Abstract
Context:
Reproductive domains of the Tehran lipid and glucose study (TLGS) are unique in that they provide reliable information on reproduction of an urban population of West Asia. The aim of this review is to present the most important reproductive findings of TLGS.Evidence Acquisition:
This review is summarizing all articles published in the context of reproductive aspects of TLGS results over the 20-year follow-up. A comprehensive databases search was conducted in PubMed (including Medline), Web of Science and Scopus for retrieving articles on the reproductive histories in context of the TLGS.Results:
The mean (SD) age at menarche and menopause was 13 (1.2) and 49.6 (4.5) years respectively. While pills were the most commonly used modern methods at the initiation of TLGS, the prevalence of condoms rose sharply and significantly over the follow up duration. Among women with history of gestational diabetes, the risk of diabetes and dyslipidemia progression were 2.44 and 1.2 fold higher than others. Prevalences of PCOS and idiopathic hirsutism among reproductive age participants of TLGS were 8.5% (95% CI: 6.8% - 10.2%) and 13.0% (95% CI: 10.9% - 15.1%), respectively. Trend of cardio-metabolic risk factors among women with PCOS showed that there were no statistically significant differences between mean changes of each cardio metabolic variables between PCOS and healthy women; PCOS status also significantly associated with increased hazard of diabetes and prediabetes among women aged younger than 40 years (HR: 4.9; 95% CI: 2.5 - 9.3, P value < 0.001)) and (HR: 1.7; 95% CI: 1.1 - 2.6), P value < 0.005), respectively.Conclusions:
The population based nature of TLGS provides a unique opportunity for valid assessment of reproductive issues, the results of which could provide new information for modification of existing guidelines.Keywords
1. Context
There are a limited number of cohort studies that provided the population based evidences on reproductive histories of both male and females in reproductive life span and research in this area has been clearly insufficient. Reproductive disturbances and their consequences are mainly defined according to data derived from clinical settings or case control studies that suffer from many shortcomings. For instance, the current definition for polycystic ovary syndrome (PCOS) seems to be premature and may have unwanted negative influence on research, clinical practice, and quality of life. As a result societies on reproductive endocrinology endorse the immediate and considerable need for future research into PCOS, its phenotypes and morbidities in population settings (1).
Data collected on reproductive domains in Tehran lipid and glucose study (TLGS) is one of the unique data that provide reliable information on reproduction of an urban population of West Asia. Using valid tools for assessment of various aspects of reproductive history including structured questionnaire, comprehensive physical exam, thoughtful checking of previous medical history and hospital records and universal biochemical and hormonal assessment, provide a valuable data set for investigating some gaps in knowledge in the context of a population-based cohort. Menarcheal and menopausal age and their influencing factors, contraceptive behaviors of participants and its trend, infertility, pregnancy complications and androgen excess disorders are some topics that been reported in this paper.
2. Evidence Acquisition
This review was summarizes all articles published in the context of reproductive aspects of TLGS over the 20-year follow-up. For this purpose, a comprehensive dataset search was conducted in PubMed [including Medline], Web of Science and Scopus for retrieving papers on the reproductive histories in context of the TLGS. The following MeSH keywords were used for the search: “menarche” OR “menopause” OR “contraception” OR “diabetes, dyslipidemia, hypertension progression after pregnancy” OR “infertility” OR “androgen excess disorders” OR “polycystic ovary syndrome” OR “hirsutism” OR “menstrual cycle irregularity” OR “cardio-metabolic disturbance” AND “Tehran lipid and glucose study”.
In TLGS a comprehensive questionnaires on reproductive lifespan including menarche, menopause, menstrual regularity, parity, abortion, type and duration of contraception usage, infertility, and lactation were collected through face to face interviews by trained staff. Pregnancy complications were assessed based on self-reports using the standard definition for each complications. Androgen excess manifestation were evaluated using valid tools. The hirsutism scores were evaluated using the modified Ferriman-Gallwey (mFG) scoring scale. Acne was assessed based on its type, number and distribution. Blood samples of participants were collected at each visit. Serum concentrations of biochemical parameters were measured immediately and rest of the sera was stored at -80°C for future assessments. For purpose of hormonal assessments, fasting venous blood sampling were collected on the third day of spontaneous or progesterone withdrawal menstrual period. Transvaginal ultrasound scans of the ovaries was performed for non-virgin participants by an experienced specialist in the same day as the blood samples were obtained.
3. Results
3.1. Menarcheal Age; Trend and Its Influencing Factors
The first menstruation or menarche, the latest event in female’s puberty, is a landmark in the reproductive life span of a woman, which is influenced by various genetic and environmental factors, including race, socio-economic status of family, geographic region, nutritional status, physical activity and body mass index (BMI), psychological factors and smoking (2, 3). The TLGS study has 6 follow-up phases at approximately 3 year intervals. Age at menarche was assessed in the second phase of study, using face to face interview, assessments that were repeated in each phase of TLGS; mean (SD) age at menarche in TLGS participants was 13 (1.2) years (3); there was a negative secular trend resulting a reduction in age at menarche from 13.8 years to 12.9 years in women born between 1930 - 1990. This negative secular trend was associated with a positive trend in mean of height. In this respect, while the mean age at menarche reduced by 0.15 years per decade; mean height elevated by 0.99 cm per decade (4). Contradictory data have been documented on the secular trend of age at menarche in different societies; while studies from Denmark and the Netherlands show no any decrease in age at menarche since 1960 (5, 6), studies from Brazil, the USA, South China, Mexico and Korea reported a significant negative trend in the age of puberty and menarche (7-9).
Among the influencing factors, data collected in TLGS revealed that maternal education, and maternal age at menarche, pre-menarcheal BMI, pre-menarcheal nutrition affect menarcheal age; the risk of earlier menarche was increased in participants with higher milk consumption [OR: 2.28; 95% CI: 1.03 - 5.05], Calcium [OR:3.20; 95% CI: 1.39 - 7.42], magnesium [OR: 2.43; 95% CI: 1.12 - 5.27] and phosphor [OR: 3.37; 95 % CI: 1.44 - 7.87) after adjusting for energy and protein consumption and maternal age at menarche (10). There was no significant correlation between age at menarche and mother’s job, physical activity, lipid profiles and calorie intake during pre-menarcheal years (2). Among all of these factors, premenarcheal BMI is modifiable and may prevent early or late menarche (3).
Timing of menarche has several potential health implications during women’s life. In this respect, early menarche is associated with breast cancer (11), T2 diabetes mellitus (MD) (12), metabolic syndrome (MetS), cardiovascular events (CVD) and all-cause mortality (13). The results of TLGS showed that early menarche (< 11 years) was related to increased risk of DM and pre-DM, compared to the control group (13 - 14 years); (OR = 3.55; 95% CI: 1.6 - 7.8 and OR = 2.55; 95% CI: 1.4 - 4.8), respectively. This added risk remained unchanged after adjustment for other potential confounders including family history of DM, parity, educational level, anthropometric characteristics of age, BMI and waist circumference (WC), smoking status, physical activity, and duration of hormonal use (14).
3.2. Menopause and Its Cardio-Metabolic Consequences and Prediction of Menopausal Age
Menopause is an important milestone in the reproductive life of women. There is remarkable variation in the age at menopause. Age at menopause is naturally influenced by a variety of racial, environmental, genetic, behavioral and physiological factors. In TLGS, menopause was defined as the time of cessation of menstrual cycle for 12 consecutive-months, not due to vasectomy or any other biological or physiological factors; this information was collected from the first phase of study through direct interviews, and again in each further phase of TLGS.
Mean age at menopause of TLGS participants was 49.6 (4.5) years (15). There was a significant correlation between age at menopause, age at menarche, smoking history, and parity; it was lower among ever smoker, but increased with increasing age of menarche, and parity. However, we did not found any association between age at menopause and other demographic, anthropometric or reproductive characteristics of participants during reproductive period. In agreement with our findings, Mohammad et al. were reported the similar menopausal age in a national survey (mean 50.4, median 49.6 years). There was a positive secular trend (16); means for menopausal age of women born in the 1930s, 1940s, and 1950s were 48.5, 49.5 and 49.9 years respectively, a trend that remained even after adjusting for possible confounding factors. Worldwide data on secular trends of menopausal age are inconsistent, although they increased by approximately one year in Finland, Sweden and the USA in women born after the 1930s (17, 18), these findings differ to those of other studies conducted on 3944 white women (19) and a systematic review of observational studies (20).
Menopausal age is directly associated with the number of remaining both ovarian, indicating that serum concentration of anti mullerian hormone (AMH) can be considered a predictor for estimating age at menopause. The ongoing status of TLGS and the availability of frozen base line serum enables us to use statistical modeling for prediction of age of menopause using AMH serum concentration (21-23). Accurate estimation of menopausal age could identify those at higher risk of CVD, osteoporosis, and all cancers related to early or late menopause. Moreover, the estimation of the age at menopause is an extremely important information for women who plan to delay their childbearing. Identification of women who reached menopause during the study follow ups gives us the opportunity to check the reliability of our models. In the Bland and Altman method, the median difference between actual and predicted age at menopause was 0.51 years (SD = 2.45, range, -3 5.4 to 9.2 years) showing good agreement between the two. Additionally the graphical representation of menopause-free survival, using the Kaplan Meier plot showed similarity of survival experiences when actual and predicted ages at menopause were compared (Figure 1).Furthermore, collaboration with the Department of Reproductive Medicine of the University Medical Centre Utrecht, UMCU, Netherlands enabled us to assess the external validity of our model.
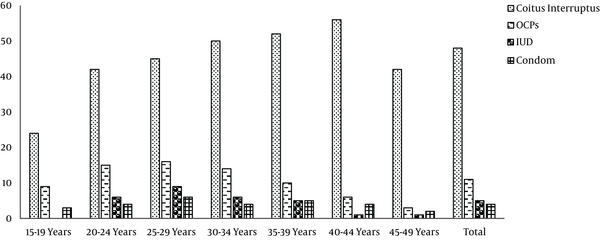
Percentages of family planning methods used by couples based on age groups in the first phase of the Tehran Lipid and Glucose
Reproductive aged women with various follicular reserve statuses may have different risk for cardiovascular events; to assess this assumption, we calculated age-specific anti-Müllerian hormone for individual reproductive age participant of the TLGS to consider the silent coronary artery disease (CAD) among women with various ovarian reserve statuses. We founded that anti-Müllerian hormone was not a marker for silent CAD.
Menopause was associated with increasing the risk of cardio-metabolic disturbances including decreasing high density lipoprotein cholesterol (HDL-C) and increasing diastolic blood pressure (DBP), low density lipoprotein cholesterol (LDL-C), triglycerides (TG), total cholesterol (24), fasting plasma glucose (FPG) and waist circumference (WC). The prevalence of metabolic syndrome during menopausal transition in TLGS was 53%, 54%, and 69% in pre-menopause, menopause, and postmenopause respectively (25, 26). The risk of diabetes and hypertension was increased in postmenopausal women (27). Despite elevated serum levels of TC and LDL-C after menopause in TLGS (26), there was no increase in numbers of cardiovascular events, possibly due to the lack of adequate years of follow up of cohort members.
The cardio-metabolic consequences of surgical menopause was compared with natural menopause in the TLGS and results revealed that metabolic disturbances were more common among menopausal women undergoing vasectomy compared to natural menopause (46.2 vs. 20.5 % respectively, P value < 0.01) (28).
In TLGS we found that menopausal status per se is related to elevated serum concentration of nitric oxide. Nitric oxide plays an important role in the protection from cardiovascular disease and its progression (29, 30).
3.3. Trend of Contraception Behavior
Contraception methods of couples have an important influence on the women health; improving the autonomy of women in modern societies, substantially influencing gender equality, increasing women’s empowerment and reduces the poverty and mortality (31). During the previous decades, Iranian population experienced the largest and fastest fertility decline around the world. Iran’s traditionally pronatalist culture provides a favorable environment for high fertility. The 1986 national census showed a more than 3 percent annual population growth rate (32). The antinatalist policy and socio-political changes toward low fertility in Iran was officially initiated in 1988 (33). In this respect, Statistical Center of Iran in 1998 (same year as TLGS initiation) confirmed the signs of below-replacement fertility in Iran after the Population Growth Estimation Survey (PGES) in 1998 (same year as TLGS initiation). Despite Iran’s successful family planning programs, Iran has overshot the target. The level of reduction in the total fertility rate to below birth replacement status has become a serious concern for politicians, encouraging the support of the pronatalist policy to increase population growth rates.
In the first phase of TLGS, the use of contraceptive methods during the last three months including OCPs, (LD contraceptive, HD contraceptive, Dian, Minipill and Triphasic pills), intrauterine devices (IUDs), Norplant, condoms, coitus interruptus and other methods were determined by a standard questionnaire of TLGS, during face-to-face interviews. The scientific committee of TLGS proposed the modification of reproduction data including contraception methods after phase 2 (3 years after initiation). In this respect, data on the contraceptive methods of vasectomy, tubal ligation, depo-provera injections and whether or not contraception usage were ontained and documented separately. The ongoing nature of TLGS enables us to assess trends of contraception use in Iran, especially after the recent population policies implementation.
According to the results of the first phase of TLGS, coitus interrupts, COCs, condom and IUD were used by 48, 11,4 and 5% of the individuals, respectively; 32% used no contraception, with no significant differences in contraceptive use behavior among various age groups (Figure 1) (34).
However the contraceptive behaviors of TLGS participants changed overtime; while pills were the most commonly used modern methods at initiation of the TLGS, condom was the most commonly used method in the last phase of study; the proportion of condom use sharply and significantly increased from 10.9% in 2002 to 21.9% in 2011 (Table 1) (35).
Prevalence Percentages of Women by Contraceptive Use, Based on TLGS Phase
| Year | N | Modern, n (%) | Traditionala, n (%) | None, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pill | IUD | Female Sterilization | Male Sterilization | Condom | Injection | Total | ||||
| 2002 | 2506 | 377 (15) | 433 (17.2) | 228 (9) | 214 (8.5) | 275 (10.9) | 14 (0.5) | 1541 (61.4) | 645 (25.7) | 320 (12.7) |
| 2005 | 2529 | 323 (12.7) | 385 (15.2) | 214 (8.4) | 226 (8.9) | 385 (15.2) | 10 (0.3) | 1543 (61) | 778 (30.7) | 208 (8.2) |
| 2008 | 2594 | 248 (9.5) | 323 (12.4) | 181 (6.9) | 216 (8.3) | 519 (20) | 10 (0.3) | 1497 (57.7) | 849 (32.7) | 248 (8) |
| 2011 | 2525 | 153 (6) | 198 (7.8) | 172 (6.8) | 203 (8) | 553 (21.9) | 10 (0.3) | 1289 (51) | 874 (34.6) | 362 (14.3) |
3.4. Oral Contraceptive Usage and Cardio-Metabolic Disturbance
Since contraceptive usage have been showed as a risk factor for vascular thromboembolism and cardio-metabolic disorders, the impact of oral contraceptives on some cardio-metabolic parameters including hypertension, hypercholesterolemia, hypertriglyceridemia, obesity and metabolic syndrome was determined in TLGS.
According to duration of oral contraceptive usage in four sub-groups, i.e. non-users, less than 11 month users, 12 - 35 month users, and ≥ 36 month users; cardio-metabolic parameters did not have statistical significant between these sub-groups, after further adjustment for potential confounders of age, parity and education, except for hypercholesterolemia which was significantly 1.5 times higher among women who used OCPs for more than 36 months compared to others (OR 1.5; 95 % CI: 1.01 - 2.2) showing the cardio-metabolic safety of COPs consumption for less than 3 years in TLGS women (36).
3.5. Infertility
The prevalence and causes of primary infertility were assessed in TLGS population. Infertility was defined as inability to conceive after a 12 months of sexual intercourse, without using any contraception. Primary infertility was defined as the “incapacity to become pregnant within one year of exposure to pregnancy among women aged 15 - 49 years”. The overall prevalence of primary infertility was 17.3%. According to the logistic regression analysis, primary infertility was independently associated with age (OR: 1.3; 95% CI: 1.1 - 13.6, P value = 0.001), BMI (OR: 1.9; 95% CI: 1.8 - 4.1, P value < 0.001), smoking status (OR: 1.4; 95% CI: 1.3 - 3.5, P value = 0.012) and higher educational level (OR: 2.2; 95% CI: 1.1 - 5.5, P value = 0.03). Our prevalence however is lower than those of low-to-middle-income countries (34.1%, 95% CI: 30.3 - 39.3%) (37), and is higher than those of western countries which is approximately 11.5% (95% CI: 10.2, 12.9) (38).
3.6. Incident Diabetes Type 2 and Dyslipidemia in Relation to Previous Gestational Diabetes Mellitus: Contributions of the Tehran Lipid and Glucose Study
Several studies have investigated cardio-metabolic disturbances following GDM. However, a 20-year follow-up evaluation of GDM outcomes is one of the unique contributions provided by TLGS in this field. In the present review, we highlight our findings on T2DM and dyslipidemia incidence and major influencing factors after a history of GDM.
3.6.1. Type 2 Diabetes After Gestational Diabetes
In TLGS, gestational diabetes (GDM) women were assessed based on a self-reporting questionnaire asking about their history of GDM. At initiation of TLGS, GDM was defined based on the World Health Criteria (39) and universal screening was part of routine prenatal care. In TLGS, the median (IQR) for follow-up years of GDM and non-GDM were 12.1 (8.09 - 13.51) and 11.62 (6.2 - 13.1), respectively. The odds of diabetes progression was more that two fold higher in women with prior GDM, compared without history of GDM. The adjusted odds was 2.1 (P value < 0.001, CI: 1.5 - 3).
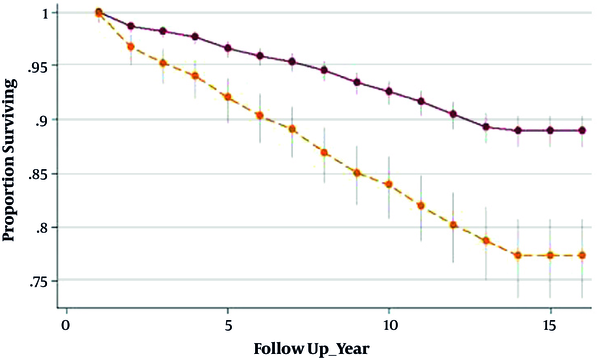
Survivor function indicated significantly different results between GDM and non-GDM women, indicating shorter interval until T2DM progression in GDM-affected women compared with their healthy counterparts, (6.95 years [IQ: 4.22 - 10.71]) vs. (8.45 years [IQ: 5.08 - 10.89]), (P value < 0.001), respectively (Figure 2). The total cumulative incidence rate of diabetes at the median follow-up time was 9/1000 (CI = 5/1000 - 12/1000) and 4/1000 (CI = 1/1000 - 6/1000) in GDM and non-GDM women, respectively.
Survivor function (95% CI) using Kaplan-Meier analysis between GDM and non-GDM groups. Upper line: (non-GDM) non-gestational diabetes mellitus group; Lower line: (GDM) gestational diabetes mellitus group
We found that each one unit increment in BMI raised the odds of T2DM for a minimum of 0.08% and maximum of 15% (OR: 1.13, CI: 1.08 - 1.15). Moreover, of the several predisposing factors, family history of T2DM was associated with 1.7 fold higher risk of emerging diabetes in GDM group (OR: 1.76, CI: 1.24 - 2.5).
3.6.2. Dyslipidemia as a Consequence of Gestational Diabetes
Of a total of 4076 reproductive aged women, with at least one history of pregnancy, 2604 subjects were excluded due to baseline dyslipidemia, and missing or incomplete data. Of the remaining 1472 women, 289 had history of prior GDM and 1183 did not have history of GDM. Median (IQR) for follow-up years in women with and without prior GDM were 7.48 (2.1 - 12.4) and 8 (2.5 - 12.3), respectively. The odds ratio of dyslipidemia development was more than 1.2 fold higher in women with prior GDM. However after adjustment for age, BMI and smoking, the GDM effect was no longer significant (OR = 1.04, CI: (0.87, 1.24, P value > 0.05).
Adjusting for age, BMI and baseline values of lipid indices revealed no significant difference in mean changes of total cholesterol, triglyceride, HDL-c and LDL-c between groups. Data indicated that overtime the trend of lipid parameters changed and the probability of progressing dyslipidemia was not significantly different in women with and without prior history of GDM (Figure 3).
Probability pattern of dyslipidemia incidence within follow-ups. - - -: Non-gestational diabetes mellitus group; ___: Gestational diabetes mellitus group
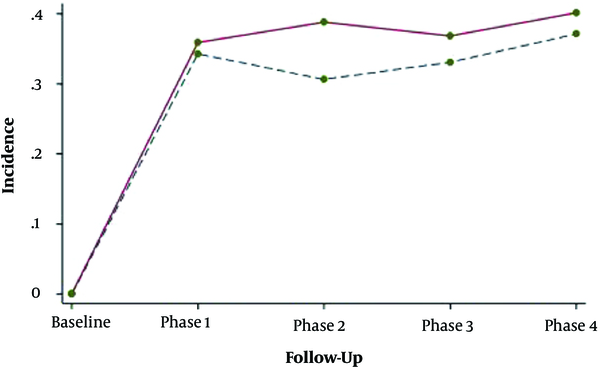
We found that despite the non-significant trend of mean changes between groups, overall lipid profiles of the patients were worse than healthy women within follow-ups. Moreover, the impact of GDM on dyslipidemia disappeared when other covariates were added to the model, which may highlight the major effect of variables like BMI or smoking on lipid dysfunction in comparison with the GDM per se.
3.7. Hypertension After Hypertensive Pregnancy Disorders (HPD)
Hypertensive pregnancy disorders are defined as the onset of hypertension (HTN) with or without proteinuria after 20 weeks’ gestation. In TLGS, hypertensive pregnancy disorders history was evaluated every 3 years based on self-reports through face-to-face interviews, any controversial data was corrected using their medical documents. According to the JNC-VI criteria chronic hypertension was defined as mean systolic blood pressure ≥ 140 mm Hg, mean systolic blood pressure ≥ 90 mm Hg or current use of antihypertensive medication (40). While hypertension and proteinuria associated with preeclampsia generally resolve soon after delivery, several studies have been demonstrated increased risk of future CVD especially HTN in affected women (41). We found that HPD are significantly associated with subsequent hypertension. At the end of a follow up, the cumulative incidences of hypertension in women with prior HPD was significantly higher than their age- and BMI matched controls (41.4 vs 19.5% for HTN). Women with prior of hypertensive disorders, compared to age- and BMI matched controls without HPD had a 2-fold increased risk for hypertension (95% CI: 1.4 - 3.2) (42).
We have also assessed future hypertension incidence and trend of blood pressure changes after 15 years follow-up in women with and without previous preeclampsia (PE). In agreement with findings of previous studies, results of these published data revealed that women with prior PE have a higher rate of hypertension progression compared to the non-PE one; mean changes of blood pressure differed significantly in the preeclampsia group compared without PE, after adjustment for potential variable. These findings could help policy makers and health care providers to manage women with a history of HPD, who are at risk for development of hypertension.
3.8. Androgen Excess Disorders; Insight from TLGS
3.8.1. Hirsutism
The absence of anovulation and/or hyperandrogenemia and the presence of terminal hair in females in a male-like pattern, is considered as idiopathic hirsutism (IH) (43). In TLGS, data regarding hirsutism was collected by a standard questionnaire and hirsutism was evaluated using the modified Ferriman-Gallwey (mFG) scoring and was diagnosed as m FG ≥ 8. The estimated prevalence of IH was 13.0% (95% CI: 10.9% - 15.1%) in participants of TLGS (44). Hirsutism is an important clinical sign of hyperandrogenism. It potentially influences the perception of a woman regarding her femininity and decreasing her self-esteem and leading to a reduction in the quality of life. Hence, we investigated the concordance between the patient’s self assumptions of hirsutism with clinical diagnosis and noted the chin area to be the most concordant site in predicting the women feelings of hirsutism. However, mFG system is still difficult to use in the clinical evaluation. Using population based data collected in the TLGS enables us to simplify mFG from 8 points to 3 (lip, abdomen and thighs); this subset had optimum sensitivity and specificity (91.5% and 92%, respectively) and a positive predictive value (PPV) of 72.2% and concordance percentage (91.9%), in comparison to original mFG score (45).
Results of TLGS study showed that among 12 androgenic sensitive body areas, the chin and skin had the highest area under curve of (0.81; CI; 0.78 - 0.84).
Despite the heavy burden of idiopathic hirsutism and its influence on the quality of life wellbeing of women, and its metabolic and cardiovascular consequences have not yet been clarified; TLGS is the first population based study that provides reliable information on this issue. Our findings demonstrate that the age and BMI adjusted prevalence of metabolic syndrome and insulin resistance (IR) was comparable in women with and without IH (30% vs. 23.9 and 25.7% vs. 22.5%, respectively).
3.8.2. Menstrual Cycle Irregularity and Cardio-Metabolic Disturbance
The menstrual cycles of all reproductive age participants of TLGS were assessed in detail from initiation of the study and re-checked in further phases of the study using structured questionnaires, valuable information which enables us to evaluate the impact of menstrual cycle irregularity on cardio-metabolic disturbances. So, TLGS as a long term prospective study, let us to assess the risk of metabolic disturbances in women with a history of menstrual irregularity.
It is revealed that women with history of menstrual irregularity had an increased risk for diabetes mellitus type 2 (DM2) (age adjusted HR: 2.01; CI: 1.59 - 3.50), which remained significant after the adjustment for BMI, FBS, parity and family history of diabetes (HR, 1.73; 95% CI: 1.14 - 2.64).
In addition, the results showed that menstrual irregularity was associated with prediabetes after adjustment for confounders (HR, 1.33; 95% CI: 1.05 - 1.69) (46).
Irregular menstrual cycles as one of the important components of PCOS, might be related to diabetes and cardiovascular diseases (47). A cohort study showed that the improvement of ovulatory cycles in PCOS patients could reduce in their risk for CVD and MetS (48). As a result our study revealed that a history of irregular menstrual cycles may be considered as a risk factor for development of DM and pre-DM, suggesting a comprehensive screening program and advocating healthy lifestyles in this group of women.
3.8.3. Polycystic Ovary Syndrome (PCOS)
PCOS is a heterogeneous disorder may leade to reproductive and metabolic disturbances. In TLGS, women aged, 15 - 49 years, were precisely screened for PCOS using validated questionnaire covering data on demographic characteristics, and reproductive, obstetrics and gynecological history, with emphasis on regularity of menstrual cycle, hyperandrogenic symptoms, and family history of irregular menstrual cycles, physical exams and biochemical/hormonal assessments. In TLGS, menstrual cycle irregularities due to oligo-ovulation or on-ovulation and either biochemical or clinical hyperandrogenism, were accessed after exclusion of other hyperandrogenism related disorders such as hyperprolactinemia and thyroid or adrenal disorders. As a result the prevalence of PCOS among reproductive age participants of TLGS was 8.5% (95% CI: 6.8% - 10.2%) (44) defined according to NIH criteria.
3.9. PCOS and Cardio-Metabolic Disorders: Insights from the Tehran Lipid and Glucose Study
The association between CVD outcomes and PCOS is still controversial. While some studies showed significant differences in CVD outcomes between PCOS and healthy controls, others resulted that there is no association between them. It is suggested that traditional cardiovascular/metabolic risk factors including insulin resistance, impaired fasting glucose, central obesity, dyslipidemia and hypertension, more common among women with PCOS in the general female population, are related to higher risk of mortality and morbidity (49). A meta-analysis revealed that PCOS patients had higher prevalence of impaired glucose tolerance, diabetes and MetS in both BMI and non-BMI matched studies (50). Nevertheless, most of this evidence was derived from clinical-based settings studies which did not included the milder PCOS phenotypes or their results did not compare with healthy controls with short length of follow up time.
Despite much literature available on prevalence, there are limited studies addressing the incidence of cardio-metabolic risk factors in PCOS population. TLGS, a large prospective population-based study gives us the opportunity to compare the trend as well as incidence and hazard ratio of cardio-metabolic risk factors among this population and healthy controls.
In this respect, trend of cardio-metabolic risk factors showed that there were no statistically significant differences between mean changes of cardio metabolic feature including waist circumference, lipid, blood pressure and sugar profiles. While triglyceride was elevated in the high BMI subgroup of women with PCOS, it reduced over time in both subgroups of non-PCOS women. The overall odd of insulin resistance in women with PCOS was more than threefold (95% CI: 1.3 - 8.9) higher that of the controls. In addition, the odds of IR reduced by 11% (95% CI: 2% - 19%) per year in PCOS, compared with healthy women.
In is concluded that while the insulin level and IR prevalence were higher in reproductive-aged PCOS patients compared to healthy one, the difference of these risk factors dilluted overtime. Thus, we concluded that the metabolic disturbances of women with PCOS in later life may be lower than those initially anticipated (48).
In addition, incidence rate and hazard ratios were estimatyed separately for those ≤ 40 years and > 40 years for diabetes and pre-diabetes, based on extended cox proportional hazards regression model with age scale. Incidence per 1000 person years of diabetes in PCOS and healthy women was (12.9 per 1000 person years, 95% CI (8.4 - 19.7)) and (4.9 per 1000 person years, 95% CI (3.9 - 6.2), respectively. Also, the incidence rate of diabetes per 1000 person years (95% CI) among ≤ 40 years women with PCOS and healthy subjects were 13.4 (8.6 - 20.8) and 4.2 (3.2 - 5.4) respectively.
The incidence per 1000 person years of pre-diabetes in PCOS and healthy women was (29.7 per 1000 person years, 95% CI (21.5 - 41)) and (25.9 per 1000 person years, 95% CI (23.2 - 29) respectively. the incidence rate of diabetes per 1000 person years (95% CI) among ≤ 40 years women with PCOS and healthy subjects were 13.4 (8.6 - 20.8) and 4.2 (3.2 - 5.4) respectively. The incidence rate of pre-DM per 1000 person years (95% CI) among ≤ 40 years women with PCOS and healthy subjects were 29.7 (21.5 - 41) and 25.9 (23.2 - 29), respectively.
The incidence rates of hypertension, metabolic-syndrome, dyslipidemia and obesity were 13.9, 21.0, 46.1, 24.6, 50.6 and 13.8, 22.7, 46.0, 24.0 per 1000 person-years for PCOS and controls, respectively. Women with PCOS, aged ≤ 40 years had an adjusted higher risk of developing hypertension and metabolic-syndrome (HR: 2.08; 95% CI, 1 - 3.9; P < 0.026) and (HR1.81; 95% CI, 1.1 - 2.9; P < 0.019), respectively), a risk which however disappeared after the age of 40 year (in press).
The incidence and risk of cardio-metabolic outcomes were higher than in the general healthy women, although these risks diluted in the late reproductive period; women with PCOS may not be at additional risk for cardio-metabolic outcomes than the non-POCS population, suggesting that in prevention strategies, routine screening for diabetes may not be reinforced for PCOS patients at late reproductive ages if they had not been glucose intolerant during this period.
4. Conclusions
The population based nature of TLGS provides a unique opportunity for valid assessment of reproductive issues, the results of which could provide new information for modification of existing guidelines. Using valid tools for assessment of various aspects of reproductive history including structured questionnaire, comprehensive physical exams, meticulous checking of previous medical history and hospital records and standard validated biochemical and hormonal assessments were the main strengths of study facilitating a reliable results. However, relying on self-reporting for assessment of some aspects of reproductive histories such as contraceptive use and obstetrics characteristics could be a potential limitation in our study.
Acknowledgements
References
-
1.
Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E, et al. American association of clinical endocrinologists, American college of endocrinology, and androgen excess and pcos society disease state clinical review: Guide to the best practices in the evaluation and treatment of polycystic ovary syndrome--part 1. Endocr Pract. 2015;21(11):1291-300. [PubMed ID: 26509855]. https://doi.org/10.4158/EP15748.DSC.
-
2.
Farahmand M, Ramezani Tehrani F, Azizi F. Whether age of menarche is influenced by body mass index and lipoproteins profile? A retrospective study. Iran J Reprod Med. 2012;10(4):337-42. [PubMed ID: 25246895]. [PubMed Central ID: PMC4165951].
-
3.
Ramezani Tehrani F, Mirmiran P, Gholami R, Moslehi N, Azizi F. Factors influencing menarcheal age: Results from the cohort of tehran lipid and glucose study. Int J Endocrinol Metab. 2014;12(3). e16130. [PubMed ID: 25237321]. [PubMed Central ID: PMC4166004]. https://doi.org/10.5812/ijem.16130.
-
4.
Tehrani FR, Mirmiran P, Zahedi-Asl S, Nakhoda K, Azizi F. Menarcheal age of mothers and daughters: Tehran lipid and glucose study. East Mediterr Health J. 2010;16(4):391-5. [PubMed ID: 20795422]. https://doi.org/10.26719/2010.16.4.391.
-
5.
Juul A, Teilmann G, Scheike T, Hertel NT, Holm K, Laursen EM, et al. Pubertal development in Danish children: Comparison of recent European and US data. Int J Androl. 2006;29(1):247-55. discussion 286-90. [PubMed ID: 16466546]. https://doi.org/10.1111/j.1365-2605.2005.00556.x.
-
6.
Mul D, Fredriks AM, van Buuren S, Oostdijk W, Verloove-Vanhorick SP, Wit JM. Pubertal development in the Netherlands 1965-1997. Pediatr Res. 2001;50(4):479-86. [PubMed ID: 11568291]. https://doi.org/10.1203/00006450-200110000-00010.
-
7.
Hwang JY, Shin C, Frongillo EA, Shin KR, Jo I. Secular trend in age at menarche for South Korean women born between 1920 and 1986: The Ansan study. Ann Hum Biol. 2003;30(4):434-42. [PubMed ID: 12881142]. https://doi.org/10.1080/0301446031000111393.
-
8.
Mahachoklertwattana P, Suthutvoravut U, Charoenkiatkul S, Chongviriyaphan N, Rojroongwasinkul N, Thakkinstian A, et al. Earlier onset of pubertal maturation in Thai girls. J Med Assoc Thai. 2002;85 Suppl 4:S1127-34. [PubMed ID: 12549786]. https://doi.org/10.1080/03014460400018077.
-
9.
Malina RM, Pena Reyes ME, Tan SK, Little BB. Secular change in age at menarche in rural Oaxaca, southern Mexico: 1968--2000. Ann Hum Biol. 2004;31(6):634-46. [PubMed ID: 15799231].
-
10.
Ramezani Tehrani F, Moslehi N, Asghari G, Gholami R, Mirmiran P, Azizi F. Intake of dairy products, calcium, magnesium, and phosphorus in childhood and age at menarche in the Tehran lipid and glucose study. PLoS One. 2013;8(2). e57696. [PubMed ID: 23451261]. [PubMed Central ID: PMC3581542]. https://doi.org/10.1371/journal.pone.0057696.
-
11.
Velie EM, Nechuta S, Osuch JR. Lifetime reproductive and anthropometric risk factors for breast cancer in postmenopausal women. Breast Dis. 2005;24:17-35. [PubMed ID: 16917137]. https://doi.org/10.3233/BD-2006-24103.
-
12.
He C, Zhang C, Hunter DJ, Hankinson SE, Buck Louis GM, Hediger ML, et al. Age at menarche and risk of type 2 diabetes: Results from 2 large prospective cohort studies. Am J Epidemiol. 2010;171(3):334-44. [PubMed ID: 20026580]. [PubMed Central ID: PMC2842205]. https://doi.org/10.1093/aje/kwp372.
-
13.
Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94(12):4953-60. [PubMed ID: 19880785]. https://doi.org/10.1210/jc.2009-1789.
-
14.
Farahmand M, Tehrani FR, Dovom MR, Azizi F. Menarcheal age and risk of type 2 diabetes: A community-based cohort study. J Clin Res Pediatr Endocrinol. 2017;9(2):156-62. [PubMed ID: 27840328]. [PubMed Central ID: PMC5463289]. https://doi.org/10.4274/jcrpe.3370.
-
15.
Farahmand M, Tehrani FR, Pourrajabi L, Najafi M, Azizi F. Factors associated with menopausal age in Iranian women: Tehran lipid and glucose study. J Obstet Gynaecol Res. 2013;39(4):836-41. [PubMed ID: 23279558]. https://doi.org/10.1111/j.1447-0756.2012.02050.x.
-
16.
Mohammad K, Sadat Hashemi SM, Farahani FK. Age at natural menopause in Iran. Maturitas. 2004;49(4):321-6. [PubMed ID: 15531128]. https://doi.org/10.1016/j.maturitas.2004.02.006.
-
17.
Rodstrom K, Bengtsson C, Milsom I, Lissner L, Sundh V, Bjourkelund C. Evidence for a secular trend in menopausal age: A population study of women in Gothenburg. Menopause. 2003;10(6):538-43. [PubMed ID: 14627863]. https://doi.org/10.1097/01.GME.0000094395.59028.0F.
-
18.
Mollaei E, Hoseinpour F, Fasihi S, Ziaei T. [Age of menarche and its relationship with some factors in the schoolgirls of Gorgan in 2006]. Sci J Nurs Midwifery Facult Bouye Gorgan. 2010;7(1):48-54. Persian.
-
19.
Pakarinen M, Raitanen J, Kaaja R, Luoto R. Secular trend in the menopausal age in Finland 1997-2007 and correlation with socioeconomic, reproductive and lifestyle factors. Maturitas. 2010;66(4):417-22. [PubMed ID: 20537824]. https://doi.org/10.1016/j.maturitas.2010.04.005.
-
20.
McKinlay SM. The normal menopause transition: An overview. Maturitas. 1996;23(2):137-45. [PubMed ID: 8735352]. https://doi.org/10.1016/0378-5122(95)00985-X.
-
21.
Tehrani FR, Solaymani-Dodaran M, Azizi F. A single test of antimullerian hormone in late reproductive-aged women is a good predictor of menopause. Menopause. 2009;16(4):797-802. [PubMed ID: 19225427]. https://doi.org/10.1097/gme.0b013e318193e95d.
-
22.
Tehrani FR, Solaymani-Dodaran M, Tohidi M, Gohari MR, Azizi F. Modeling age at menopause using serum concentration of anti-mullerian hormone. J Clin Endocrinol Metab. 2013;98(2):729-35. [PubMed ID: 23316087]. https://doi.org/10.1210/jc.2012-3176.
-
23.
van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: A longitudinal study. Fertil Steril. 2005;83(4):979-87. [PubMed ID: 15820810]. https://doi.org/10.1016/j.fertnstert.2004.11.029.
-
24.
Gunderson EP, Chiang V, Pletcher MJ, Jacobs DR, Quesenberry CP, Sidney S, et al. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: The coronary artery risk development in young adults study. J Am Heart Assoc. 2014;3(2). e000490. [PubMed ID: 24622610]. [PubMed Central ID: PMC4187501]. https://doi.org/10.1161/JAHA.113.000490.
-
25.
Ainy E, Mirmiran P, Zahedi Asl S, Azizi F. Prevalence of metabolic syndrome during menopausal transition Tehranian women: Tehran lipid and glucose study (TLGS). Maturitas. 2007;58(2):150-5. [PubMed ID: 17768019]. https://doi.org/10.1016/j.maturitas.2007.07.002.
-
26.
Ramezani Tehrani F, Behboudi-Gandevani S, Ghanbarian A, Azizi F. Effect of menopause on cardiovascular disease and its risk factors: A 9-year follow-up study. Climacteric. 2014;17(2):164-72. [PubMed ID: 23895384]. https://doi.org/10.3109/13697137.2013.828197.
-
27.
Azizi F, Ainy E. Coronary heart disease risk factors and menopause: A study in 1980 Tehranian women, the Tehran lipid and glucose study. Climacteric. 2003;6(4):330-6. [PubMed ID: 15006254]. https://doi.org/10.1080/cmt.6.4.330.336.
-
28.
Farahmand M, Ramezani Tehrani F, Bahri Khomami M, Noroozzadeh M, Azizi F. Surgical menopause versus natural menopause and cardio-metabolic disturbances: A 12-year population-based cohort study. J Endocrinol Invest. 2015;38(7):761-7. [PubMed ID: 25722224]. https://doi.org/10.1007/s40618-015-0253-3.
-
29.
Tehrani FR, Behboudi-Gandevani S, Ghasemi A, Azizi F. Menopause status as the main factor explaining the gender differences of serum nitric oxide concentrations in middle-aged population. Arch Gynecol Obstet. 2015;291(1):159-63. [PubMed ID: 25047269]. https://doi.org/10.1007/s00404-014-3338-x.
-
30.
Ramezani Tehrani F, Behboudi-Gandevani S, Ghasemi A, Azizi F. Association between serum concentrations of nitric oxide and transition to menopause. Acta Obstet Gynecol Scand. 2015;94(7):708-14. [PubMed ID: 25867606]. https://doi.org/10.1111/aogs.12655.
-
31.
Behboudi-Gandevani S, Ziaei S, Khalajabadi-Farahani F, Jasper M. Iranian primigravid women's awareness of the risks associated with delayed childbearing. Eur J Contracept Reprod Health Care. 2013;18(6):460-7. [PubMed ID: 24011122]. https://doi.org/10.3109/13625187.2013.832195.
-
32.
Motlaq ME, Eslami M, Yazdanpanah M, Nakhaee N. Contraceptive use and unmet need for family planning in Iran. Int J Gynaecol Obstet. 2013;121(2):157-61. [PubMed ID: 23473347]. https://doi.org/10.1016/j.ijgo.2012.11.024.
-
33.
Sadat-Hashemi SM, Ghorbani R, Majdabadi HA, Farahani FK. Factors associated with contraceptive use in Tehran, Iran. Eur J Contracept Reprod Health Care. 2007;12(2):148-53. [PubMed ID: 17559013]. https://doi.org/10.1080/13625180601143462.
-
34.
Azizi F, Ainy E, Mirmiran P, Habibian S. Contraceptive methods and risk factors of cardiovascular diseases in Tehranian women: Tehran lipid and glucose study. Eur J Contracept Reprod Health Care. 2002;7(1):1-6. [PubMed ID: 12041858]. https://doi.org/10.1080/713604284.
-
35.
Behboudi-Gandevani S, Ramezani Tehrani F, Cheraghi L, Noroozzadeh M, Farahmand M, Azizi F. Trends of contraception use among married reproductive age women: Tehran lipid and glucose cohort study 2002-2011. Sex Reprod Healthc. 2017;12:116-22. [PubMed ID: 28477923]. https://doi.org/10.1016/j.srhc.2017.04.003.
-
36.
Farahmand M, Ramezani Tehrani F, Rostami Dovom M, Hashemi S, Azizi F. The impact of oral contraceptives on cardiometabolic parameters. J Endocrinol Invest. 2016;39(3):277-83. [PubMed ID: 26223383]. https://doi.org/10.1007/s40618-015-0346-z.
-
37.
Rostami Dovom M, Ramezani Tehrani F, Abedini M, Amirshekari G, Hashemi S, Noroozzadeh M. A population-based study on infertility and its influencing factors in four selected provinces in Iran (2008-2010). Iran J Reprod Med. 2014;12(8):561-6. [PubMed ID: 25408706]. [PubMed Central ID: PMC4233315].
-
38.
Bushnik T, Cook JL, Yuzpe AA, Tough S, Collins J. Estimating the prevalence of infertility in Canada. Hum Reprod. 2012;27(3):738-46. [PubMed ID: 22258658]. [PubMed Central ID: PMC3279129]. https://doi.org/10.1093/humrep/der465.
-
39.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539-53. [PubMed ID: 9686693]. https://doi.org/10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S.
-
40.
Gifford R. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1-S22. [PubMed ID: 10920346]. https://doi.org/10.1067/mob.2000.107928.
-
41.
Sibai BM, el-Nazer A, Gonzalez-Ruiz A. Severe preeclampsia-eclampsia in young primigravid women: Subsequent pregnancy outcome and remote prognosis. Am J Obstet Gynecol. 1986;155(5):1011-6. [PubMed ID: 3777042]. https://doi.org/10.1016/0002-9378(86)90336-4.
-
42.
Hashemi S, Ramezani Tehrani F, Mehrabi Y, Azizi F. Hypertensive pregnancy disorders as a risk factor for future cardiovascular and metabolic disorders (Tehran lipid and glucose study). J Obstet Gynaecol Res. 2013;39(5):891-7. [PubMed ID: 23438323]. https://doi.org/10.1111/j.1447-0756.2012.02069.x.
-
43.
Azziz R, Carmina E, Sawaya ME. Idiopathic hirsutism. Endocr Rev. 2000;21(4):347-62. [PubMed ID: 10950156]. https://doi.org/10.1210/edrv.21.4.0401.
-
44.
Tehrani FR, Rashidi H, Azizi F. The prevalence of idiopathic hirsutism and polycystic ovary syndrome in the Tehran lipid and glucose study. Reprod Biol Endocrinol. 2011;9:144. [PubMed ID: 22044512]. [PubMed Central ID: PMC3214199]. https://doi.org/10.1186/1477-7827-9-144.
-
45.
Ramezani Tehrani F, Minooee S, Azizi F. Validation of a simplified method to assess hirsutism in the Iranian population. Eur J Obstet Gynecol Reprod Biol. 2014;174:91-5. [PubMed ID: 24393448]. https://doi.org/10.1016/j.ejogrb.2013.12.008.
-
46.
Rostami Dovom M, Ramezani Tehrani F, Djalalinia S, Cheraghi L, Behboudi Gandavani S, Azizi F. Menstrual cycle irregularity and metabolic disorders: A population-based prospective study. PLoS One. 2016;11(12). e0168402. [PubMed ID: 27992506]. [PubMed Central ID: PMC5161370]. https://doi.org/10.1371/journal.pone.0168402.
-
47.
Panidis D, Tziomalos K, Chatzis P, Papadakis E, Delkos D, Tsourdi EA, et al. Association between menstrual cycle irregularities and endocrine and metabolic characteristics of the polycystic ovary syndrome. Eur J Endocrinol. 2013;168(2):145-52. [PubMed ID: 23109645]. https://doi.org/10.1530/EJE-12-0655.
-
48.
Ramezani Tehrani F, Montazeri SA, Hosseinpanah F, Cheraghi L, Erfani H, Tohidi M, et al. Trend of cardio-metabolic risk factors in polycystic ovary syndrome: A population-based prospective cohort study. PLoS One. 2015;10(9). e0137609. [PubMed ID: 26360602]. [PubMed Central ID: PMC4567354]. https://doi.org/10.1371/journal.pone.0137609.
-
49.
Cho LW, Randeva HS, Atkin SL. Cardiometabolic aspects of polycystic ovarian syndrome. Vasc Health Risk Manag. 2007;3(1):55-63. [PubMed ID: 17583175]. [PubMed Central ID: PMC1994046].
-
50.
Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347-63. [PubMed ID: 20159883]. https://doi.org/10.1093/humupd/dmq001.
