Abstract
Background:
Sepsis is one of the major causes of disability and death in the pediatric population globally. Although metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) has been reported to be associated with the survival of adult patients with sepsis, its prognostic value in children has not been identified.Objectives:
The present study aimed to evaluate the role of MALAT1 in the prognosis of severe sepsis in children.Methods:
A total of 60 children with severe sepsis were included in this research. Serum level of MALAT1 was assessed at baseline, and the survival data were recorded during a follow-up of 28 days. These participants were categorized into high or low MALAT1 groups based on the median value. Multivariate Cox regression was performed to explore the association of MALAT1 level with the survival of pediatric patients with sepsis after controlling for potential confounding factors.Results:
The 28-day mortality rate of severe sepsis was 35%. The expression of MALAT1 in the non-survivors was significantly higher than in the survived patients (P < 0.01). The multivariate Cox proportional hazards model, showed that a higher MALAT1 expression was associated with a higher risk of mortality in patients with severe sepsis (HR = 6.70; 95% CI: 1.65 - 27.2; P < 0.01).Conclusions:
According to our results, MALAT1 might be a promising marker for predicting the prognosis of severe pediatric sepsis.Keywords
1. Background
Sepsis is defined as a life-threatening dysfunction of the organs caused by the disturbed response of the host to infection (1, 2). In children, infections commonly lead to sepsis, including blood, skin, lung, and urinary tract infections. A systematic review and meta-analysis of studies on population-based sepsis incidence in children published during 1979 - 2016 reported an annual incidence of 1.2 million cases of sepsis in children on a global scale (3). Although several effective therapies have been applied, its mortality rate still reaches 29 - 70% (4, 5). Moreover, the sepsis survivors suffer from disability, morbidity, and readmission (6-8). Therefore, exploring novel and accurate biomarkers is necessary to improve prognosis in pediatric sepsis patients (9, 10).
Several prognostic scores and biomarkers have been evaluated in pediatric patients with sepsis to suggest more rigorous monitoring or to predict the risk of early deterioration. Currently, the second-generation pediatric index of mortality or pediatric risk of mortality score is used to assess the severity and prognosis of pediatric sepsis internationally, while the pediatric critical illness score (PCIS) is more commonly used in China (11, 12). Hu et al. generated a disease severity scoring model for pediatric sepsis in China that comprises prothrombin time, D-dimer, total bilirubin, total serum protein, uric acid, PaO2/FiO2 ratio, and myoglobin, indicating that patients with higher scores had a higher risk of severe sepsis (13). In addition, several biomarkers, such as lactate, procalcitonin, high-sensitivity C-reactive protein, pancreatic stone protein, interleukin-6 (IL-6), cardiac dysfunction, and high serum ferritin, have attracted attention in low- and middle-income countries because of being inexpensive (11, 14-16). However, so far, none of these biomarkers showed sufficient sensitivity or specificity to be pervasively applied in clinical practice (17), and it is necessary to explore more novel biomarkers. Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), also known as non-coding nuclear-enriched abundant transcript 2 (NEAT2), is a large, infrequently spliced non-coding RNA that is highly conserved amongst mammals and highly expressed in the nucleus (18). MALAT1 was identified in multiple physiological processes, and evidence indicates that MALAT1 also closely relates to various pathological processes, ranging from diabetes complications to cancers. MALAT1 is reported to regulate the secretion of inflammatory cytokines in the immune and inflammatory systems (19). It is associated with infection and immune-mediated inflammatory diseases by regulating the inflammatory response through multiple target genes and signaling pathways (20-23). Recent studies indicated that MALAT1 was significantly associated with the survival of adult patients with sepsis (24, 25).
2. Objectives
However, to our knowledge, its prognostic value in children has not been identified. Therefore, this research aimed to assess the association of MALAT1 level with the prognosis of severe sepsis in children.
3. Methods
3.1. Patients
This prospective, observational study was conducted on consecutive pediatric patients (≤ 18 years) with severe sepsis admitted into The Fifth Affiliated Hospital of Zunyi Medical University, Zhuhai, China, during Jan. 2017-Dec. 2020. All participants were diagnosed with sepsis according to the criteria established by the International Pediatric Sepsis Consensus Conference. Severe sepsis was defined as sepsis complicated with organ dysfunction or tissue hypoperfusion (26). The exclusion criteria included blood specimen not taken at admission, refusal of parents, not being available for the 28-day follow-up after hospital discharge, malignancy, congenital heart disease, coronary artery disease, chronic cardiac dysfunction, chronic renal insufficiency, and immunodeficiency.
This study was approved by the Ethics Committee of The Fifth Affiliated Hospital of Zunyi Medical University, Zhuhai, China (Code: 2016052).
3.2. Data Collection and Measurement of MALAT-1 Expression
The baseline characteristics of patients, including age, gender, body mass index (BMI), infection sites, etiology, PCIS, and length of pediatric intensive care unit (PICU) stay, were obtained from medical records. Survival was also recorded and measured.Peripheral blood specimens of 5 mL were collected from each patient at admission. Total RNA was elicited using TRIzol reagent (Invitrogen, Shanghai, China) following the manufacturer's protocol. Next, total RNA was reversely transcribed to complementary DNAs by PrimeScript RT reagent Kit (Takara, Shanghai, China), and qPCR was performed using TB Green Fast qPCR Mix (Takara, Shanghai, China). The relative mRNA expression of MALAT-1 was calculated according to the 2-ΔΔCt formula using GAPDH as the internal reference.
3.3. Statistical Analysis
We assumed that approximately 20 of 60 enrolled patients would die by the time of the primary analysis (with a two-sided type I error rate of 5%), providing a power of more than 90% to detect a hazard ratio of 2.0. The continuous variables with normal distribution were presented as mean ± SD and compared by the unpaired t-test, and those with non-normal distribution were expressed as median (range) and compared by the Mann-Whitney U test. Moreover, the categorical variables were expressed as numbers (percentage) and compared by the χ² test. Kaplan-Meier method was used to estimate survival distribution. Multivariate Cox regression was applied to evaluate the association of MALAT1 with the mortality risk of pediatric sepsis after controlling for potential confounders, including age, gender, BMI, infection sites, etiology, PCIS, and length of PICU stay. The SPSS statistical software version 20 was used for all analyses. P-value < 0.05 was considered statistically significant.
4. Results
A total of 64 patients were assessed for eligibility, out of which four cases were excluded due to the refusal of parents (n = 2), loss to follow-up (n = 1), and malignancy (n = 1). Finally, 60 patients included in the analysis were divided into low and high MALAT1 groups (30 in each group) based on the median value of 3.1.
Among 60 pediatric patients with severe sepsis, 31 were boys, and 29 were girls. The 28-day mortality rate was 35%. Table 1 summarizes the baseline demographic and clinical characteristics of patients according to survival status or MALAT level. It is shown that the distributions of age, gender, BMI, infection sites (chest, abdomen, combined, or others), etiology (Gram-positive or -negative, fungus, or others), and PCIS were not significantly different between survivors and non-survivors or between patients with low and high MALAT1 levels (P > 0.05). The etiology of sepsis was described in detail; it is shown that the distribution of microbial pathogens, including Gram-positive bacteria (Staphylococcus aureus, Streptococcus species, and Enterococcus species), Gram-negative bacteria (Pseudomonas species, Escherichia coli, Klebsiella species), and fungi (Candida parapsilosis and Candidiasis albicans) were not significantly different between survivors and non-survivors or between patients with low and high MALAT1 levels (P > 0.05). Patients with lower MALAT1 levels had a significantly shorter PICU stay compared to those with higher MALAT1 levels (P < 0.01); however, the difference in the PICU stay was not significant between survivors and non-survivors (P > 0.05).
| Variables | Total | Survival Status | MALAT1 Level | ||||
|---|---|---|---|---|---|---|---|
| Survival | Death | P-Value | High MALAT1 | Low MALAT1 | P-Value | ||
| No. | 60 | 39 | 21 | 31 | 29 | ||
| Age (mo) | 30.5 (2 - 150) | 32 (2 - 150) | 27 (3 - 145) | 0.53 | 32 (2 - 145) | 30 (5 - 150) | 0.66 |
| BMI (kg/m2) | 14.7 (7.9 - 22.0) | 15.5 (8.5 - 22.0) | 14.5 (7.9 - 21.3) | 0.94 | 13.5 (7.9 - 22) | 15.5 (8.9 - 21.6) | 0.23 |
| Gender | 0.79 | 0.60 | |||||
| Boys | 31 (51.7) | 21 (53.8) | 10 (47.6) | 16 (51.6) | 15 (51.7) | ||
| Girls | 29 (48.3) | 18 (46.2) | 11 (52.4) | 15 (48.4) | 14 (48.3) | ||
| Infection sites | 0.06 | ||||||
| Chest | 25 (41.7) | 17 (43.6) | 8 (38.1) | 0.79 | 12 (38.7) | 16 (55.2) | |
| Abdomen | 14 (23.3) | 8 (20.5) | 6 (28.6) | 0.53 | 9 (29.0) | 2 (6.9) | |
| Combined | 11 (18.3) | 7 (17.9) | 4 (19.0) | 1.00 | 7 (22.6) | 4 (13.8)) | |
| Other | 10 (16.7) | 7 (17.9) | 3 (14.3) | 1.00 | 3 (9.0) | 7 (24.1) | |
| Etiology | 0.97 | 0.66 | |||||
| Gram-positive bacteria | 32 (53.3) | 21 (53.8) | 11 (52.4) | 16 (51.6) | 16 (55.2) | ||
| Staphylococcus aureus | 17 (28.3) | 13 (33.3) | 4 (19.1) | 10 (32.3) | 7 (24.1) | ||
| Streptococcus species | 10 (16.7) | 6 (15.4) | 4 (19.0) | 4 (12.9) | 6 (20.7) | ||
| Enterococcus species | 5 (8.3) | 4 (10.3) | 1 (4.8) | 2 (6.5) | 3 (10.3) | ||
| Gram-negative bacteria | 19 (31.7) | 12 (30.8) | 7 (33.3) | 11 (35.5) | 8 (27.6) | ||
| Pseudomonas species | 10 (16.7) | 7 (17.9) | 3 (14.3) | 6 (19.4) | 5 (17.2) | ||
| Escherichia coli | 6 (10) | 3 (7.7) | 3 (14.3) | 3 (9.7) | 3 (10.3) | ||
| Klebsiella species | 3 (5.0) | 2 (5.1) | 1 (4.8) | 2 (6.5) | 1 (3.4) | ||
| Fungus | 5 (8.3) | 3 (7.7) | 2 (9.5) | 3 (9.7) | 2 (6.9) | ||
| Candida parapsilosis | 3 (5.0) | 2 (5.1) | 1 (4.8) | 1 (3.2) | 2 (6.9) | ||
| Candidiasis albicans | 2 (3.3) | 1 (2.6) | 1 (4.8) | 2 (6.5) | 0 | ||
| Other | 4 (6.7) | 3 (7.7) | 1 (4.8) | 1 (3.2) | 3 (10.3) | ||
| PCIS | 58.2 ± 15.1 | 64.3 ± 13.9 | 46.9 ± 10.7 | 0.11 | 56.9 ± 13.5 | 59.6 ± 17.1 | 0.10 |
| Length of PICU stay (d) | 5 (2 - 15) | 5 (2 - 15) | 6 (2 - 15) | 0.65 | 8 (2 - 15) | 3 (2 - 9) | < 0.01 |
| Mortality | 21 (35.0) | - | - | - | 15 (48.4) | 6 (20.7) | 0.03 |
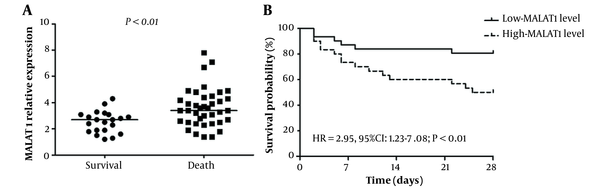
Figure 1A illustrates that the MALAT1 relative expression level was significantly higher in non-survivors compared to survivors (P < 0.01). These 60 patients were divided into low and high MALAT1 groups (30 in each group) based on the median value of 3.1. As shown in the Kaplan-Meier survival curves (Figure 1B), subjects with higher MALAT1 levels had a worse survival than those with lower MALAT1 levels (HR = 2.95, 95% CI: 1.23 - 7.08; P < 0.01). Multivariate Cox proportional-hazards regression analyses showed that a higher MALAT1 level (HR = 6.70; 95% CI: 1.65 - 27.2; P < 0.01) was significantly related to increased mortality risk, and a higher PCIS (HR = 0.90, 95% CI: 0.86 - 0.95; P < 0.01) was significantly associated with a lower risk of mortality (Table 2).
A, Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) mRNA expression levels in survivors and non-survivors; B, Kaplan-Meier survival curves in high- and low-MALAT1 levels
Multivariate Cox Regression Analysis for Investigating the Association of MALAT1 with the Risk of 28-Day Mortality in Pediatric Patients with Severe Sepsis a
| Variables | HR | 95% CI | P-Value |
|---|---|---|---|
| MLALT-1 level | 6.70 | 1.65 - 27.2 | < 0.01 |
| Age | 1.00 | 0.98 - 1.02 | 0.85 |
| Girl | 1.97 | 0.73 - 5.34 | 0.18 |
| BMI | 1.06 | 0.94 - 1.20 | 0.32 |
| Infection sites | 0.84 | 0.55 - 1.27 | 0.41 |
| Etiology | 1.16 | 0.63 - 2.16 | 0.63 |
| PCIS | 0.90 | 0.86 - 0.95 | < 0.01 |
| Length of PICU stay | 0.87 | 0.73 - 1.03 | 0.11 |
5. Discussion
Antibiotics are specific medications for sepsis treatment as sepsis is a blood infection. However, mortality remains high despite antibiotic therapy, fluid resuscitation, and assisted ventilation. The lack of laboratory diagnostic modality to triage and classify the patients as high- and low-risk complicates the clinical management. Therefore, reliable biomarkers are urgently required to accurately stratify the stages of sepsis, predict the prognosis, and apply specific treatments based on the patient category (27). LncRNA MALAT1 has been found to dysregulate and influence biologic or pathologic processes in several inflammation-related diseases, including sepsis (28, 29). However, the prognostic value of MALAT1 for sepsis was rarely reported, especially in the pediatric population. To our knowledge, the present study is the first to evaluate the predictive value of MALAT1, indicating that pediatric patients with a higher MALAT1 level were significantly associated with a higher risk of 28-day mortality.
Three studies have assessed the association of MALAT1 with survival in adult patients with sepsis and reported conflicting results. A study by Geng et al. (25) showed that the 28-day mortality rate was 30.5%. Kaplan-Meier curves and the log-rank test indicated that the accumulating survival was worse in patients with the high expression of lncRNA MALAT1 than in patients with LncRNA MALAT1 low expression (P < 0.001). In another study by Chen et al. (24), multivariate logistic regression revealed that high MALAT1 expression was an independent risk factor for sepsis (P < 0.001), septic shock (P = 0.030), and poor prognosis (P = 0.015). Huang and Zhao by a univariate logistic regression, revealed that lnc-MALAT1 high expression was associated with raised mortality (OR = 2.263, 95% CI: 2.107 - 29.798; P = 0.024) (30). However, the association in the multivariate logistic regression was not significant (OR = 1.433, 95% CI: 0.535 - 3.840; P = 0.474). The prognostic value of MALAT1 in pediatric patients with sepsis has not been reported. The present study is the first to evaluate the predictive value of MALAT1, indicating that a high MALAT1 level in pediatric patients was significantly associated with a higher risk of 28-day mortality.
The current research had some limitations. First, it was performed in a single center with a limited patient number, which may present insufficient data and cause selection bias. Moreover, several potential confounders, such as socioeconomic status (e.g., family income, insurance status, and parental education level) and receiving vitamin D that have been reported to be associated with the progression and mortality of pediatric sepsis were not collected in the present study (31-34), which may cause bias. In addition, detailed mechanisms of MALAT1 in sepsis had not yet been fully elucidated. Consequently, large-scale clinical studies with more samples and long-term follow-up and an in vivo study for evaluating the molecular mechanisms should be performed to verify our findings.
5.1. Conclusions
In conclusion, MALAT1 might be a promising marker for predicting the prognosis of severe pediatric sepsis.
References
-
1.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-10. [PubMed ID: 26903338]. [PubMed Central ID: PMC4968574]. https://doi.org/10.1001/jama.2016.0287.
-
2.
Siroosbakht S, Aarabi N, Rezakhaniha B. Bathing or Not Bathing: Which Is Better for Umbilical Cord Separation Time and Bacterial Colonization in Neonates? Arch Pediatr Infect Dis. 2021;9(2). e104100. https://doi.org/10.5812/pedinfect.104100.
-
3.
Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6(3):223-30. [PubMed ID: 29508706]. https://doi.org/10.1016/S2213-2600(18)30063-8.
-
4.
Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis*. Crit Care Med. 2014;42(3):625-31. [PubMed ID: 24201173]. [PubMed Central ID: PMC4313930]. https://doi.org/10.1097/CCM.0000000000000026.
-
5.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228. [PubMed ID: 23361625]. [PubMed Central ID: PMC7095153]. https://doi.org/10.1007/s00134-012-2769-8.
-
6.
Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787-94. [PubMed ID: 20978258]. [PubMed Central ID: PMC3345288]. https://doi.org/10.1001/jama.2010.1553.
-
7.
Shah FA, Pike F, Alvarez K, Angus D, Newman AB, Lopez O, et al. Bidirectional relationship between cognitive function and pneumonia. Am J Respir Crit Care Med. 2013;188(5):586-92. [PubMed ID: 23848267]. [PubMed Central ID: PMC3827700]. https://doi.org/10.1164/rccm.201212-2154OC.
-
8.
Yende S, Linde-Zwirble W, Mayr F, Weissfeld LA, Reis S, Angus DC. Risk of cardiovascular events in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;189(9):1065-74. [PubMed ID: 24456535]. [PubMed Central ID: PMC4098105]. https://doi.org/10.1164/rccm.201307-1321OC.
-
9.
Melendez E, Bachur R. Advances in the emergency management of pediatric sepsis. Curr Opin Pediatr. 2006;18(3):245-53. [PubMed ID: 16721143]. https://doi.org/10.1097/01.mop.0000193305.55635.ff.
-
10.
Lestari IN, Yoel C, Lubis M. The Association between the Level of Antithrombin III and Mortality in Children with Sepsis. Open Access Maced J Med Sci. 2019;7(6):959-61. [PubMed ID: 30976340]. [PubMed Central ID: PMC6454173]. https://doi.org/10.3889/oamjms.2019.211.
-
11.
Tonial CT, Costa CAD, Andrades GRH, Crestani F, Bruno F, Piva JP, et al. Performance of prognostic markers in pediatric sepsis. J Pediatr (Rio J). 2021;97(3):287-94. [PubMed ID: 32991837]. https://doi.org/10.1016/j.jped.2020.07.008.
-
12.
Wu Q, Nie J, Wu FX, Zou XL, Chen FY. Prognostic Value of High-Sensitivity C-Reactive Protein, Procalcitonin and Pancreatic Stone Protein in Pediatric Sepsis. Med Sci Monit. 2017;23:1533-9. [PubMed ID: 28358790]. [PubMed Central ID: PMC5384617]. https://doi.org/10.12659/msm.900856.
-
13.
Hu L, Zhu Y, Chen M, Li X, Lu X, Liang Y, et al. Development and Validation of a Disease Severity Scoring Model for Pediatric Sepsis. Iran J Public Health. 2016;45(7):875-84. [PubMed ID: 27516993]. [PubMed Central ID: PMC4980341].
-
14.
Shahkar L, Keshtkar A, Mirfazeli A, Ahani A, Roshandel G. The role of IL-6 for predicting neonatal sepsis: a systematic review and meta-analysis. Iran J Pediatr. 2011;21(4):411-7. [PubMed ID: 23056824]. [PubMed Central ID: PMC3446138].
-
15.
Zurek J, Vavrina M. Procalcitonin Biomarker Kinetics to Predict Multiorgan Dysfunction Syndrome in Children With Sepsis and Systemic Inflammatory Response Syndrome. Iran J Pediatr. 2015;25(1). e324. [PubMed ID: 26199699]. [PubMed Central ID: PMC4505981]. https://doi.org/10.5812/ijp.324.
-
16.
M. Ismail A, Ahmed El Sayed Ahmed Abu Elela M, Nashaat Roshdy Ahmed I, Mohamed Sabry Mahmoud N. Cardiac Dysfunction and Serum Ferritin Level as Early Prognostic Markers in Children with Sepsis: A Cross-sectional Study. Iran J Pediatr. 2021;31(6). e114738. https://doi.org/10.5812/ijp.114738.
-
17.
Lanziotti VS, Povoa P, Soares M, Silva JR, Barbosa AP, Salluh JI. Use of biomarkers in pediatric sepsis: literature review. Rev Bras Ter Intensiva. 2016;28(4):472-82. [PubMed ID: 28099644]. [PubMed Central ID: PMC5225923]. https://doi.org/10.5935/0103-507X.20160080.
-
18.
Wu Y, Huang C, Meng X, Li J. Long Noncoding RNA MALAT1: Insights into its Biogenesis and Implications in Human Disease. Curr Pharm Des. 2015;21(34):5017-28. [PubMed ID: 26205289]. https://doi.org/10.2174/1381612821666150724115625.
-
19.
Wu T, Wu D, Wu Q, Zou B, Huang X, Cheng X, et al. Knockdown of Long Non-Coding RNA-ZFAS1 Protects Cardiomyocytes Against Acute Myocardial Infarction Via Anti-Apoptosis by Regulating miR-150/CRP. J Cell Biochem. 2017;118(10):3281-9. [PubMed ID: 28295592]. https://doi.org/10.1002/jcb.25979.
-
20.
Vergadi E, Vaporidi K, Tsatsanis C. Regulation of Endotoxin Tolerance and Compensatory Anti-inflammatory Response Syndrome by Non-coding RNAs. Front Immunol. 2018;9:2705. [PubMed ID: 30515175]. [PubMed Central ID: PMC6255943]. https://doi.org/10.3389/fimmu.2018.02705.
-
21.
Yang H, Liang N, Wang M, Fei Y, Sun J, Li Z, et al. Long noncoding RNA MALAT-1 is a novel inflammatory regulator in human systemic lupus erythematosus. Oncotarget. 2017;8(44):77400-6. [PubMed ID: 29100395]. [PubMed Central ID: PMC5652787]. https://doi.org/10.18632/oncotarget.20490.
-
22.
Shaker OG, Mahmoud RH, Abdelaleem OO, Ibrahem EG, Mohamed AA, Zaki OM, et al. LncRNAs, MALAT1 and lnc-DC as potential biomarkers for multiple sclerosis diagnosis. Biosci Rep. 2019;39(1):BSR20181335. [PubMed ID: 30514825]. [PubMed Central ID: PMC6331681]. https://doi.org/10.1042/BSR20181335.
-
23.
Pan F, Zhu L, Lv H, Pei C. Quercetin promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by upregulating lncRNA MALAT1. Int J Mol Med. 2016;38(5):1507-14. [PubMed ID: 28026003]. https://doi.org/10.3892/ijmm.2016.2755.
-
24.
Chen J, He Y, Zhou L, Deng Y, Si L. Long noncoding RNA MALAT1 serves as an independent predictive biomarker for the diagnosis, severity and prognosis of patients with sepsis. Mol Med Rep. 2020;21(3):1365-73. [PubMed ID: 31922243]. https://doi.org/10.3892/mmr.2020.10923.
-
25.
Geng F, Liu W, Yu L. Potential role of circulating long noncoding RNA MALAT1 in predicting disease risk, severity, and patients' survival in sepsis. J Clin Lab Anal. 2019;33(8). e22968. [PubMed ID: 31301104]. [PubMed Central ID: PMC6805255]. https://doi.org/10.1002/jcla.22968.
-
26.
Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric S. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2-8. [PubMed ID: 15636651]. https://doi.org/10.1097/01.PCC.0000149131.72248.E6.
-
27.
Wang C, Liang G, Shen J, Kong H, Wu D, Huang J, et al. Long Non-Coding RNAs as Biomarkers and Therapeutic Targets in Sepsis. Front Immunol. 2021;12:722004. [PubMed ID: 34630395]. [PubMed Central ID: PMC8492911]. https://doi.org/10.3389/fimmu.2021.722004.
-
28.
Yu Z, Rayile A, Zhang X, Li Y, Zhao Q. Ulinastatin protects against lipopolysaccharide-induced cardiac microvascular endothelial cell dysfunction via downregulation of lncRNA MALAT1 and EZH2 in sepsis. Int J Mol Med. 2017;39(5):1269-76. [PubMed ID: 28405676]. https://doi.org/10.3892/ijmm.2017.2920.
-
29.
Dai L, Zhang G, Cheng Z, Wang X, Jia L, Jing X, et al. Knockdown of LncRNA MALAT1 contributes to the suppression of inflammatory responses by up-regulating miR-146a in LPS-induced acute lung injury. Connect Tissue Res. 2018;59(6):581-92. [PubMed ID: 29649906]. https://doi.org/10.1080/03008207.2018.1439480.
-
30.
Huang X, Zhao M. High expression of long non-coding RNA MALAT1 correlates with raised acute respiratory distress syndrome risk, disease severity, and increased mortality in sepstic patients. Int J Clin Exp Pathol. 2019;12(5):1877-87. [PubMed ID: 31934011]. [PubMed Central ID: PMC6947113].
-
31.
Mitchell HK, Reddy A, Perry MA, Gathers CA, Fowler JC, Yehya N. Racial, ethnic, and socioeconomic disparities in paediatric critical care in the USA. Lancet Child Adolesc Health. 2021;5(10):739-50. https://doi.org/10.1016/s2352-4642(21)00161-9.
-
32.
Gavidia R, Fuentes SL, Vasquez R, Bonilla M, Ethier MC, Diorio C, et al. Low socioeconomic status is associated with prolonged times to assessment and treatment, sepsis and infectious death in pediatric fever in El Salvador. PLoS One. 2012;7(8). e43639. [PubMed ID: 22928008]. [PubMed Central ID: PMC3425537]. https://doi.org/10.1371/journal.pone.0043639.
-
33.
Mitchell HK, Reddy A, Montoya-Williams D, Harhay M, Fowler JC, Yehya N. Hospital outcomes for children with severe sepsis in the USA by race or ethnicity and insurance status: a population-based, retrospective cohort study. Lancet Child Adolesc Health. 2021;5(2):103-12. https://doi.org/10.1016/s2352-4642(20)30341-2.
-
34.
Wang Y, Yang Z, Gao L, Cao Z, Wang Q. Effects of a single dose of vitamin D in septic children: a randomized, double-blinded, controlled trial. J Int Med Res. 2020;48(6):1-11. [PubMed ID: 32485124]. [PubMed Central ID: PMC7273772]. https://doi.org/10.1177/0300060520926890.