Abstract
Keywords
Polymyxins Pharmacodynamics Pharmacokinetics Multidrug Resistance Gram-Negative Bacteria
1. Context
Globally, the challenge of antibiotic resistance has reached the point of “new antibiotic crisis” in fighting against various life-threatening bacteria. Such a medical urgency was spotlighted in the “bad bugs, no drugs” report of the Infectious Diseases Society of America (IDSA) in 2004 (1).
At present, medical science is threatened by “superbugs”, the bacteria that are resistant to most available potential antibiotics. The World Health Organization (WHO) has identified this alarming situation as one of the three greatest threats to global human health (2). This unresolved global threat is due to the presence of multidrug-resistant (MDR) microorganisms, especially Gram-negative bacteria, mostly Pseudomonas aeruginosa, Acinetobacter baumannii, and carbapenem-resistant Enterobacteriaceae like, Klebsiella pneumonia, against which the existing antibiotics show inability in most cases. The most unfortunate news is that there is no new remarkable antibiotic in the pipeline in the upcoming years (3). The MDR organisms are defined as non-susceptible organisms that show resistance to at least one antibiotic in three or more classes of antimicrobials within its standard susceptibility spectrum (1). The term, extensively drug-resistance (XDR) is mostly used for Mycobacterium tuberculosis (TB) infections where the TB pathogen is resistant to at least four major anti-TB drugs (2). The recent emergence of MDR infections is hinting an XDR era in the nearest future if such necessary measures are not taken immediately (1, 3). As a result, with the uprising scarcity of potential antibiotics in MDR infections, treatment costs along with mortality and morbidity rates are substantially increasing day by day (4, 5).
With the increasing risk of MDR organisms, global medical concern has turned into one of the oldest groups of antibiotics “polymyxins”, a group of cationic antibiotics consisting of five different polymyxin antibiotics (A to E). However, clinical practice only uses polymyxin B and colistin, also known as polymyxin E (6, 7). Polymyxins are broadly active against Gram-negative bacteria. Polymyxins were discovered in 1947 and clinically approved for use in the late 1950s. After completing a long journey, in the mid-1970s, the interest in the polymyxin use reduced due to the disclosing of some of its potential side effects like nephrotoxicity and neurotoxicity following intravenous administration (8). Because of the alarming existence of MDR Gram-negative bacteria and the huge shortage of potential antibiotics to effectively control the situation, polymyxins came strongly again in the global consideration over the last two decades (9). Nowadays, polymyxin B and colistin are globally considered as the last-line reserve antibiotics against these “Serious” Gram-negative superbugs (10). A recent review study showed that there are many recent controversies globally regarding the use of polymyxin B and colistin against serious life-threatening superbugs; they are especially about their potential therapeutic options one over another regarding their therapeutic side effects found in different studies (11).
The objective of the current review article was to evaluate the scope of therapeutic specification of polymyxins considering the pharmacokinetic (PK) and pharmacodynamic (PD) properties of polymyxin antibiotics in Gram-negative bacterial infections. PubMed, Cochrane Library, and Scopus databases were hand-searched in October 2018 for selecting the references of relevant reviews to select articles for this review article. The keywords used were “colistin AND polymyxin B” with or without terms pharmacokinetics, pharmacodynamics, mechanism, resistance, and history.
2. Structures of Polymyxins
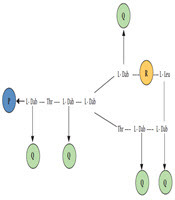
Polymyxins have a basic chemical structure similar to the structure of defensins and gramicidins, which are cationic peptides showing first-line antimicrobial properties in eukaryotic cells (12). Polymyxins are cationic polypeptides that contain a cyclic heptapeptide occupying a tripeptide side chain which is acylated at its N-terminus by a fatty acid tail (13, 14). A single amino acid in the peptide ring differentiates polymyxin B (Figure 1) and colistin (Figure 1) from each other; there are only phenylalanine and leucine in polymyxin B and colistin, respectively. Depending on the length of fatty acyl chain, the European Pharmacopoeia accepts two components of colistin-colistin A and B, and two components of polymyxin B, polymyxin B1 and B2 (Figure 1) (15). Polymyxin B is the active form and it is administered directly. On the other hand, colistin is administered as a prodrug in the form of colistimethate sodium (CMS) also known as, colistin methanesulfonate (15). Polyanionic inactive prodrug CMS is formed by reacting colistin with formaldehyde and sodium bisulfate (14, 16). In aqueous media in vitro and in biological fluids in vivo, CMS is converted into colistin; moreover, several methanesulfonate compounds are generated that are inactive in nature (17, 18).
The structures of colistimethate, colistin A and B, and polymyxin B1 and B2

3. Mechanism of Action of Polymyxins
There is no difference between polymyxin B and colistin regarding their target sites and pathways of action. Basically, polymyxins target the outer membrane of Gram-negative bacteria (19). The positively charged α,γ-diaminobutyric acid (Dab) residues of polymyxins interact with the negatively charged phosphate groups of the outer lipid A membrane of Gram-negative bacteria. As a result, this electrostatic interaction causes the displacement of divalent cations, Ca2+ and Mg2+, from the negatively charged phosphate groups (20). The displacement of Ca2+ and Mg2+ causes the chemical destabilization of lipopolysaccharides (LPS). This ultimately results in the increased permeability of the cell membrane, which allows for the leakage of the intracellular essential contents and finally leads to bacterial cell death (21, 22). Except for the mentioned LPS-target mechanism, no other killing mechanism of polymyxins has been declared to date (19).
Polymyxins also exhibit anti-endotoxin properties and exert this activity by binding with the outer membrane constituent, lipopolysaccharide (also known as endotoxin) of most Gram-negative bacteria and neutralizing its activity. Gram-negative bacteria possess endotoxins in their lipid A portions of LPS; polymyxins interact with these LPS molecules to finally neutralize (7). As the secondary mode of action of polymyxins, they inhibit type II NADH: quinone oxidoreductase (NDH-2) in the inner membrane of Gram-negative bacteria, which is a vital respiratory enzyme (23).
4. Pharmacokinetics of Polymyxins
Commercially, colistin is available in two forms, including colistin sulfate for topical and oral uses and CMS for parenteral and inhalation uses. Polymyxin B is commercially available only for parenteral and intrathecal uses. Polymyxins are not absorbed from the gastrointestinal tract (7). Colistimethate sodium is the inactive form of the drug with no antibacterial activity. By hydrolysis in vivo, it is converted into its active form colistin with 32 different sulfomethylated derivatives (15). Polymyxin B shows less inter-individual variability in plasma drug concentration and distributes well as an unbound form in the liver, lung, heart, skeletal muscles, and kidneys (24). On the other hand, colistin is poorly distributed to the pleural cavity, lung parenchyma, bones, and cerebrospinal fluid (15% to 25%) (25).
After an intravenous administration, the major portion of CMS is eliminated as an unchanged form through kidneys by glomerular filtration and active tubular secretion (Figure 2) in the first 24 h (18). A recent study showed that after an intravenous single administration of CMS, 70% of the CMS doses (1 million IU) were excreted in the urine (26). In healthy individuals, only 20% to 25% of a CMS dose is converted rapidly into active colistin through hydrolysis in the plasma (15). In vivo, the renal clearance of CMS depends on creatinine clearance but colistin clearance does not depend on creatinine clearance (25). Extensive renal tubular reabsorption causes less concentration of colistin in urine. Relatively higher concentrations of colistin are eliminated by nonrenal pathways with unclear mechanisms (19, 25). The concentration of active colistin in the urinary tract is relatively higher following an intravenous administration of CMS because profusely excreting CMS is also converted into active colistin in the kidney (the intensity of conversion is not yet established); it ultimately increases the total concentration (through primary and secondary concentration) of colistin in the urinary tract (19, 25, 26). In renal impairment, the excretion of CMS by kidneys is reduced and the major fraction of a CMS dose is converted into colistin with a more prolonged half-life (7).
Overview of pharmacokinetic pathways of CMS and colistin; the thickness of the arrows indicates the intensity of clearance; yellow color indicates the blood plasma

On the other hand, polymyxin B is the active antibacterial form of the drug. Extensive renal tubular reabsorption of polymyxin B causes less residence time in kidneys, fast turn back into the blood, and less concentration in the urine; most of its elimination is through the nonrenal pathway (Figure 3) (15). Polymyxin B accumulates substantially in the heart, liver, kidneys, muscles, and lung tissues, and it can only cross the blood-brain barrier in meningitis (7).
Overview of pharmacokinetic pathways of polymyxin B; the thickness of the arrows indicates the intensity of clearance

Colistimethate sodium exhibits a very low level of plasma protein binding whereas colistin and polymyxin B possess up to 50% and 92.4% protein binding, respectively (24, 25, 27). After an intravenous bolus administration of CMS, the peak plasma level of colistin is attained within 10 minutes but it is declined relatively more rapidly (25). After an intravenous administration, the serum half-life of CMS is approximately 1.5 - 2 h while colistin shows an estimated serum half-life of 14.4 h (15, 19, 25). Polymyxin B1 represents the major characteristics of polymyxin B (28). In both sound and impaired renal functions, polymyxin B shows relatively similar age-dependent serum elimination half-life (3.1 to 13.6) and approximately 40% unbound drug concentration is maintained in plasma (24, 28-30).
5. Pharmacodynamics of Polymyxins
Polymyxins are usually inactive to Gram-positive bacteria and only show concentration-dependent bactericidal activity against Gram-negative bacteria including MDR bacteria (7). To date, clinical pharmacokinetic and pharmacodynamic data of polymyxin B are limited compared to colistin. Most of the pharmacodynamic studies of polymyxins were in vitro studies where only colistin was considered. In multiple in vitro studies, both polymyxin B and colistin were found with potential antibacterial activity against most isolates of E. coli, K. pneumonia, Acinetobacter spp. and P. aeruginosa at clinically achievable concentrations (MIC90) of ≤ 1 µg/mL, ≤ 2 µg/mL, ≤ 2 µg/mL, and ≤ 2 µg/mL, respectively (3, 31-33). A study found that colistin possesses a concentration-dependent rapid killing property, but re-growth (within 3 h) and substantial re-growth (within 24 h) of organisms occurs substantially (31). Polymyxin B also shows a similar concentration-dependent rapid killing property and the occurrence of re-growth is also determined (34-36). Some current clinical studies suggest that colistin shows limited efficacy against most Gram-negative organisms in lung infections when administered intravenously because of its high molecular weight and relatively higher water solubility that interrupt its penetration into lung tissue and attainment of the required MIC (37, 38).
6. Toxicodynamics of Polymyxins
Most of the current studies show that nephrotoxicity is the most common side effect, with a 60% incidence rate associated with both colistin and polymyxin B following intravenous administration (29, 39, 40). A study found that within two days of initiation of polymyxins intravenous therapy, nephrotoxicity was a side effect with the fastest onset and most of the nephrotoxicity cases were recorded after 15 days of therapy (29). In comparison with polymyxin B, colistin accumulates in the kidneys more. A recent analytical study showed that nephrotoxicity occurs more frequently when colistin elicits Css,avg (average concentration achieved during a steady-state intermittent dosing interval) level of more than 2.5 mg/L and CLCr (creatinine clearance) rate of more than 80 mL/min (41, 42). Even nephrotoxicity may be developed with colistin at a low plasma concentration (≥ 2.2 mg/L) (41). Therefore, the toxicity of polymyxins, mostly nephrotoxicity, is a common incidence but, in most cases, it is a reversible phenomenon (29, 40, 41).
7. Dosage, Minimum Inhibitory Concentration (MIC), and Resistance Pattern of Polymyxins
In the United States, the recommended intravenous dose of CMS for a person with 60 kg body weight is 6.67 to 13.3 mg per kg body weight per day in two to four divided dosages. In the United Kingdom, the dose is 4 to 6 mg per kg body weight per day in three divided dosages. For CMS, the highest daily recommended intravenous dose varies from 400 to 800 mg per day (14). Commercially, polymyxin B is mostly available in the IU unit globally where 10,000 IU is equal to 1 mg and the recommended intravenous dosage of polymyxin B is 1.5 to 2.5 mg per kg body weight per day divided into two equal doses (7). Colistin shows variable plasma drug concentrations in respect of time. A recent study of 105 critically ill patients found that 50% higher first intravenous dose of CMS produced a colistin plasma concentration lower than colistin plasma concentration at the steady-state gained after the fourth dose (43). This is while with the recommended dose, polymyxin B showed relatively more sustainable plasma drug concentration than colistin (15).
The Clinical and Laboratory Standards Institute (CLSI) recommends broth micro dilution (BMD) in their guidelines as the standard reference method for the determination of MICs of polymyxins where reference MICs for E. coli and P. aeruginosa are 0.25 - 2 μg/mL and 0.5 - 4 μg/mL, respectively, and the breakpoint MIC is ≥ 2 μg/mL for Gram-negative superbugs including A. baumannii (44). Recently, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) has recommended the new MIC (4 to 8 μg/mL) of colistin for a new strain of E. coli (45). The EUCAST also recommends the MIC ≤ 2 mg/L for A. baumannii, E. coli, and K. pneumonia and MIC ≤ 4 mg/L for P. aeruginosa (44). A study under the SENTRY Antimicrobial Surveillance Program (2006 to 2009) showed that polymyxin B had in vitro characteristics similar to those of colistin against Gram-negative bacteria and the resistance rates were only < 0.1% - 1.5% (46). However, over the last few decades, the resistance trends have been increasing in an alarming rate among the most common hospital and/or community pathogens including Klebsiella pneumoniae, Acinetobacter baumannii, multiple Pseudomonas spp., and Escherichia coli worldwide (Table 1) (47-57); in some cases, carbapenems-resistant microbial isolates also showed resistance to polymyxins (47, 48), making such resistant organism-associated hospital infections difficult-to-treat (Table 1) (47).
| Antibiotics | Klebsiella pneumoniae | Acinetobacter baumannii | Escherichia coli | Pseudomonas spp. | ||||
|---|---|---|---|---|---|---|---|---|
| CS, % | CR, % | CS, % | CR, % | CS, % | CR, % | CS, % | CR, % | |
| Polymyxin B | < 5 | 6.8 - 35.5 | 3 | Insufficient data | 7.3 | 5.7 | 11.7 | Insufficient data |
| Colistin | 1.5 - 6.8 | 13 - 31.4 | 0 - 6.45 | 4.4 | 0.5 - 1.1 | Insufficient data | 2 | Insufficient data |
8. Rational Use of the Last Resort: Polymyxins
Globally, the threats of prevalent Gram-negative superbugs are increasing day-by-day; simultaneously, the scarcity of new effective antibiotics is a great concern in the upcoming years (3). Although with limited comprehensive clinical data and some mega studies, polymyxins are practically considered as the last resort antibiotics still showing potentiality against Gram-negative bacteria (58). The growing resistance mechanisms among these Gram-negative bacteria are unprecedented, such as the case of carbapenem-resistant New Delhi β-lactamase (NDM)-1 generating K. pneumonia spread to 40 countries within five years from the date of its first detection in 2008 (59). Studies show that the irrational use of antibiotics not only increases the rate of infection-associated mortality but also increases the number of MDR bacteria (60, 61). One surveillance report mentioned that sometimes clinicians use all the last-line reserve group antibiotics inappropriately, leading to the production of MDR bugs (46). Globally, the irrational use of polymyxins increases the number of polymyxins-resistant Gram-negative bacteria and this is nothing but a careless approach to the use of a few remaining last-resort antibiotics (3). For example, in China, the use of polymyxins is currently not available. In 2010, a national surveillance program was conducted in 129 hospitals in China where and it was reported that the susceptibility rates of P. aeruginosa and A. baumannii to polymyxin B are 96.4% and 97.2%, respectively (3). Injudicious initiation of antibiotics and inappropriate adjustment of antibiotic dosages in renally impaired patients have led to the emergence of resistance, which ultimately results in therapeutic failure and fatal toxicities in patients (62). A review study emphasized the intelligent use of polymyxins based on adequate pharmacokinetic and pharmacodynamic knowledge to keep the antibacterial potentiality of polymyxins against superbugs (63). In this alarming situation, the use of polymyxin-based combination therapies in moderate-to-severe infections may be an effective approach to reduce the prevalence of resistance in Gram-negative bacteria and optimize the therapeutic outcomes of anti-bacterial therapies. The research found a significant synergistic response for polymyxins when used in combination with carbapenems against MDR organisms (64). Though polymyxins have limited comprehensive clinical data, the irrational use of polymyxins, either as monotherapy or combination therapy, the goal of the therapy will not be attained, and the superbugs’ resistance scenarios will be more appalling in the nearest future (58, 63, 64).
9. Therapeutic Specification of Polymyxins
Colistin is mostly accumulated in urine whereas polymyxin B is accumulated relatively more in the bloodstream (19, 65). Studies showed that in the intravenous colistin administration in patients with healthy renal function, the targeted serum colistin concentration was not fully attained or sustained for a desirable period because of the high rate of CMS elimination through the kidneys in the early hours of dose administration (43, 66). On the other hand, serum polymyxin B concentration was not deviated from the targeted serum level in patients with impaired or healthy renal function because of its direct active form and non-renal excretion property (30, 67). A large pharmacokinetic study was conducted to determine the actual serum colistin steady-state concentration and researchers estimated the serum colistin steady-state concentration of 2.5 mg/L to an area under the curve (AUC) of 60 mg × h/L (43). The study also found that the usual colistin dosage regimen yielded sufficient serum colistin concentration with a MIC of < 1 µ/mL, which is inadequate to treat moderate-to severe-infections caused by A. baumannii. However, if a MIC of > 1 µ/mL is needed to maintain to treat infections caused by A. baumannii, then it would require a double dosage regimen of colistin, which would enhance the risk of nephrotoxicity (43). Another study was conducted on patients with MDR Gram-negative bacterial infections to administer CMS intravenously. After the analysis of urine samples at different time intervals, researchers found a high urinary concentration (up to 95.4 mg/L) of colistin in early hours (68). In 2013, a population PK analysis of polymyxin B on 24 patients was conducted where the authors used Monte Carlo analysis to establish a correlation between the dosage regimen and the achievable target of fAUC:MIC (30). Finally, research showed that a total dose of 3 mg/kg body weight (30,000 IU/kg body weight) daily maintenance can produce a MIC of < 1 µ/mL in the blood, while it is active against A. baumannii; but if a MIC of > 1 µ/mL is necessary to treat infections caused by A. baumannii, a double dosage regimen than the recommended dose is required; though polymyxin B is accumulated less than colistin in kidneys, nephrotoxicity is not a prime concern for polymyxin B (30, 41, 42, 67).
In a multi-center phase II clinical study with patients suffering from serious bloodstream infections due to XDR Gram-negative bacteria, 78.1% of the total patients were successfully treated with polymyxin B (69). In the same study, researchers assumed that colistin should be considered for urinary tract infections instead of polymyxin B because of the colistin’s comparatively higher concentration in urine after the recommended dosage, which is sufficient to maintain the targeted MIC sustainably in the urinary tract for a longer period (19, 69). Another large prospective cohort study was conducted only on patients (55% of whom aged > 60 years) with moderate to severe urinary tract infections; they were successfully treated with colistin monotherapy with a cure rate of 80% (70).
Therefore, from the above multi-clinical evidence-based discussions, a strong opinion can be evolved easily in the context of the rational use of polymyxin B and colistin; that is, in order to optimize the therapeutic effectiveness of reserve polymyxins antibiotics, their therapeutic selection should be infection site-specific considering all their PK and PD characteristics and based on recent clinical evidence. The use of polymyxins should be rationalized always focusing on the optimized therapeutic outcomes. Though the therapeutic indications of polymyxin are well recommended notwithstanding, concerning the PK and PD characteristics of polymyxin B and colistin, we suggest that polymyxin B be specified mostly in bloodstream infections whereas colistin be specified mostly in urinary tract infections caused by MDR Gram-negative bacteria. This intelligent therapeutic specification technique may predominantly enhance the therapeutic potentiality of polymyxins in life-threatening MDR Gram-negative bacterial infections; it can also be effective in slowing down the emergence of polymyxin resistance.
10. Conclusions
Nowadays, the tremendous rate of increasing antibiotic resistance among MDR Gram-negative bacteria is a threat to global human health. At present, a few antibiotics including polymyxins are still showing effectiveness against these MDR Gram-negative bacteria. The rational use of last resort polymyxin B and colistin is highly required at this moment. The comprehensive PK and PD data of polymyxin B and colistin strongly assume the possibility of achieving optimum therapeutic outcomes with the therapeutic specifications of polymyxin B and colistin for MDR Gram-negative bacteria-associated bloodstream infections and urinary tract infections, respectively. This intelligent attempt may reduce the evolvement of polymyxin resistance among Gram-negative bacteria.
References
-
1.
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1-12. [PubMed ID: 19035777]. https://doi.org/10.1086/595011.
-
2.
World Health Organization. Antibiotic resistance. 2016, [cited 2018 Sep 10]. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
-
3.
Bergen PJ, Landersdorfer CB, Zhang J, Zhao M, Lee HJ, Nation RL, et al. Pharmacokinetics and pharmacodynamics of 'old' polymyxins: What is new? Diagn Microbiol Infect Dis. 2012;74(3):213-23. [PubMed ID: 22959816]. [PubMed Central ID: PMC3477253]. https://doi.org/10.1016/j.diagmicrobio.2012.07.010.
-
4.
Montero A, Corbella X, Ariza J. Clinical relevance of Acinetobacter baumannii ventilator-associated pneumonia. Crit Care Med. 2003;31(10):2557-9. [PubMed ID: 14530770]. https://doi.org/10.1097/01.CCM.0000089937.38406.9F.
-
5.
Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867-903. [PubMed ID: 11934711]. https://doi.org/10.1164/ajrccm.165.7.2105078.
-
6.
Storm DR, Rosenthal KS, Swanson PE. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723-63. [PubMed ID: 197881]. https://doi.org/10.1146/annurev.bi.46.070177.003451.
-
7.
Gupta S, Govil D, Kakar PN, Prakash O, Arora D, Das S, et al. Colistin and polymyxin B: A re-emergence. Indian J Crit Care Med. 2009;13(2):49-53. [PubMed ID: 19881183]. [PubMed Central ID: PMC2772240]. https://doi.org/10.4103/0972-5229.56048.
-
8.
Koch-Weser J, Sidel VW, Federman EB, Kanarek P, Finer DC, Eaton AE. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann Intern Med. 1970;72(6):857-68. [PubMed ID: 5448745]. https://doi.org/10.7326/0003-4819-72-6-857.
-
9.
Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, et al. Framework for optimisation of the clinical use of colistin and polymyxin B: The Prato polymyxin consensus. Lancet Infect Dis. 2015;15(2):225-34. [PubMed ID: 25459221]. https://doi.org/10.1016/S1473-3099(14)70850-3.
-
10.
National Center for Emerging and Zoonotic Infectious Diseases. Antibiotic/antimicrobial resistance: Antibiotic resistance threats in the United States. 2018, [cited 2018 Sep 17]. Available from: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
-
11.
Ahiskali A, Gens K. The great debate: Polymyxin b versus polymyxin e. contagion, infectious diseases today, 2017. [cited 2018 Sep 10]. Available from: https://www.contagionlive.com/publications/contagion/2017/february2017/the-great-debate-polymyxin-b-versus-polymyxin-e.
-
12.
Hancock RE. Peptide antibiotics. Lancet. 1997;349(9049):418-22. [PubMed ID: 9033483]. https://doi.org/10.1016/S0140-6736(97)80051-7.
-
13.
Falagas ME, Rafailidis PI, Matthaiou DK. Resistance to polymyxins: Mechanisms, frequency and treatment options. Drug Resist Updat. 2010;13(4-5):132-8. [PubMed ID: 20843473]. https://doi.org/10.1016/j.drup.2010.05.002.
-
14.
Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, et al. Colistin: The re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6(9):589-601. [PubMed ID: 16931410]. https://doi.org/10.1016/S1473-3099(06)70580-1.
-
15.
Nation RL, Velkov T, Li J. Colistin and polymyxin B: Peas in a pod, or chalk and cheese? Clin Infect Dis. 2014;59(1):88-94. [PubMed ID: 24700659]. [PubMed Central ID: PMC4305129]. https://doi.org/10.1093/cid/ciu213.
-
16.
Barnett M, Bushby SR, Wilkinson S. Sodium sulphomethyl derivatives of polymyxins. Br J Pharmacol Chemother. 1964;23:552-74. [PubMed ID: 14256814]. [PubMed Central ID: PMC1704012]. https://doi.org/10.1111/j.1476-5381.1964.tb01610.x.
-
17.
Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob Agents Chemother. 2003;47(4):1364-70. [PubMed ID: 12654671]. [PubMed Central ID: PMC152538]. https://doi.org/10.1128/aac.47.4.1364-1370.2003.
-
18.
Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother. 2004;53(5):837-40. [PubMed ID: 15044428]. https://doi.org/10.1093/jac/dkh167.
-
19.
Poirel L, Jayol A, Nordmann P. Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev. 2017;30(2):557-96. [PubMed ID: 28275006]. [PubMed Central ID: PMC5355641]. https://doi.org/10.1128/CMR.00064-16.
-
20.
Dixon RA, Chopra I. Leakage of periplasmic proteins from Escherichia coli mediated by polymyxin B nonapeptide. Antimicrob Agents Chemother. 1986;29(5):781-8. [PubMed ID: 3015004]. [PubMed Central ID: PMC284154]. https://doi.org/10.1128/aac.29.5.781.
-
21.
Newton BA. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956;20(1):14-27. [PubMed ID: 13303920]. [PubMed Central ID: PMC180843].
-
22.
Schindler M, Osborn MJ. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry. 1979;18(20):4425-30. [PubMed ID: 226126]. https://doi.org/10.1021/bi00587a024.
-
23.
Deris ZZ, Akter J, Sivanesan S, Roberts KD, Thompson PE, Nation RL, et al. A secondary mode of action of polymyxins against Gram-negative bacteria involves the inhibition of NADH-quinone oxidoreductase activity. J Antibiot (Tokyo). 2014;67(2):147-51. [PubMed ID: 24169795]. [PubMed Central ID: PMC3943757]. https://doi.org/10.1038/ja.2013.111.
-
24.
Zavascki AP, Goldani LZ, Cao G, Superti SV, Lutz L, Barth AL, et al. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis. 2008;47(10):1298-304. [PubMed ID: 18840079]. https://doi.org/10.1086/592577.
-
25.
Michalopoulos AS, Falagas ME. Colistin: Recent data on pharmacodynamics properties and clinical efficacy in critically ill patients. Ann Intensive Care. 2011;1(1):30. [PubMed ID: 21906382]. [PubMed Central ID: PMC3224467]. https://doi.org/10.1186/2110-5820-1-30.
-
26.
Couet W, Gregoire N, Gobin P, Saulnier PJ, Frasca D, Marchand S, et al. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin Pharmacol Ther. 2011;89(6):875-9. [PubMed ID: 21544080]. https://doi.org/10.1038/clpt.2011.48.
-
27.
Abodakpi H, Gohlke J, Chang KT, Chow DS, Tam VH. Analytical and functional determination of polymyxin B protein binding in serum. Antimicrob Agents Chemother. 2015;59(11):7121-3. [PubMed ID: 26324262]. [PubMed Central ID: PMC4604353]. https://doi.org/10.1128/AAC.01815-15.
-
28.
Kwa AL, Abdelraouf K, Low JG, Tam VH. Pharmacokinetics of polymyxin B in a patient with renal insufficiency: A case report. Clin Infect Dis. 2011;52(10):1280-1. [PubMed ID: 21507928]. [PubMed Central ID: PMC3115279]. https://doi.org/10.1093/cid/cir137.
-
29.
Phe K, Lee Y, McDaneld PM, Prasad N, Yin T, Figueroa DA, et al. In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob Agents Chemother. 2014;58(5):2740-6. [PubMed ID: 24566187]. [PubMed Central ID: PMC3993221]. https://doi.org/10.1128/AAC.02476-13.
-
30.
Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: Implications for selection of dosage regimens. Clin Infect Dis. 2013;57(4):524-31. [PubMed ID: 23697744]. https://doi.org/10.1093/cid/cit334.
-
31.
Owen RJ, Li J, Nation RL, Spelman D. In vitro pharmacodynamics of colistin against Acinetobacter baumannii clinical isolates. J Antimicrob Chemother. 2007;59(3):473-7. [PubMed ID: 17289768]. https://doi.org/10.1093/jac/dkl512.
-
32.
Poudyal A, Howden BP, Bell JM, Gao W, Owen RJ, Turnidge JD, et al. In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2008;62(6):1311-8. [PubMed ID: 18922815]. https://doi.org/10.1093/jac/dkn425.
-
33.
Bergen PJ, Bulitta JB, Forrest A, Tsuji BT, Li J, Nation RL. Pharmacokinetic/pharmacodynamic investigation of colistin against Pseudomonas aeruginosa using an in vitro model. Antimicrob Agents Chemother. 2010;54(9):3783-9. [PubMed ID: 20585118]. [PubMed Central ID: PMC2934992]. https://doi.org/10.1128/AAC.00903-09.
-
34.
Abdul Rahim N, Cheah SE, Johnson MD, Yu H, Sidjabat HE, Boyce J, et al. Synergistic killing of NDM-producing MDR Klebsiella pneumoniae by two 'old' antibiotics-polymyxin B and chloramphenicol. J Antimicrob Chemother. 2015;70(9):2589-97. [PubMed ID: 26023209]. [PubMed Central ID: PMC4635649]. https://doi.org/10.1093/jac/dkv135.
-
35.
Tran TB, Cheah SE, Yu HH, Bergen PJ, Nation RL, Creek DJ, et al. Anthelmintic closantel enhances bacterial killing of polymyxin B against multidrug-resistant Acinetobacter baumannii. J Antibiot (Tokyo). 2016;69(6):415-21. [PubMed ID: 26669752]. [PubMed Central ID: PMC4911330]. https://doi.org/10.1038/ja.2015.127.
-
36.
Tam VH, Schilling AN, Vo G, Kabbara S, Kwa AL, Wiederhold NP, et al. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49(9):3624-30. [PubMed ID: 16127031]. [PubMed Central ID: PMC1195418]. https://doi.org/10.1128/AAC.49.9.3624-3630.2005.
-
37.
Yapa SWS, Li J, Patel K, Wilson JW, Dooley MJ, George J, et al. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: Targeting advantage of inhalational administration. Antimicrob Agents Chemother. 2014;58(5):2570-9. [PubMed ID: 24550334]. [PubMed Central ID: PMC3993267]. https://doi.org/10.1128/AAC.01705-13.
-
38.
Cheah SE, Wang J, Nguyen VT, Turnidge JD, Li J, Nation RL. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: Smaller response in lung infection. J Antimicrob Chemother. 2015;70(12):3291-7. [PubMed ID: 26318190]. https://doi.org/10.1093/jac/dkv267.
-
39.
Akajagbor DS, Wilson SL, Shere-Wolfe KD, Dakum P, Charurat ME, Gilliam BL. Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center. Clin Infect Dis. 2013;57(9):1300-3. [PubMed ID: 23840000]. https://doi.org/10.1093/cid/cit453.
-
40.
Kubin CJ, Ellman TM, Phadke V, Haynes LJ, Calfee DP, Yin MT. Incidence and predictors of acute kidney injury associated with intravenous polymyxin B therapy. J Infect. 2012;65(1):80-7. [PubMed ID: 22326553]. https://doi.org/10.1016/j.jinf.2012.01.015.
-
41.
Sorli L, Luque S, Grau S, Berenguer N, Segura C, Montero MM, et al. Trough colistin plasma level is an independent risk factor for nephrotoxicity: A prospective observational cohort study. BMC Infect Dis. 2013;13:380. [PubMed ID: 23957376]. [PubMed Central ID: PMC3765824]. https://doi.org/10.1186/1471-2334-13-380.
-
42.
Forrest A, Silveira FP, Thamlikitkul V, Garonzik SM, Mandragos K, Shoham S, et al. Toxicodynamics for colistin-associated changes in creatinine clearance. Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington DC. ASM Press; 2014. p. 5-9.
-
43.
Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55(7):3284-94. [PubMed ID: 21555763]. [PubMed Central ID: PMC3122440]. https://doi.org/10.1128/AAC.01733-10.
-
44.
Clinical and Laboratory Standards Institute. Performance standard for antimicrobial susceptibility testing; twenty-fifth informational supplement, 2015. 2018, [cited 2018 Sep 15]. Available from: http://file.qums.ac.ir/repository/mmrc/CLSI2015.pdf.
-
45.
Carretto E, Brovarone F, Russello G, Nardini P, El-Bouseary MM, Aboklaish AF, et al. Clinical validation of SensiTest colistin, a broth microdilution-based method to evaluate colistin MICs. J Clin Microbiol. 2018;56(4). [PubMed ID: 29343542]. [PubMed Central ID: PMC5869822]. https://doi.org/10.1128/JCM.01523-17.
-
46.
Gales AC, Jones RN, Sader HS. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: Results from the SENTRY Antimicrobial Surveillance Program (2006-09). J Antimicrob Chemother. 2011;66(9):2070-4. [PubMed ID: 21715434]. https://doi.org/10.1093/jac/dkr239.
-
47.
Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161-8. [PubMed ID: 26603172]. https://doi.org/10.1016/S1473-3099(15)00424-7.
-
48.
Lekunberri I, Balcazar JL, Borrego CM. Detection and quantification of the plasmid-mediated mcr-1 gene conferring colistin resistance in wastewater. Int J Antimicrob Agents. 2017;50(6):734-6. [PubMed ID: 28842282]. https://doi.org/10.1016/j.ijantimicag.2017.08.018.
-
49.
Moubareck CA, Mouftah SF, Pal T, Ghazawi A, Halat DH, Nabi A, et al. Clonal emergence of Klebsiella pneumoniae ST14 co-producing OXA-48-type and NDM carbapenemases with high rate of colistin resistance in Dubai, United Arab Emirates. Int J Antimicrob Agents. 2018;52(1):90-5. [PubMed ID: 29530587]. https://doi.org/10.1016/j.ijantimicag.2018.03.003.
-
50.
Giamarellou H. Epidemiology of infections caused by polymyxin-resistant pathogens. Int J Antimicrob Agents. 2016;48(6):614-21. [PubMed ID: 27865627]. https://doi.org/10.1016/j.ijantimicag.2016.09.025.
-
51.
Kuo SC, Huang WC, Wang HY, Shiau YR, Cheng MF, Lauderdale TL. Colistin resistance gene mcr-1 in Escherichia coli isolates from humans and retail meats, Taiwan. J Antimicrob Chemother. 2016;71(8):2327-9. [PubMed ID: 27076107]. https://doi.org/10.1093/jac/dkw122.
-
52.
European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe 2017. 2017, [cited 2019 Oct 22]. Available from: https://ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2017.
-
53.
Li B, Ke B, Zhao X, Guo Y, Wang W, Wang X, et al. Antimicrobial resistance profile of mcr-1 positive clinical isolates of Escherichia coli in China from 2013 to 2016. Front Microbiol. 2018;9:2514. [PubMed ID: 30405572]. [PubMed Central ID: PMC6206212]. https://doi.org/10.3389/fmicb.2018.02514.
-
54.
Bartolleti F, Seco BM, Capuzzo Dos Santos C, Felipe CB, Lemo ME, Alves Tda S, et al. Polymyxin B resistance in carbapenem-resistant Klebsiella pneumoniae, Sao Paulo, Brazil. Emerg Infect Dis. 2016;22(10):1849-51. [PubMed ID: 27648951]. [PubMed Central ID: PMC5038415]. https://doi.org/10.3201/eid2210.160695.
-
55.
Mirnejad R, Heidary M, Bahramian A, Goudarzi M, Pournajaf A. Evaluation of polymyxin B susceptibility profile and detection of drug resistance genes among Acinetobacter baumannii clinical isolates in Tehran, Iran during 2015-2016. Mediterr J Hematol Infect Dis. 2018;10(1). e2018044. [PubMed ID: 30002800]. [PubMed Central ID: PMC6039082]. https://doi.org/10.4084/MJHID.2018.044.
-
56.
Gales AC, Jones RN, Sader HS. Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: Report from the SENTRY Antimicrobial Surveillance Programme (2001-2004). Clin Microbiol Infect. 2006;12(4):315-21. [PubMed ID: 16524407]. https://doi.org/10.1111/j.1469-0691.2005.01351.x.
-
57.
Goli HR, Nahaei MR, Ahangarzadeh Rezaee M, Hasani A, Samadi Kafil H, Aghazadeh M. Emergence of colistin resistant Pseudomonas aeruginosa at Tabriz hospitals, Iran. Iran J Microbiol. 2016;8(1):62-9. [PubMed ID: 27092226]. [PubMed Central ID: PMC4833742].
-
58.
Huttner B, Jones M, Rubin MA, Neuhauser MM, Gundlapalli A, Samore M. Drugs of last resort? The use of polymyxins and tigecycline at US veterans affairs medical centers, 2005-2010. PLoS One. 2012;7(5). e36649. [PubMed ID: 22615789]. [PubMed Central ID: PMC3353942]. https://doi.org/10.1371/journal.pone.0036649.
-
59.
Johnson AP, Woodford N. Global spread of antibiotic resistance: The example of New Delhi metallo-beta-lactamase (NDM)-mediated carbapenem resistance. J Med Microbiol. 2013;62(Pt 4):499-513. [PubMed ID: 23329317]. https://doi.org/10.1099/jmm.0.052555-0.
-
60.
Deris ZZ, Harun A, Shafei MN, Rahman RA, Johari MR. Outcomes and appropriateness of management of nosocomial Acinetobacter bloodstream infections at a teaching hospital in northeastern Malaysia. Southeast Asian J Trop Med Public Health. 2009;40(1):140-7. [PubMed ID: 19323046].
-
61.
Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057-98. [PubMed ID: 24252483]. https://doi.org/10.1016/S1473-3099(13)70318-9.
-
62.
Kumar A, Singh NP. Antimicrobial dosing in critically ill patients with sepsis-induced acute kidney injury. Indian J Crit Care Med. 2015;19(2):99-108. [PubMed ID: 25722552]. [PubMed Central ID: PMC4339912]. https://doi.org/10.4103/0972-5229.151018.
-
63.
Deris ZZ. The multidrug-resistant Gram-negative superbugs threat require intelligent use of the last weapon. Malays J Med Sci. 2015;22(5):1-6. [PubMed ID: 28239263]. [PubMed Central ID: PMC5295735].
-
64.
Bergen PJ, Bulman ZP, Saju S, Bulitta JB, Landersdorfer C, Forrest A, et al. Polymyxin combinations: Pharmacokinetics and pharmacodynamics for rationale use. Pharmacotherapy. 2015;35(1):34-42. [PubMed ID: 25630411]. [PubMed Central ID: PMC5215892]. https://doi.org/10.1002/phar.1537.
-
65.
Kassamali Z, Jain R, Danziger LH. An update on the arsenal for multidrug-resistant Acinetobacter infections: Polymyxin antibiotics. Int J Infect Dis. 2015;30:125-32. [PubMed ID: 25461655]. https://doi.org/10.1016/j.ijid.2014.10.014.
-
66.
Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother. 2009;53(8):3430-6. [PubMed ID: 19433570]. [PubMed Central ID: PMC2715599]. https://doi.org/10.1128/AAC.01361-08.
-
67.
Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, et al. Pharmacokinetics of polymyxin B in patients on continuous venovenous haemodialysis. J Antimicrob Chemother. 2013;68(3):674-7. [PubMed ID: 23179561]. https://doi.org/10.1093/jac/dks437.
-
68.
Luque S, Escano C, Sorli L, Li J, Campillo N, Horcajada JP, et al. Urinary concentrations of colistimethate and formed colistin after intravenous administration in patients with multidrug-resistant Gram-Negative bacterial infections. Antimicrob Agents Chemother. 2017;61(8). [PubMed ID: 28559275]. [PubMed Central ID: PMC5527601]. https://doi.org/10.1128/AAC.02595-16.
-
69.
Ngamprasertchai T, Boonyasiri A, Charoenpong L, Nimitvilai S, Lorchirachoonkul N, Wattanamongkonsil L, et al. Effectiveness and safety of polymyxin B for the treatment of infections caused by extensively drug-resistant Gram-negative bacteria in Thailand. Infect Drug Resist. 2018;11:1219-24. [PubMed ID: 30154668]. [PubMed Central ID: PMC6108402]. https://doi.org/10.2147/IDR.S169939.
-
70.
Karakkattu J, Mohan A, James E, Kumar A. Effectiveness and safety of colistin in multi drug resistant urinary tract infections. J Appl Pharm Sci. 2017;7(9):148-52.