Abstract
Background:
Detection of the clinical phase of chronic hepatitis B virus (HBV) infection is highly important in determining the treatment protocol. The aim of this study was to evaluate the diagnostic value of high-sensitivity C-reactive protein (hs-CRP) level as a non-invasive parameter in distinguishing different phases of chronic HBV infection.Methods:
In this cross-sectional study, 163 patients with chronic HBV infection were studied in Zahedan, Iran in 2015. The patients were classified into different phases of chronic HBV infection, based on the criteria recommended by the European association for the study of the liver (EASL). The serum level of hs-CRP was compared between different groups, using Mann-Whitney U test and Kruskal-Wallis test. Receiver operating characteristic (ROC) curve analysis was also used to investigate the predictive value of serum hs-CRP level in distinguishing cases of active HBV infection from inactive carriers.Results:
The present study included data from 163 patients with chronic HBV infection (males: 110, 68.3%; females: 53, 31.7%). Based on the findings, hs-CRP level was 2.3 ± 5.8 mg/L in patients with chronic HBV infection and 1.6 ± 2.5 mg/L in inactive carriers (P = 0.449). The optimal hs-CRP cut-off point for differentiation of chronic carriers was identified as 0.27 mg/L, with sensitivity, specificity, and positive and negative predictive values of 62.7%, 48.1%, 15.1%, and 99.9%, respectively.Conclusions:
The present study showed that serum hs-CRP level is not a predictive marker for the clinical phase of chronic HBV infection. Given its low specificity and sensitivity, hs-CRP level should be evaluated along with the viral load and other variables for determining the severity of chronic HBV infection.Keywords
Hepatitis B High-Sensitivity C-Reactive Protein Viral load Real-Time PCR
1. Background
Hepatitis B virus (HBV), which can lead to cirrhosis and hepatic cancer, is the world's most common liver infection, affecting millions of people worldwide (1). Unlike most infected infants and children developing chronic infection, most infected adults can overcome HBV infection without facing any problems (2, 3).
According to statistics, nearly 360 million people remain chronically infected carriers of HBV infection. In fact, three quarters of the world’s population live in areas with high levels of infection (4, 5). Nearly 3% of the Iranian population (approximately 200,000 - 300,000) suffer from chronic HBV infection (6). Based on the reports, approximately 25% of the general population in Sistan and Baluchestan province, Iran had previous exposure to HBV, and 3.38% were chronic cases of HBV infection (7).
Chronic HBV infection is a dynamic condition which fluctuates through time, probably due to interactions between the virus and the patient's immune system (8). C-reactive protein (CRP) is a member of the class of acute-phase reactants, released by hepatocytes in response to acute injury, infection, or other inflammatory stimuli. CRP expression in hepatocytes is regulated by cytokines, such as interleukin-1 (IL-1), interlukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) (9). This enhancement is due to the rise in the plasma concentration of IL-6, which is predominantly produced by macrophages.
A variety of serious diseases are associated with high serum levels of CRP. Several researchers have suggested that patients with elevated basal levels of CRP are at an increased risk of diabetes, hypertension, cardiovascular diseases, and elevated levels of liver enzymes (10, 11). According to a study by Al-Ajeeli KS, the median CRP titer was significantly higher in patients, compared to healthy controls (11). The pathogenesis of viral hepatitis seems to be mediated by the immune response to virus-infected hepatocytes, where cytokines play an important role in eradicating the virus.
According to a comparative study by Shima et al. the intensity of CRP expression was closely associated with the progression of HBV in patients, unlike hepatitis C virus infection (12). In addition, Yuan et al. showed that serum hs-CRP concentration can be used as an inflammatory marker for the diagnosis of patients with chronic and severe HBV infection, accompanied by spontaneous bacterial peritonitis (4). On the other hand, the results of a study by Gedik et al. indicated no significant correlation between serum CRP level and serum HBV viral load in patients with chronic HBV infection (13).
According to a previous study, CRP level can serve as a diagnostic biomarker for alpha-fetoprotein-negative HBV-related hepatocellular carcinoma (14). The serum level of CRP might reflect an inflammatory reaction, activated as a process in response to TNF or other local tissue damage. Moreover, cancer cells produce cytokines via autocrine pathways, such as IL-8 and IL-6, which in turn induce CRP production (15).
With this background in mind, this study was designed to determine the diagnostic value of hs-CRP level in differentiation of patients with different stages of chronic HBV infection. In addition, we aimed to determine the relationship between the serum level of hs-CRP and HBV viral load.
2. Methods
In this cross-sectional study, a total of 163 patients with HBV infection not receiving antiviral therapy were included. None of the patients were co-infected with hepatitis A virus, hepatitis C virus, hepatitis delta virus, hepatitis E virus, or human immunodeficiency virus.
All indicators, including the patient's age, biochemical parameters, and hepatitis B e antigen (HBeAg) level, were determined. Blood samples (5 mL) were collected from all the patients. The serum samples were stored at 2 - 8°C for 48 hours and then frozen at -20°C (or below). HBV DNA was extracted [DynaBioTM Viral Nucleic Acid (DNA/RNA) Extraction Mini Kit], and the viral load was determined according to the manufacturer’s instructions (DynaBioTM HBV quantitative real-time PCR Kit). Also, hs-CRP level was measured by particle-enhanced immunonephelometry, using the Nephstar Ultrasensitive CRP Kit (Goldsite, England, United Kingdom). The sensitivity limit was 0.25 mg/L and the upper limit was set at 18 mg/L.
The patients were categorized into four groups, based on the clinical phase of HBV infection (i.e., immune-tolerant, immune-clearance, HBeAg-negative, and low-replicative phases), according to the criteria presented in Table 1 (16, 17). The classification of these patients was in accordance with the European association for the study of the liver (EASL) criteria presented in 2012 (18). The serum hs-CRP concentration was compared among four groups of patients.
The Clinical Phases of Chronic HBV Infection
| Clinical Stages of Chronic HBV Infection | HBV DNA titer, IU/mL | HBeAg | ALT |
|---|---|---|---|
| Immune tolerance | > 107 IU/mL | + | < ULN |
| Immune clearance | > 2000 | + | > 2 ULN |
| HBeAg-negative hepatitis | > 2000 | - | > 2 ULN |
| Low replicative (inactive carrier) | < 2000 | - | < ULN |
In addition, the patients included in the study were divided into two groups:
1) Inactive carriers with persistently normal serum alanine aminotransferase (ALT) level, i.e., below the laboratory-defined upper limit of normal (ULN), and low HBV DNA level (< 2000 IU/mL)
2) Active hepatitis group with persistently or intermittently increased serum ALT (> ULN) and HBV DNA (> 20,000 IU/mL) levels.
2.1. Statistical Analysis
Kolmogorov-Smirnov test results showed that none of the measured variables had a normal distribution; therefore, non-parametric tests were used for data analysis. For univariate analysis, variables were compared between the groups, using Mann-Whitney U test, while Kruskal-Wallis test was used for multivariate comparisons. Receiver operating characteristic (ROC) curve analysis was also used to investigate the efficacy of serum hs-CRP level in distinguishing cases of active HBV infection from inactive carriers. Statistical analysis was performed, using SPSS version 20.
3. Results
In this study, we analyzed the data collected from 163 patients with chronic HBV infection, including 111 (68.1%) males and 52 (31.9%) females. The viral load, hs-CRP level, and ALT level were assessed in all the participants (Table 2).
The Viral Load and Serum Levels of hs-CRP and ALT in Patients with Chronic HBV Infection
| Variables | No. | Minimum | Maximum | Mean ± Std. Deviation |
|---|---|---|---|---|
| Viral load | 163 | 0.00 | 14750000000.00 | 220508156.26 ± 1407206854.64 |
| Hs-CRP | 163 | 0.00 | 20.00 | 1.89 ± 3.64 |
| ALT | 163 | 6.00 | 189.00 | 36.72 ± 28.03 |
A total of 9, 22, 28, and 104 patients were evaluated in groups of immune tolerance, immune clearance, HBeAg-negative, and inactive carriers, respectively. No statistically significant difference was found between the four groups in terms of serum hs-CRP concentration (Table 3). Similarly, no statistically significant difference was found in the serum level of hs-CRP between the inactive carrier and active hepatitis groups (Table 4).
Comparison of the Serum Level of hs-CRP Among the Participants According to the Clinical Phases of HBV Infectiona
| Patient Groups | Mean ± Std. Deviation | Median | IQR | P Valuea |
|---|---|---|---|---|
| Immune tolerance | 2.46 ± 4.07 | 0.16 | 3.44 | 0.825 |
| Immune clearance | 3.37 ± 6.70 | 0.85 | 1.71 | |
| HBeAg-negative hepatitis B | 1.55 ± 3.51 | 0.42 | 0.95 | |
| Inactive carrier | 1.62 ± 2.56 | 0.34 | 2.25 |
Comparison of the Serum Level of hs-CRP Between the Inactive Carrier and Active Hepatitis Groups
| Patient Groups | No. | Mean ± Std. Deviation | Median | IQR | P Valuea |
|---|---|---|---|---|---|
| Inactive carrier | 104 | 1.624 ± 2.564 | 0.343 | 2.251 | 0.449 |
| Active hepatitis | 50 | 2.35 ± 5.18 | 0.643 | 1.55 |
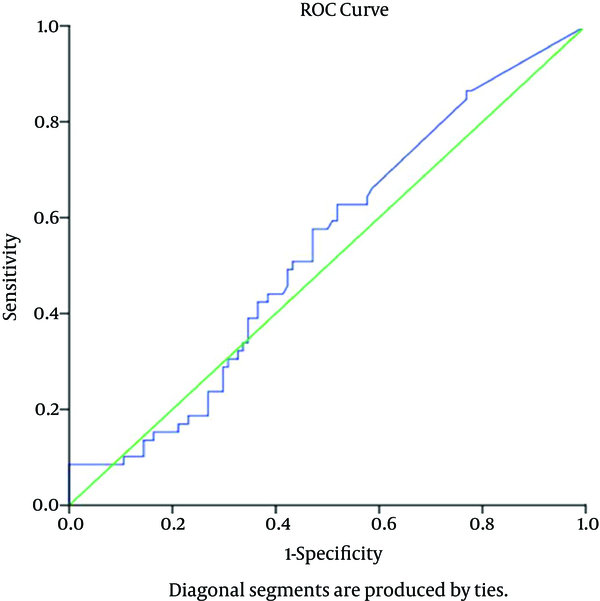
The ROC curve analysis was also used to calculate the sensitivity, specificity, positive predictive value, and negative predictive value for different hs-CRP levels. Youden’s J index and minimum d statistics were calculated to identify the optimal cut-off value for hs-CRP level and distinguish chronic active cases (i.e., immune clearance and HBeAg-negative HBV) from inactive HBV carriers (low-replicative cases).
Based on the analysis, hs-CRP concentration of ≥ 0.270 mg/L was found to be the optimal cut-off point. In our study, the area under the curve was 0.535 (95% CI: 0.489 - 0.581), which was not statistically significant (P < 0.462) (Figure 1). The sensitivity, specificity, and positive and negative predictive values for this hs-CRP level were 62.7%, 48.1%, 15.1%, and 99.9% in the diagnosis of chronic active carriers, respectively.
ROC Curve for Serum hs-CRP Level
4. Discussion
Hepatitis is a necro-inflammatory reaction, caused by different viruses. Cytokines play an important role in the early immune response against viruses and chronic development of the disease. Hepatic Kupffer cells produce pro-inflammatory cytokines such as TNF-α, IL-1, and IL-3. Also, the liver produces acute-phase proteins by stimulating the production of serum cytokines (IL-6).
Little is known about the relationship between hs-CRP secretion and progression of liver inflammation. According to the results of the present study, hs-CRP level was 2.3 ± 5.8 in patients with chronic hepatitis and 1.6 ± 2.5 in inactive carriers (P = 0.449). The optimal cut-off point of hs-CRP for differentiation of chronic carriers was identified as 0.27 mg/L, with sensitivity, specificity, and positive and negative predictive values of 62.7%, 48.1%, 15.1%, and 99.9%, respectively. No statistically significant difference was found in the serum level of hs-CRP between the inactive carrier and active hepatitis groups (i.e., immune clearance and HBeAg-negative groups). Similarly, hs-CRP serum level was not recognized as a predictive factor for the clinical phase of chronic HBV infection due to low specificity and sensitivity.
In line with the results of the present study, Gedik et al. in 2006 indicated no significant correlation between CRP level and HBV viral load in patients with chronic HBV infection (13). Also, in a study carried out by Yilmaz et al. the serum level of hs-CRP differed between hepatitis and control groups, although this difference was not statistically significant. In this study, hs-CRP level of > 0.56 mg/dL showed 100% specificity and 12% sensitivity in the diagnosis of chronic hepatitis (area: 0.71; P = 0.002) (9). Also, according to a comparative study by Shima et al. CRP expression was correlated with the progression of HBV infection (12).
Yuan et al. showed that serum CRP concentration could be used as an inflammatory marker for the diagnosis of chronic and severe HBV patients with spontaneous bacterial peritonitis (4). Also, in a study conducted by Lui et al. in 2016, the efficiency of combined evaluation of liver stiffness and serum CRP level was investigated in patients with HBV-related liver cirrhosis. The serum CRP level was significantly higher in the hepatocellular carcinoma group, compared to the liver cirrhosis group (P < 0.01). Based on this study, concurrent evaluation of serum CRP level and liver stiffness was recommended for the detection of HBV stage (19).
Based on the present findings, there was no significant relationship between hs-CRP level and clinical stages of chronic HBV infection in inactive and active hepatitis groups. However, the relatively small sample size in some groups (with the exception of chronic carriers) might have been insufficient for our comparative study. According to the results of the present study, we cannot recommend a strategy without liver biopsy to guide treatment in patients with chronic hepatitis or to distinguish a variety of groups with chronic clinical hepatitis. We suggest that larger sample sizes and more homogeneous groups of HBV patients be used in future studies.
Acknowledgements
References
-
1.
Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97-107. [PubMed ID: 14996343].
-
2.
Kumar M, Singh T, Sinha S. Chronic hepatitis B virus infection and pregnancy. J Clin Exp Hepatol. 2012;2(4):366-81. [PubMed ID: 25755458]. https://doi.org/10.1016/j.jceh.2012.09.001.
-
3.
Yalcin K, Degertekin H, Yildiz F, Celik Y. Markers of disease activity in chronic hepatitis B virus infection. Clin Invest Med. 2003;26(1):27.
-
4.
Yuan LY, Ke ZQ, Wang M, Li Y. Procalcitonin and C-reactive protein in the diagnosis and prediction of spontaneous bacterial peritonitis associated with chronic severe hepatitis B. Ann Lab Med. 2013;33(6):449-54. [PubMed ID: 24205495]. https://doi.org/10.3343/alm.2013.33.6.449.
-
5.
Hashemi-Shahri SM, Sharifi-Mood B, Khalili M. Review of the Prevention of the Hepatitis B Virus Infection Transmission From Mother to Child During Pregnancy. Int J Infect. 2015;2(3). https://doi.org/10.17795/iji26626.
-
6.
Alian S, Masoudzadeh A, Khoddad T, Dadashian A, Ali Mohammadpour R. Depression in hepatitis B and C, and its correlation with hepatitis drugs consumption (interfron/lamivodin/ribaverin). Iran J Psychiatry Behav Sci. 2013;7(1):24-9. [PubMed ID: 24644496].
-
7.
Alavian SM. Hepatitis B is a Serious Health Problem in Some Parts of Iran; Sistan and Baluchestan Province. Int J Infect. 2015;2(2). https://doi.org/10.17795/iji-17937.
-
8.
Moudi B, Heidari Z, Mahmoudzadeh-Sagheb H, Hashemi M, Metanat M, Khosravi S, et al. Association Between IL-10 Gene Promoter Polymorphisms (-592 A/C, -819 T/C, -1082 A/G) and Susceptibility to HBV Infection in an Iranian Population. Hepat Mon. 2016;16(2). ee32427. [PubMed ID: 27148384]. https://doi.org/10.5812/hepatmon.32427.
-
9.
Yilmaz S, Bayan K, Tuzun Y, Dursun M, Kaplan A, Ozmen S, et al. Replacement of hystological findings: serum hyaluronic acid for fibrosis, high-sensitive C-reactive protein for necroinflamation in chronic viral hepatitis. Int J Clin Pract. 2007;61(3):438-43. [PubMed ID: 17313611]. https://doi.org/10.1111/j.1742-1241.2006.00912.x.
-
10.
Zhang X, Zoulim F, Habersetzer F, Xiong S, Trepo C. Analysis of hepatitis B virus genotypes and pre-core region variability during interferon treatment of HBe antigen negative chronic hepatitis B. J Med Virol. 1996;48(1):8-16. [PubMed ID: 8825704]. https://doi.org/10.1002/(SICI)1096-9071(199601)48:1<8::AID-JMV2>3.0.CO;2-E.
-
11.
Al-Ajeeli KS. Assessment of C-reactive protein titer in patients with chronic hepatitis B virus infection. Iraqi J Comm Med. 2011;24(4):291-4.
-
12.
Shima M, Nakao K, Kato Y, Nakata K, Ishii N, Nagataki S. Comparative study of C-reactive protein in chronic hepatitis B and chronic hepatitis C. Tohoku J Exp Med. 1996;178(3):287-97. [PubMed ID: 8727711].
-
13.
Gedik M, Ozekinci T, Ozbek E, Atmaca S, Yilmaz S. Lack of correlation between CRP and hepatitis B viral load in serum of patients with chronic HBV. J Infect. 2007;54(2):204. [PubMed ID: 16780953]. https://doi.org/10.1016/j.jinf.2006.04.009.
-
14.
She S, Xiang Y, Yang M, Ding X, Liu X, Ma L, et al. C-reactive protein is a biomarker of AFP-negative HBV-related hepatocellular carcinoma. Int J Oncol. 2015;47(2):543-54. [PubMed ID: 26058824]. https://doi.org/10.3892/ijo.2015.3042.
-
15.
Heikkila K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health. 2007;61(9):824-33. [PubMed ID: 17699539]. https://doi.org/10.1136/jech.2006.051292.
-
16.
McMahon BJ. Natural history of chronic hepatitis B - clinical implications. Medscape J Med. 2008;10(4):91. [PubMed ID: 18504503].
-
17.
Song LW, Liu PG, Liu CJ, Zhang TY, Cheng XD, Wu HL, et al. Quantitative hepatitis B core antibody levels in the natural history of hepatitis B virus infection. Clin Microbiol Infect. 2015;21(2):197-203. [PubMed ID: 25658546]. https://doi.org/10.1016/j.cmi.2014.10.002.
-
18.
European Association For The Study Of The L. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167-85. [PubMed ID: 22436845]. https://doi.org/10.1016/j.jhep.2012.02.010.
-
19.
Liu XY, Ma LN, Yan TT, Lu ZH, Tang YY, Luo X, et al. Combined detection of liver stiffness and C-reactive protein in patients with hepatitis B virus-related liver cirrhosis, with and without hepatocellular carcinoma. Mol Clin Oncol. 2016;4(4):587-90. [PubMed ID: 27073669]. https://doi.org/10.3892/mco.2016.742.