Abstract
Background:
Infant milk powder (IMP) is a very suitable medium for the growth of micro-organisms such as Bacillus cereus. B. cereus is a spore-producer that can survive easily during the pasteurization process to cause some health problems.Objectives:
This study was carried out to determine the antibiotic resistance pattern of B. cereus isolates producing metallo-β-lactamase (MBL) enzymes in IMP samples.Methods:
After preparing 50 samples of IMP, they were cultured to detect the presence of B. cereus. The Kirby-Bauer method was used to determine antibiotic susceptibility and the DDST method to phenotypically detect MBL enzymes. MBL genes blaVIM, blaSMP-1, and blaIMP were genotypically examined in the isolates by the PCR method.Results:
Most samples of IMP had a high level of contamination with B. cereus when compared to national and international standards. The highest percentage of resistance was related to ceftazidime and oxacillin (100%) and the lowest resistance to tetracycline (21.4%) and ciprofloxacin (26.3%). All B. cereus isolates were the producers of MBL in the DDST test. None of the isolates was positive for the blaSPM gene. Of the other MBL genes, the blaVIM gene was seen in all isolates (100%) and the blaIMP gene in 16 isolates (84.21%).Conclusions:
Antibiotic resistance is on the rise in the study region due to the production of MBL enzymes. Among MBL-producing isolates, the frequency of the blaVIM gene was more than that of blaIMP and blaSPM-1.Keywords
Bacillus cereus Milk Metallo-β-lactamase Antibiotic Resistance blaVIM
1. Background
Infant milk powder (IMP) is a global food source for growing infants. Nowadays, most babies are fed artificially after being fed with IMP under the age of one year. IMP is one of the most important sources of food in the first months of infant’s development. Given the infants’ weak immune system, the quality of IMP is of vital importance. Due to non-sterility of IMP, it is a perfect environment for the growth of microorganisms. Among the microorganisms that can cause infections and complications such as bacteremia, meningitis, and sepsis, are Bacillus cereus, Staphylococcus aureus, Clostridium perfringens, Escherichia coli, and Enterobacter sakazakii (1). B. cereus is a pathogen with an increasing trend in the contamination of IMP. It is a Gram-positive, spore-forming, aerobic, facultative anaerobic bacillus from the Bacillaceae family.
The optimum temperature for the growth of B. cereus is between 30 and 35°C but it can grow at temperatures up to 48°C or under 7°C. D100 of the spore forms of B. cereus is 2.2 to 5.4 minutes that indicates their high resistance to heat (2, 3). The enterotoxins of this bacterium can produce two types of the food-poisoning syndrome: diarrhea (diarrheal type) and nauseous type (emetic type) (4, 5). In addition to being pathogenic, B. cereus is responsible for food decay because of proteolytic, lipolysis, and amylolytic activity (6). Several antibiotics are used to treat B. cereus infection. However, excessive consumption of antibiotics for treating bacterial infections has led to the generation of antibiotic-resistant strains as one of the great problems in the medical field. This is due to the induction of antibiotic inactivating enzymes or mutations in coding genes, cell wall canals (external membrane purines), secretion systems, or transmission of plasmids among bacteria (7).
Carbapenems are selective drugs for the treatment of infections caused by penicillin- or cephalosporin-resistant bacteria due to their wide range of activity and hydrolysis stability against most β-lactamases and metallo-β-lactamase (MBL) enzymes. Carbapenems include imipenem, meropenem, and ertapenem (8). Carbapenems can inhibit the synthesis of the bacterial cell wall, increase the permeability of the outer membrane of bacteria, and affect their secretion system. Resistance to carbapenems is caused by various mechanisms of which, the production of MBL enzymes is most notably (7-9). Based on molecular studies, MBL belongs to the group B Ambler and can hydrolyze a range of beta-lactams (anti-cell wall antibiotics) including cephalosporins (third and fifth generations). Moreover, they can create resistance to aminoglycosides but cannot hydrolyze monobactams (only with a beta-lactam ring) such as aztreonam. Six types of MBL have been detected including SPM, VIM, IMP, SIM, GIM, and AIM, the most common of which include IMP and VIM. The encoding genes of MBL are located on class 1 integron on a plasmid with the ability to its transmission. Sometimes, the transposable elements of the genes (transposon and integron) can be part of a chromosome, making them easily transmissible among different bacteria (9, 10). These enzymes require a metal cofactor (often Zn). They are not inhibited by beta-inhibitors such as sulbactam, tazobactam, and clavulanic acid but inhibited only by chelating agents such as ethylene diamine tetra-acetic acid (EDTA), 2-mercapto propionic acid, sodium mercaptoacetic acid, and dipicolinic acid. These inhibitors can be used for the phenotypic diagnosis of MBL-producing strains in the laboratory (11). EDTA is preferred to other inhibitors, as it is an easily available compound and EDTA-containing disks are suitable for use for up to 16 weeks at -20°C. The Bar E-test is also used to identify bacterial genera producing MBL, but with the high cost and less repeatable results. PCR is currently used for confirmation as a sensitive and reliable method (12).
Microbial resistance is often investigated in bacteria isolated from clinical specimens but it has been less studied in bacteria isolated from food. This is while there is a possibility of transferring resistance from strains in IMP to other strains. Moreover, it is important to identify species producing MBL.
2. Objectives
Therefore, the present study was designed to investigate the antibiotic resistance profile of B. cereus strains isolated from IMP to elucidate the possibility of resistance transmission to other strains.
3. Methods
3.1. Isolation and Identification of B. cereus
First, 50 samples of IMP were prepared for microbial evaluation. To do so, 25-g samples were collected under sterile conditions, added to 225 mL of peptone water, and incubated for 24 hours. The suspension was mixed well and a portion of 0.1 mL was cultured on B. cereus selective medium (MYP Agar) plates and incubated overnight at 37°C. Large pink colonies (non-mannitol-fermenting) with a sedimentary halo (due to the production of lecithinase) were considered as suspected colonies. To identify the bacterium, gram staining and biochemical tests were performed, including catalase test, growth under anaerobic conditions, growth at 45°C, lecithinase C test, beta-hemolysis on blood agar with 5% sheep blood, MR-VP, mobility test, susceptibility testing to penicillin 10 (IU), and induction of nitrate (13).
3.2. Antibiotic Susceptibility Testing
The disc diffusion (Kirby-Bauer) method was used following the CLSI guidelines to evaluate the antibiotic resistance profile of the isolates against ceftizoxime (30 µg), amikacin (30 µg), gentamicin (10 µg), ciprofloxacin (30 µg), piperacillin (30 µg), ceftriaxone (30 µg), cefotaxime (30 µg), meropenem (10 µg), ceftazidime (30 µg), imipenem (10 µg), clindamycin (2 µg), vancomycin (30 µg), oxacillin (1 µg), ampicillin (10 µg), tetracycline (30 µg), and methicillin (5 µg) (14). The antibiotics were purchased from Padtan Teb Company.
3.3. Confirmatory Test for the Production of MBL Enzyme
A phenotypic method (DDST) was used to determine the presence of MBL enzymes. Prior to this stage, a screening test for MBL production was performed. In accordance with the principles of CLSI, in this preliminary test, the sensitivity of B. cereus isolates was measured to carbapenems (imipenem and meropenem). An inhibition zone diameter of ≤ 23 mm indicated a suspected strain producing MBL. After this stage, a phenotypic confirmatory test was conducted to confirm the production of MBL (15). A bacterial suspension was prepared for this purpose and cultured on the surface of Moeller-Hinton agar plates. In the next step, antibiotic disks were placed on this medium. First, imipenem (10 µg) and meropenem (10 µg) were impregnated with EDTA at a concentration of 0.5 µL, and placed on the plate containing Muller-Hinton medium inoculated with the test isolate. Imipenem and meropenem discs without EDTA were placed next to the disks impregnated with EDTA at a distance of 20 mm apart. After 24 hours of incubation at 37°C, the results were recorded and compared. If a difference of more than 7 mm in the inhibition zone diameter was observed between the disks with EDTA and those without EDTA, it would be positive for the presence of MBL in the isolate (16).
3.4. DNA Extraction
The boiling method was used to extract DNA. First, fresh colonies were extracted from a culture medium containing pure B. cereus, suspended in 100 μL of sterile deionized or distilled water, and boiled for 15 minutes at 100°C. Then, it was centrifuged at 10000 rpm for 10 minutes. The supernatant containing the bacterial DNA was used for PCR and the precipitate was set aside. A biophotometer (USA, Bio-Rad) was used to confirm the degree of DNA purity in the extracted specimen.
3.5. PCR
The PCR method was used to study the frequency of MBL genes blaVIM, blaSMP-1, and blaIMP in the phenotypically positive isolates. A B. cereus strain producing MBL was used as a positive control. The specific primers are listed in Table 1. The primers were designed and provided by a manufacturer (Arka Teb Ruham).
Primers Used for MBL Genes Detection
| Primer Name | Sequence (5’ to 3’) | Size (bp) |
|---|---|---|
| IMP (F) | ACCGCAGCAGAGTCTTTGCC | 578 |
| IMP (R) | ACAACCAGTTTTGCCTTACC | |
| VIM (F) | AGTGGTGAGTATCCGACA | 836 |
| VIM (R) | ATGAAAGTGCGTGGAGAC | |
| SPM-1 (F) | GCGTTTTGTTTGTTGCTC | 786 |
| SPM-1 (R) | TTGGGGATGTGAGACTAC |
Amplification was done in the following thermal cycling conditions: 5 minutes at 95°C and 30 cycles of amplification consisting of 30 seconds at 95°C, 30 seconds at 49°C, and 1 minute at 72°C, followed by a final extension for 5 minutes at 72°C. The amplified products were analyzed after electrophoresis on a 1% agarose gel. A B. cereus strain carrying blaVIM was used as control.
The data were presented by descriptive statistics such as means and percentages. They were analyzed using SPSS software (V. 20 SPSS) by the chi-square test at a significance level of 0.05.
4. Results
4.1. Isolation and Identification of B. cereus
In this study, 50 IMP samples were examined for the presence of B. cereus of which, 19 were contaminated with B. cereus. Of these, seven samples (36.84%) had more than 100 CFU/g, six contained 10 - 100 CFU/g, and the remaining six samples showed less than 10 CFU/g of B. cereus.
4.2. Antibiotic Susceptibility Testing
Antibiotic susceptibility of the isolates was tested against various antibiotics, the results of which are given in Table 2.
| Antibiotics | Susceptible | Intermediate | Resistant |
|---|---|---|---|
| Imipenem | 0 | 3 (15.8) | 16 (84.2) |
| Ciprofloxacin | 13 (68.4) | 1 (5.3) | 5 (26.3) |
| Gentamicin | 12 (63.2) | 0 | 7 (36.8) |
| Cefotaxime | 0 | 2 (10.5) | 17 (89.5) |
| Ceftazidime | 0 | 0 | 19 (100) |
| Ceftizoxime | 0 | 3 (15.8) | 16 (84.2) |
| Piperacillin | 0 | 1 (5.3) | 18 (94.7) |
| Clindamycin | 2 (10.5) | 2 (10.5) | 15 (78.9) |
| Vancomycin | 11 (57.9) | 1 (5.3) | 7 (36.8) |
| Oxacillin | 0 | 0 | 19 (100) |
| Ampicillin | 0 | 1 (5.3) | 18 (94.7) |
| Tetracycline | 11 (57.9) | 4 (21.1) | 4 (21.1) |
| Methicillin | 0 | 1 (5.3) | 18 (94.7) |
The isolates from IMP samples had the highest percentage of resistance to ceftazidime and oxacillin (100%) and the least resistance to tetracycline (21.4%) and ciprofloxacin (26.3%). Moreover, 16 samples (84.2%) were resistant to carbapenem.
4.3. Preliminary Test Results for the Production of MBL
If an isolate showed a inhibition zone diameter of ≤ 23 mm against imipenem, it was considered a suspected strain of MBL producer. Therefore, three intermediate resistant isolates and 16 resistant isolates to imipenem were suspected to produce MBL.
4.4. Phenotypic Confirmatory Test Results for the Production of MBL
The DDST method was used to identify the presence of MBL enzymes. It showed that all the B. cereus isolates were the producers of MBL enzymes. As Table 3 shows, three (15.78%) intermediate resistant isolates and 16 (84.21%) resistant isolates to imipenem were the producers of MBL by the DDST phenotypic method. Thus, all of the B. cereus isolates resistant to imipenem in the disk diffusion method were confirmed as MBL producers in DDST.
The Frequency of MBL Producers in Imipenem-Resistant Isolates in the Disk Diffusion Method
| Antibiotic Susceptibility | MBL Producera |
|---|---|
| Intermediate resistant to impenem | 3 (78.15) |
| Resistant to imipenem | 16 (21.84) |
| Total | 19 (100) |
4.5. PCR Test Results
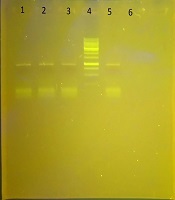
In this study, B. cereus isolates were examined for the presence of genes blaVIM, blaSPM, and blaIMP by the PCR molecular method. In this study, the blaSPM gene was detected in none of the isolates. Of the other MBL genes, the blaVIM gene was observed in all the isolates (100%) and the blaIMP gene in 16 isolates (84.21%). The results of the electrophoresis of the PCR product for the metallo-β-lactamase genes are shown in Figure 1.
Gel electrophoresis of the PCR product for the blaVIM gene; Lane 4, DNA ladder; Lanes 1, 2, 3, PCR product for the blaVIM gene (836 bp); Lane 5, positive control; Lane 6, negative control.

Table 4 shows that all imipenem-resistant isolates (16 isolates) possessed the blaIMP gene. The blaIMp gene was not found in intermediate resistant isolates. There was a statistically significant relationship between blaIMP presence and resistance to imipenem (P < 0.005).
The Frequency Distribution of blaIMP Gene Based on Imipenem Resistance in B. cereus Isolatesa
| blaIMP-Negative | blaIMP-Positive | P Value | |
|---|---|---|---|
| Intermediate resistant to imipenem | 3 (100) | 0 (0) | 0.001 |
| Resistant to imipenem | 0 (0) | 16 (100) |
5. Discussion
B. cereus can cause food poisoning and its spores are scattered widely in nature. The results of this study showed that out of 50 IMP samples, six samples were contaminated with less than 10 CFU/g, six samples with 10 - 100 CFU/g, and seven samples with more than 100 CFU/g from B. cereus. B. cereus is authorized in most foods as 102 - 103 CFU/g and the national standards limit its presence in IMP as 102 CFU/g. Because the microbial flora is not complete in the baby’s intestine, it is possible that B. cereus is reproduced due to different climatic conditions and improper maintenance of the IMP, so the further remedy is needed to amend this difficulty. According to similar studies in Iran, B. cereus is the most bacterial isolate from IMP. In the current study, in general, 50 samples were examined, 19 of which were contaminated with B. cereus. Rahimi Fard et al. in Isfahan found that 42% of infants’ food samples were contaminated with B. cereus (17). Rahimi Fard et al. in another study of 60 samples of IMP tested for B. cereus contamination showed that 15 samples were over the authorized limits (18). Similar results have been achieved in other countries. Reyes et al. by evaluating 56 samples of IMP and rice-based diets found that 35 (62.5%) samples were contaminated with B. cereus (19). Deeb et al. in Egypt showed that the rate of contamination with B. cereus was 56.9% in milk powder (20). Di pinto et al. identified 11 out of 60 samples contaminated with Bacillus spp., with B. cereus being detected in 5 out of 11 positive samples (21). Logan in 2012 found B. cereus to be one of the agents producing toxins in food leading to diarrhea and vomiting (22). The differences in the formulas, the quality of raw milk, the season of production, sampling method, and procedure of conducting experiments are the factors involved in the level of contamination, which may have led to differences in the results of various studies. B. cereus spores can easily contaminate milk from unhealthy dairy and create biofilms in milk pasteurization facilities in the dairy industry. Moreover, its heat-resistant spores can remain in the heat-treated milk.
The current study also determined the frequency of antibiotic resistance in the isolates producing MBL from IMP samples. The results of antibiotic susceptibility testing showed that 16 (84.2%) isolates were resistant to imipenem. It was also found that 81.2% of the imipenem-resistant isolates were sensitive to ciprofloxacin. Ciprofloxacin is the most effective antibiotic in the treatment of bacteria and the rate of resistance to this antibiotic was less than the rate to other antibiotics.
To perform its catalytic activity, MBL needs a metal co-factor (ZU), which is inhibited by EDTA and thiol compounds. In this study, EDTA was used to identify MBL-producing strains (23). The results of the DDST phenotypic method for the detection of MBL in this study showed that all intermediate resistant and resistant strains to imipenem were MBL producers. In this study, B. cereus isolates were examined for the presence of genes blaVIM, blaIMP, and blaSPM-1 by the PCR molecular method. blaSPM-1 was seen in none of the isolates. Among the other MBL genes, the blaVIM gene was observed in all isolates (100%) and the blaIMP gene in 84.21% of the isolates.
In 2018, Torkar and Bedenic examined the sensitivity and antimicrobial properties of MBLs in environmental and clinical isolates of B. cereus. All strains were sensitive to imipenem and 98.5% to meropenem. The blaVIM gene was identified in 21.2% of the isolates (15). The results of the study by Ghazaei in 2018 on Bacillus subtilis isolated from raw milk and cheese samples showed that 25 (52.52%) isolates from raw milk and 16 (44.43%) isolates from cheese samples produced MBL by the phenotypic method (16).
The research results showed that the rate of antibiotic resistance in bacteria producing beta-lactamases has increased significantly, which creates serious limitations to use different antibiotics. Bacillus is able to produce spores resistant to temperature. It often enters milk through different processing stages. Therefore, it is very important to identify bacilli producing beta-lactamases, as transmitting these microorganisms through the milk and dairy products may create resistance to antibiotics. In recent years, many epidemics have been reported with the organisms producing broad-spectrum beta-lactamases all over the world. Khosravi in Ahvaz in 2007 performed E-test and PCR on 100 isolates of Pseudomonas aeruginosa and showed that eight (19.51%) out of 41 strains resistant to imipenem were the producers of MBL and carrier of the blaVIM gene (24).
In a study by Pena et al. in Portugal, only 26 isolates (19.4%) of 40 positive isolates of P. aeruginosa identified by the DDST method were confirmed with PCR to possess the blaVIM gene (25). Yusefi et al. in 2010 performed DDST on the isolates from the northwest of Iran and only 37.5% of the 139 strains had MBLs. Of these, 23.1% were positive in PCR; 18 isolates carried blaVIM and 6 isolates carried blaIMP (26). In a study by Cheng et al. in China in 2008, it was observed that the class 1 integron gene was present in 43.5% of the VIM strains and in one IMP-positive isolate (27). There are differences in our study and other studies, which can be due to other resistant mechanisms to MBLs, differences in geographical regions and sample types, and multiple/high resistance of isolates to antibiotics.
5.1. Conclusions
This study showed that antibiotic resistance is increasing. Food contamination with bacteria resistant to antibiotics will increase the transmission of antibiotic resistance to the intestinal bacterial flora of consumers, which needs to be considered more as a risk factor. There is clinical importance for MBL production as it generates multiple resistance to medication used in treating infections of B. cereus. Therefore, modifying antibiotic therapy regimens is essential to reduce the prevalence of MBL genes, especially in recent years that microbial resistance has been on the rise in most species of bacteria. As B. cereus is an important contaminant of IMP, it is necessary to pay special attention to quality control of imported IMP, exact supervision on production units, and applying good manufacture practice (GMP) and hazard analysis and critical control point (HACCP).
References
-
1.
Askarizade N, Pourzakariya Z, Najafpoor A. [Fluoride content of packaged milk and infant formulas in Tehran]. Res Dent Sci. 2014;11(2):108-11. Persian.
-
2.
Murray PR, Rosenthal KS, Pfaller MA. Medical microbiology. 7th ed. St. Louis: Mosby; 2012.
-
3.
Shirmohammadi E, Saeidi S, Mohasseli T, Rahimian Boogar A. Antibacterial effects of silver nanoparticles produced by satureja hortensis extract on isolated Bacillus cereus from soil of Sistan plain. Int J Infect. 2014;1(3). https://doi.org/10.17795/iji-21944.
-
4.
Ajello L, Hay RG. Topley and Wilson's microbiology and microbial infections, 8 volume set. 10th ed. Hardcover; 2005. 400 p.
-
5.
Vilas-Boas GT, Peruca AP, Arantes OM. Biology and taxonomy of Bacillus cereus, Bacillus anthracis, and Bacillus thuringiensis. Can J Microbiol. 2007;53(6):673-87. [PubMed ID: 17668027]. https://doi.org/10.1139/W07-029.
-
6.
Roy A, Moktan B, Sarkar PK. Characteristics of Bacillus cereus isolates from legume-based Indian fermented foods. Food Control. 2007;18(12):1555-64. https://doi.org/10.1016/j.foodcont.2006.12.006.
-
7.
Rao PN, Prasad SR. Detection of extended-spectrum beta-lactamases. Indian J Med Microbiol. 2016;34(2):251-2. [PubMed ID: 27080789]. https://doi.org/10.4103/0255-0857.180363.
-
8.
Bonomo RA. Beta-lactamases: A focus on current challenges. Cold Spring Harb Perspect Med. 2017;7(1). [PubMed ID: 27742735]. [PubMed Central ID: PMC5204326]. https://doi.org/10.1101/cshperspect.a025239.
-
9.
Kazeminezhad B, Bostanmanesh Rad A, Gharib A, Zahedifard S. blaVIM and blaIMP genes detection in isolates of carbapenem resistant P. aeruginosa of hospitalized patients in two hospitals in Iran. Iran J Pathol. 2017;12(4):392-6.
-
10.
Bahar MA, Jamali S, Samadikuchaksaraei A. Imipenem-resistant Pseudomonas aeruginosa strains carry metallo-beta-lactamase gene bla(VIM) in a level I Iranian burn hospital. Burns. 2010;36(6):826-30. [PubMed ID: 20045260]. https://doi.org/10.1016/j.burns.2009.10.011.
-
11.
Bora A, Sanjana R, Jha BK, Mahaseth SN, Pokharel K. Incidence of metallo-beta-lactamase producing clinical isolates of Escherichia coli and Klebsiella pneumoniae in central Nepal. BMC Res Notes. 2014;7:557. [PubMed ID: 25146590]. [PubMed Central ID: PMC4148948]. https://doi.org/10.1186/1756-0500-7-557.
-
12.
Drieux L, Brossier F, Sougakoff W, Jarlier V. Phenotypic detection of extended-spectrum beta-lactamase production in Enterobacteriaceae: Review and bench guide. Clin Microbiol Infect. 2008;14 Suppl 1:90-103. [PubMed ID: 18154532]. https://doi.org/10.1111/j.1469-0691.2007.01846.x.
-
13.
Chika K, Ifeanyichukwu I, Malachy U, Benigna O, Adaora CE, Araka O, et al. Phenotypic detection of AmpC enzymes and antimicrobial susceptibility of Klebsiella spp. isolated from abattoir. Int J Appl Microbiol Biotechnol Res. 2016;4:117-21.
-
14.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement. CLSI document M100-S17. Wayne, PA: CLSI; 2011.
-
15.
Torkar KG, Bedenic B. Antimicrobial susceptibility and characterization of metallo-beta-lactamases, extended-spectrum beta-lactamases, and carbapenemases of Bacillus cereus isolates. Microb Pathog. 2018;118:140-5. [PubMed ID: 29551437]. https://doi.org/10.1016/j.micpath.2018.03.026.
-
16.
Ghazaei C. [Phenotypic detection of beta and metallo lactamases enzymes in Bacillus subtilis strains isolated from raw milk and cheese samples]. Vet Res Biol Prod. 2018;31(3):28-35. Persian. https://doi.org/10.22092/vj.2018.115854.1371.
-
17.
Rahimi E, Abdos F, Momtaz H, Baghbadorani ZT, Jalali M. Bacillus cereus in infant foods: Prevalence study and distribution of enterotoxigenic virulence factors in Isfahan province, Iran. ScientificWorldJournal. 2013;2013:292571. [PubMed ID: 23781153]. [PubMed Central ID: PMC3678457]. https://doi.org/10.1155/2013/292571.
-
18.
Rahimifard N, Fatholahzadeh B, Noory Z. Bacillus cereus contamination in infant laboratory. Tehran Univ Med J. 2007;65(8):64-8.
-
19.
Reyes JE, Bastias JM, Gutierrez MR, Rodriguez Mde L. Prevalence of Bacillus cereus in dried milk products used by Chilean School Feeding Program. Food Microbiol. 2007;24(1):1-6. [PubMed ID: 16943088]. https://doi.org/10.1016/j.fm.2006.04.004.
-
20.
Deeb A, Al-Hawary II, Aman IM, Shahine DMHA. Bacteriological investigation on milk powder in the Egyptian market with emphasis on its safety. Global Vet. 2010;4(5):424-33.
-
21.
Di Pinto A, Bonerba E, Bozzo G, Ceci E, Terio V, Tantillo G. Occurence of potentially enterotoxigenic Bacillus cereus in infant milk powder. Eur Food Res Tech. 2013;237(2):275-9. https://doi.org/10.1007/s00217-013-1988-8.
-
22.
Logan NA. Bacillus and relatives in foodborne illness. J Appl Microbiol. 2012;112(3):417-29. [PubMed ID: 22121830]. https://doi.org/10.1111/j.1365-2672.2011.05204.x.
-
23.
Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-beta-lactamases: The quiet before the storm? Clin Microbiol Rev. 2005;18(2):306-25. [PubMed ID: 15831827]. [PubMed Central ID: PMC1082798]. https://doi.org/10.1128/CMR.18.2.306-325.2005.
-
24.
Khosravi AD, Mihani F. Detection of metallo-beta-lactamase-producing Pseudomonas aeruginosa strains isolated from burn patients in Ahwaz, Iran. Diagn Microbiol Infect Dis. 2008;60(1):125-8. [PubMed ID: 17900848]. https://doi.org/10.1016/j.diagmicrobio.2007.08.003.
-
25.
Pena A, Donato AM, Alves AF, Leitao R, Cardoso OM. Detection of Pseudomonas aeruginosa producing metallo-beta-lactamase VIM-2 in a central hospital from Portugal. Eur J Clin Microbiol Infect Dis. 2008;27(12):1269-71. [PubMed ID: 18629554]. https://doi.org/10.1007/s10096-008-0579-2.
-
26.
Yousefi S, Farajnia S, Nahaei MR, Akhi MT, Ghotaslou R, Soroush MH, et al. Detection of metallo-beta-lactamase-encoding genes among clinical isolates of Pseudomonas aeruginosa in northwest of Iran. Diagn Microbiol Infect Dis. 2010;68(3):322-5. [PubMed ID: 20846807]. https://doi.org/10.1016/j.diagmicrobio.2010.06.018.
-
27.
Cheng X, Wang P, Wang Y, Zhang H, Tao C, Yang W, et al. Identification and distribution of the clinical isolates of imipenem-resistant Pseudomonas aeruginosa carrying metallo-beta-lactamase and/or class 1 integron genes. J Huazhong Univ Sci Technolog Med Sci. 2008;28(3):235-8. [PubMed ID: 18563313]. https://doi.org/10.1007/s11596-008-0301-8.