisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(12):5801-7. [PubMed ID: 14671172]. https://doi.org/10.1210/jc.2003-030562.