Abstract
Background:
Juvenile Idiopathic Arthritis (JIA) is accompanied by growth failure, mostly occurring due to chronic inflammation and use of corticosteroids for treatment. The aim of our study was to determine the prevalence of short stature in JIA patients and possible systemic disorders which may affect the growth pattern in this group of patients.Methods:
In this cross-sectional study erformed from June 2014 to May 2015, JIA patients with a history of more than one-year treatment were examined by an endocrinologist and based on their height standard deviation score (SDS) two groups were determined: Group A > -2SD and group B < -2SD. Complete blood count, thyroid function tests and 25OHD3 level were assessed in both groups, but other laboratory tests, including liver and renal function tests, growth hormone stimulation test, urine analysis and culture, as well as left hand and wrist X-ray for bone age determination, were done in group B.Results:
Of 117 JIA patients who were enrolled, 41 patients were under -2SD (19% of pauciarticular, 62% of poly articular and 33% of systemic onset). The mean height SDS in group B was -3.48 ± 1.28 (compared to -0.9 ± 0.8 in their parents). We found hypovitaminosis D in 73% of our patients. The prevalence of subclinical hypothyroidism was 7.4% (5% of group A and 9.7% of group B). Twenty-four percent (10 patients) of group B did not respond to growth hormone (GH) stimulation test and 14.6% of them (6 patients) were possibly GH resistant. Liver function tests and renal function tests were normal in all the patients. There was no difference between 2 groups in hypothyroidism and hypovitaminosis D but polyarticular type of the disease was associated with short stature (P Value < 0.000).Conclusions:
Growth failure is common in JIA patients. So they, especially those with polyarticular type, need to be visited periodically by an endocrinologist.Keywords
Juvenile Idiopathic Arthritis Growth Hormone Vitamin D Deficiency Hypothyroidism
1. Background
Juvenile idiopathic arthritis (JIA), the most common rheumatologic disorder in children, can be associated with growth failure (1, 2). Different proinflammatory cytokines (IL-1, IL-6, TNFα), long term treatment with corticosteroids, malnutrition and stress (related to being chronically ill or handicapped) may be responsible for growth retardation (1-4)
The proportion of the patients suffering from short stature in different studies ranges from 10 - 40 percent (5). Growth retardation is more common in systemic subtype and in patients with many joints affected (6, 7).
In addition to GH/IGF1 axis problems, other endocrinopathies such as hypothyroidism (8-10), hypovitaminosis D (11-13), hyperinsulinism, hyperglycemia (14, 15), hyperlipidemia (16, 17) and delayed puberty (5, 18-20) may be associated with JIA.
Regarding the prevalence of growth failure as a complication of JIA, few data are available in developing countries.
This study describes the growth status of JIA patients in our center.
2. Methods
This cross sectional study includes all JIA patients with a history of more than one year treatment between June 2014 - May 2015. They were divided into three subtypes based on the International League of Association for Rheumatology (ILAR) criteria (oligoarticular, polyarticular and systemic).
Patients were excluded if they had an auto inflammatory disease before JIA diagnosis, or were treated with growth hormone and if they were in remission longer than one year.
Height and weight of all the patients were measured and standard deviation scores were calculated. Based on their height standard deviation score (SDS) 2 groups were determined: Group A > -2SD and group B < -2SD.
For group B patients, a data sheet was completed including: demographic information, past medical history, drug history and height of the parents.
Complete blood count (CBC), thyroid function tests (because of the high probability of other autoimmune disorders in JIA) and 25(OH)D3 (because of the high prevalence of hypovitaminosis D in our country and its effect on the treatment of JIA patients) were checked in both groups but other lab tests including liver function tests, renal function tests, calcium, phosphorus, alkaline phosphatase, zinc level, fasting blood sugar (FBS), insulin growth factor 1 (IGF1), growth hormone (GH) stimulation test, urine analysis and urine culture were done in group B to evaluate other causes of short stature.
In GH stimulation test, GH was measured at baseline and after 30, 60, 90 minutes of oral clonidine tablets (150 µg/m2). Based on the result of this test, patients were divided into 3 categories:
1. Normal response (IGF1 normal and GH > 10 µU/mL after stimulation in at least one sample)
2. No response (GH didn’t increase more than 10 µU/mL after stimulation)
3. Possibly resistant to GH (GH increased more than 10 µU/mL after stimulation but IGF1 was lower than normal)
Clinical hypothyroidism was considered if free T4 was below normal with TSH increased. Subclinical hypothyroidism was defined as elevated TSH with normal free T4.
Serum 25(OH)D3 was measured by ELISA method. Levels between 10 - 30 ng/mL were considered as insufficient and below 10 ng/mL as deficient,
In hyperglycemic patients OGTT was done. Ferritin, TIBC, and serum iron were measured in anemic patients.
Bone age was determined by comparing left hand and wrist x-ray with Greulich and Pyle atlas of skeletal maturity by an experienced pediatric radiologist.
For analyses SPSS version 22 was used. Descriptive statistics was used to assess the demographic variables and characteristics of the disease. Numerical variables were compared with the Student's t test and multiple group analysis was done by one way analysis of variance (ANOVA).
For comparing nominal variables, chi-square test was used. P value <0.05 was considered as statistically significant.
Parents o all participants signed the informed consent. This study protocol was approved by the ethical committee of Tehran University of Medical Sciences.
3. Results
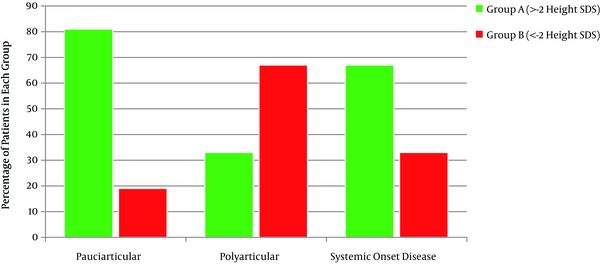
Out of 117 patients suffering from JIA, 60% (71 patients) were female. Thirty five percent of them were under -2 height SDS (Group B), with 19% oligoarticulars, 62% polyarticulars and 33% with systemic onset disease (totally 41 patients) (Figure 1). There was a strong correlation between patients’ height SDS and JIA subtypes (P = 0.000). The mean age of group B was 9.7 ± 4.47 years. Duration of their disease was 5.4 ± 3.96 years. The mean height SDS was -3.48 ± 1.28 and the mean mid parental height SDS was -0.9 ± 0.8. Older patients and patients with longer duration of the disease had lower height SDS (P = 0.001, P = 0.000). There was no correlation between patient’s height SDS with their mid parental height SDS, birth weight and birth length. BMI in 5% of group A and14.6% of group B was under 5 percentiles (underweight), on the other hand 27% of group A and 21.9% of group B were overweight (85% < BMI < 95%) and obese (> 95%) (Table 1). Comparing BMI in the two groups, considering sex and subtypes of the disease, revealed no statistically significant difference.
Growth status in different types of juvenile of idiopathic arthritis
Mean of Demographic Data of Short Stature JIA Patients
| Variable | Mean ± Standard Deviation | Minimum | Maximum |
|---|---|---|---|
| Birth weight, g | 3018 ± 644.23 | 1350 | 4350 |
| Birth length, cm | 48.4 ± 2.97 | 37 | 52 |
| Age, y | 9.70 ± 4.47 | 1.5 | 21.33 |
| Duration of disease, y | 5.41 ± 3.96 | 1 | 16 |
| Height SDS | -3.48 ± 1.28 | -7 | -2 |
| Mid parental height SDS | -0.92 ± 0.86 | -2.64 | 0.64 |
| BMI | 17.34 ± 3.66 | 12.62 | 30 |
| Bone age, y | 7.45 ± 3.94 | 0.83 | 17 |
The mean bone age of group B was 7.45 ± 3.94 which was about 2 years and one month behind the mean chronological age. Patients with more difference between chronological age and bone age had lower height SDS (Table 1).
In GH stimulation test, 56% had normal response, 14.6% did not respond and 24.4% showed possibility of GH resistance. Groups with no normal response to GH stimulation test had the lowest height SDS (P = 0.046).
Vitamin D level was insufficient in 61% and deficient in 11.4 % of all the patients. Group B who were suffering from hypovitaminosis D (insufficient + deficient) had lower height SDS too, although it was not statistically significant (P = 0.095).
Subclinical hypothyroidism was found in 7.4% of patients (9.7 % of group B and 5% of group A). Clinical hypothyroidism was not detected in any of the participants. All of the subclinical hypothyroidism patients had normal responses in GH stimulation test.
There was no difference in mean hemoglobin value between the two groups. Anemia was found in 36.6% of group B (1/3 iron deficiency anemia and 2/3 anemia of chronic disease). Iron deficiency anemia was more common in girls, and anemia of chronic disease was more common in boys) (P = 0.016).Group B patients with anemia of chronic disease had lower mean height SDS (P = 0.000) and longer duration of disease (P = 0.03).
Zinc level was below normal in 12.2% of group B patients. Liver function tests and renal function tests were normal in all of them. Turner syndrome was diagnosed in one of the group B patients.
4. Discussion
JIA is the most common rheumatologic disease in pediatrics, which is associated with significant negative effects on growth (1, 2). Short stature was detected in 1/3 of all JIA patients in our center which is greater than the number in the studies of Souza with 10.4% and Uettwiller with 19% (21, 22). About half of the patients included in this study were oligoarticular, but the most affected subtype was polyarticular (with 60%), which was statistically significant (P < 0.000). Zak found short stature in 11% of his polyarticular patients, which is less than what we found in our study (6). Furthermore, because of exclusion of short stature children who had received GH treatment in our study, the exact prevalence of growth failure is more than what we detected and it is so high that requires more investigations to determine its causes, especially in polyarticular subtype.
The proportion of female to male ratio was 1.7/1 in oligoarticulars, 1.6/1 in polyarticulars and 1/1 in systemic onset disease. This ratio is greater in other studies (23). Genetic factors may be involved in this issue.
The decreased height SDS, that didn’t have any correlation with birth weight, birth length and mid-parental height SDS shows the essential role of environmental factors.
About one-fourth of our patients were overweight and obese, which may be correlated to the immobility due to pain and corticosteroids used for the treatment, but due to the similarity of its prevalence between our two groups and the other studies in healthy children of our society, it seems that changing lifestyle in our country is the main reason (24-26). The prevalence of obesity and overweight in Pelajo’s and Gronlund’s studies was about 30% which is comparable to ours too (27, 28).Obesity may have a negative effect on the joints.
Chronic inflammation, subclinical hypothyroidism, decreased GH and GH resistance may be causes of delayed bone age of these patients.
Since autoimmunity has an important role in the pathophysiology of JIA, other autoimmune disorders were investigated in these patients (8). Alpagini reported more thyroid autoantibodies in JIA patients than that in general population 9. The prevalence of subclinical hypothyroidism in our patients was 7.4% compared to 9% in Stagi’s and 5% in Unsel’s study (8). As Unsal found, there was no significant difference in subclinical hypothyroidism between the two groups (29). So, subclinical hypothyroidism would not have an important role in growth retardation in JIA patients.
Osteopenia is more common in JIA especially in polyarticular and systemic onset types (30). Vitamin D deficiency and elevated PTH that may aggravate osteopenia and growth, is more common too (11, 31). Some researchers have attributed vitamin D level to disease activity (13, 32). The prevalence of hypovitaminosis D in our study was 73%, roughly the same as Bouddi’s research result (11). Since hypovitaminosis D is really common in the general population of our society, we cannot attribute it to JIA. The prevalence of hypovitaminosis D was 86% in 9 - 12 years old children in Neyestani’s study (33). Rabbani et al found vitamin D insufficiency in 53.6% of girls and 11.3% of 7 - 18 years old boys of Tehran (34). Although treatment of vitamin D deficiency in JIA is very helpful.
The group that didn’t respond to GH stimulation test (24.5%) and those with GH resistance (14.6%) had lower height SDS. These patients may have resistance to GH or IGF1. In both of them GH treatment may be helpful. In the former group, GH treatment compensates their deficiency and in latter one, it may overcome their resistance. Simon and Becthold showed that treatment with GH can improve final height in JIA patients (4, 35).
Previous studies have stressed on intermittent monitoring of BS and HbA1C especially in those taking GH and corticosteroids simultaneously because insulin level was higher in this group than in other JIA patients (14, 15). Hyperglycemia was not common in our study and hyperglycemic children had normal fasting and 2 h pp blood sugar later in observation.
Improving nutritional status is one of the steps of treatment in JIA patients with short stature, although anemia seems not to be a predictor of growth failure in this disease. Zinc is a modulator of the immune response and chronic deficiency of it increases proinflammatory cytokines (36, 37). The prevalence of zinc deficiency in general population is not clear, but in particular JIA patients with zinc deficiency will benefit from zinc treatment.
Growth failure in our JIA patients was not the result of liver or renal dysfunction.
Because of the negative influence of short stature on self-related health and life satisfaction, prevention of growth failure or its treatment can improve self-esteem and emotional well-being in the affected children (38, 39).
4.1. Conclusions
Growth failure and endocrine disorders are common among JIA patients. According to new treatment options and increasing lifespan in these patients, meticulous attention to decline of growth velocity as one of the major complications of the disease is essential. The most important predictor of growth retardation in JIA patients is the subtype with the poorest prognosis in polyarticulars. Therefore, they need to be visited by an endocrinologist periodically.
Acknowledgements
References
-
1.
Mondal R, Sarkar S, Das NK, Chakravorti S, Hazra A, Sabui T, et al. Growth of children with juvenile idiopathic arthritis. Indian Pediatr. 2014;51(3):199-202. [PubMed ID: 24736907].
-
2.
Cooke DW, Divall SA, Radovick S. Normal and aberrant growth. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Williams Textbook of Endocrinology. Philadelphia: Saunders; 2011. 975 p.
-
3.
MacRae VE, Farquharson C, Ahmed SF. The pathophysiology of the growth plate in juvenile idiopathic arthritis. Rheumatology (Oxford). 2006;45(1):11-9. [PubMed ID: 16148018]. https://doi.org/10.1093/rheumatology/kei091.
-
4.
Simon D, Fernando C, Czernichow P, Prieur AM. Linear growth and final height in patients with systemic juvenile idiopathic arthritis treated with longterm glucocorticoids. J Rheumatol. 2002;29(6):1296-300. [PubMed ID: 12064849].
-
5.
Umlawska W, Prusek-Dudkiewicz A. Growth retardation and delayed puberty in children and adolescents with juvenile idiopathic arthritis. Arch Med Sci. 2010;6(1):19-23. [PubMed ID: 22371715]. https://doi.org/10.5114/aoms.2010.13501.
-
6.
Zak M, Muller J, Karup Pedersen F. Final height, armspan, subischial leg length and body proportions in juvenile chronic arthritis. A long-term follow-up study. Horm Res. 1999;52(2):80-5. [PubMed ID: 10681637]. https://doi.org/10.1159/000023439.
-
7.
Saha MT, Verronen P, Laippala P, Lenko HL. Growth of prepubertal children with juvenile chronic arthritis. Acta Paediatr. 1999;88(7):724-8. [PubMed ID: 10447130].
-
8.
Stagi S, Giani T, Simonini G, Falcini F. Thyroid function, autoimmune thyroiditis and coeliac disease in juvenile idiopathic arthritis. Rheumatology (Oxford). 2005;44(4):517-20. [PubMed ID: 15695302]. https://doi.org/10.1093/rheumatology/keh531.
-
9.
Alpigiani MG, Cerboni M, Bertini I, d'Annunzio G, Haupt R, Iester A, et al. Endocrine autoimmunity in young patients with juvenile chronic arthritis. Clin Exp Rheumatol. 2002;20(4):565-8. [PubMed ID: 12175117].
-
10.
Robazzi TC, Adan LF, Pimentel K, Guimaraes I, Magalhaes Filho J, Toralles MB, et al. Autoimmune endocrine disorders and coeliac disease in children and adolescents with juvenile idiopathic arthritis and rheumatic fever. Clin Exp Rheumatol. 2013;31(2):310-7. [PubMed ID: 23406715].
-
11.
Bouaddi I, Rostom S, El Badri D, Hassani A, Chkirate B, Abouqal R, et al. Vitamin D concentrations and disease activity in Moroccan children with juvenile idiopathic arthritis. BMC Musculoskelet Disord. 2014;15:115. [PubMed ID: 24690195]. https://doi.org/10.1186/1471-2474-15-115.
-
12.
Goshayeshi L, Saber H, Sahebari M, Rezaieyazdi Z, Rafatpanah H, Esmaily H, et al. Association between metabolic syndrome, BMI, and serum vitamin D concentrations in rheumatoid arthritis. Clin Rheumatol. 2012;31(8):1197-203. [PubMed ID: 22581277]. https://doi.org/10.1007/s10067-012-1995-3.
-
13.
Moghimi J, Sadeghi A, Malek M, Ghorbani R. Relationship between disease activity and serum levels of vitamin D and parathyroid hormone in rheumatoid arthritis. Endocr Regul. 2012;46(2):61-6. [PubMed ID: 22540853].
-
14.
Simon D, Prieur AM, Quartier P, Charles Ruiz J, Czernichow P. Early recombinant human growth hormone treatment in glucocorticoid-treated children with juvenile idiopathic arthritis: a 3-year randomized study. J Clin Endocrinol Metab. 2007;92(7):2567-73. [PubMed ID: 17488793]. https://doi.org/10.1210/jc.2006-2877.
-
15.
Bismuth E, Chevenne D, Czernichow P, Simon D. Moderate deterioration in glucose tolerance during high-dose growth hormone therapy in glucocorticoid-treated patients with juvenile idiopathic arthritis. Horm Res Paediatr. 2010;73(6):465-72. [PubMed ID: 20407232]. https://doi.org/10.1159/000313589.
-
16.
Marangoni RG, Hayata AL, Borba EF, Azevedo PM, Bonfa E, Schainberg CG. Decreased high-density lipoprotein cholesterol levels in polyarticular juvenile idiopathic arthritis. Clinics (Sao Paulo). 2011;66(9):1549-52. [PubMed ID: 22179157].
-
17.
Urban M, Pietrewicz E, Gorska A, Glowinska B. [Lipids and homocysteine level in juvenile idiopathic arthritis]. Pol Merkur Lekarski. 2004;17(99):235-8. [PubMed ID: 15628048].
-
18.
El Badri D, Rostom S, Bouaddi I, Hassani A, Chkirate B, Amine B, et al. Sexual maturation in Moroccan patients with juvenile idiopathic arthritis. Rheumatol Int. 2014;34(5):665-8. [PubMed ID: 23553519]. https://doi.org/10.1007/s00296-013-2737-9.
-
19.
Aggarwal B, Bhalla AK, Singh S. Sexual maturation in boys with juvenile rheumatoid arthritis. Rheumatol Int. 2011;31(11):1419-21. [PubMed ID: 20429008]. https://doi.org/10.1007/s00296-010-1473-7.
-
20.
Maher SE, Ali FI. Sexual maturation in Egyptian boys and girls with juvenile rheumatoid arthritis. Rheumatol Int. 2013;33(8):2123-6. [PubMed ID: 23430157]. https://doi.org/10.1007/s00296-013-2683-6.
-
21.
Souza L, Machado SH, Bredemeier M, Brenol JC, Xavier RM. Effect of inflammatory activity and glucocorticoid [corrected] use on nutritional variables in patients with juvenile idiopathic arthritis. J Rheumatol. 2006;33(3):601-8. [PubMed ID: 16511929].
-
22.
Uettwiller F, Perlbarg J, Pinto G, Bader-Meunier B, Mouy R, Compeyrot-Lacassagne S, et al. Effect of biologic treatments on growth in children with juvenile idiopathic arthritis. J Rheumatol. 2014;41(1):128-35. [PubMed ID: 24293576]. https://doi.org/10.3899/jrheum.130311.
-
23.
Wu EY, Van Mater H, Rabinovich CE. Juvenile idiopathic arthritis. In: Kliegman RM, Stanton BF, Schor NF, Geme JW, Behrman RE, editors. Nelson Text Book oF Pediatrics. Philadelphia: Saunders; 2011. p. 1499-509.
-
24.
Agha-Alinejad H, Farzad B, Salari M, Kamjoo S, Harbaugh BL, Peeri M. Prevalence of overweight and obesity among Iranian preschoolers: Interrelationship with physical fitness. J Res Med Sci. 2015;20(4):334-41. [PubMed ID: 26109987].
-
25.
Djalalinia S, Kelishadi R, Qorbani M, Peykari N, Kasaeian A, Nasli-Esfahani E, et al. A Systematic Review on the Prevalence of Overweight and Obesity, in Iranian Children and Adolescents. Iran J Pediatr. 2016;26(3). e2599. [PubMed ID: 27617064]. https://doi.org/10.5812/ijp.2599.
-
26.
Salehiniya H, Yazdani K, Barekati H, Asadi Lari M. The Prevalence of Overweight and Obesity in Children Under 5 Years in Tehran, Iran, in 2012: A Population-Based Study. Res Cardiovasc Med. 2016;5(1). e30425. [PubMed ID: 26889459]. https://doi.org/10.5812/cardiovascmed.30425.
-
27.
Pelajo CF, Lopez-Benitez JM, Miller LC. Obesity and disease activity in juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2012;10(1):3. [PubMed ID: 22240096]. https://doi.org/10.1186/1546-0096-10-3.
-
28.
Gronlund MM, Kaartoaho M, Putto-Laurila A, Laitinen K. Juvenile idiopathic arthritis patients with low inflammatory activity have increased adiposity. Scand J Rheumatol. 2014;43(6):488-92. [PubMed ID: 25178152]. https://doi.org/10.3109/03009742.2014.918171.
-
29.
Unsal E, Oren O, Salar K, Makay B, Abaci A, Ozhan B, et al. The frequency of autoimmune thyroid disorders in juvenile idiopathic arthritis. Turk J Pediatr. 2008;50(5):462-5. [PubMed ID: 19102051].
-
30.
Stagi S, Bertini F, Cavalli L, Matucci-Cerinic M, Brandi ML, Falcini F. Determinants of vitamin D levels in children, adolescents, and young adults with juvenile idiopathic arthritis. J Rheumatol. 2014;41(9):1884-92. [PubMed ID: 25086083]. https://doi.org/10.3899/jrheum.131421.
-
31.
Pelajo CF, Lopez-Benitez JM, Kent DM, Price LL, Miller LC, Dawson-Hughes B. 25-hydroxyvitamin D levels and juvenile idiopathic arthritis: is there an association with disease activity? Rheumatol Int. 2012;32(12):3923-9. [PubMed ID: 22198692]. https://doi.org/10.1007/s00296-011-2287-y.
-
32.
Zakeri Z, Sandoughi M, Mashhadi MA, Raeesi V, Shahbakhsh S. Serum vitamin D level and disease activity in patients with recent onset rheumatoid arthritis. Int J Rheum Dis. 2016;19(4):343-7. [PubMed ID: 24134402]. https://doi.org/10.1111/1756-185X.12181.
-
33.
Neyestani TR, Hajifaraji M, Omidvar N, Eshraghian MR, Shariatzadeh N, Kalayi A, et al. High prevalence of vitamin D deficiency in school-age children in Tehran, 2008: a red alert. Public Health Nutr. 2012;15(2):324-30. [PubMed ID: 21356149]. https://doi.org/10.1017/S1368980011000188.
-
34.
Rabbani A, Alavian SM, Motlagh ME, Ashtiani MT, Ardalan G, Salavati A, et al. Vitamin D insufficiency among children and adolescents living in Tehran, Iran. J Trop Pediatr. 2009;55(3):189-91. [PubMed ID: 18775944]. https://doi.org/10.1093/tropej/fmn078.
-
35.
Bechtold S, Ripperger P, Hafner R, Said E, Schwarz HP. Growth hormone improves height in patients with juvenile idiopathic arthritis: 4-year data of a controlled study. J Pediatr. 2003;143(4):512-9. [PubMed ID: 14571231]. https://doi.org/10.1067/S0022-3476(03)00390-1.
-
36.
Bonaventura P, Benedetti G, Albarede F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015;14(4):277-85. [PubMed ID: 25462582]. https://doi.org/10.1016/j.autrev.2014.11.008.
-
37.
Xin L, Yang X, Cai G, Fan D, Xia Q, Liu L, et al. Serum Levels of Copper and Zinc in Patients with Rheumatoid Arthritis: a Meta-analysis. Biol Trace Elem Res. 2015;168(1):1-10. [PubMed ID: 25869414]. https://doi.org/10.1007/s12011-015-0325-4.
-
38.
Jafari-Adli S, Qorbani M, Heshmat R, Ranjbar SH, Taheri E, Motlagh ME, et al. Association of short stature with life satisfaction and self-rated health in children and adolescents: the CASPIAN-IV study. J Pediatr Endocrinol Metab. 2016;29(11):1299-306. [PubMed ID: 27754967]. https://doi.org/10.1515/jpem-2016-0215.
-
39.
Geisler A, Lass N, Reinsch N, Uysal Y, Singer V, Ravens-Sieberer U, et al. Quality of life in children and adolescents with growth hormone deficiency: association with growth hormone treatment. Horm Res Paediatr. 2012;78(2):94-9. [PubMed ID: 22907471]. https://doi.org/10.1159/000341151.
