Abstract
Background:
Esophageal atresia and tracheoesophageal fistula (EA/TEF) is a known correctable anomaly of the esophagus, and its prognosis depends on multiple factors. Studies investigating the EA/TEF post-operative outcomes among the Iranian population are limited to small sample sizes, and the current prevalence of coexisting anomalies and predictors of poor prognosis in the Iranian population is still unclear.Objectives:
This study aimed to investigate the predictors of in-hospital mortality in neonates with EA/TEF at our center within a 12-year period.Methods:
In this retrospective cohort study, we investigated neonates with EA/TEF admitted/referred to a tertiary referral center in Tehran, Iran, from March 2008 to April 2020. Neonates with chromosomal anomalies or age > 10 days at operation date were excluded. Baseline characteristics, associated anomalies, type of EA, and transmission distance were compared in the study population. We followed the neonates for incurring in-hospital mortality.Results:
We included 233 neonates in the final analysis. The mean age at operation was 3.1 ± 1.7 days, and 111 (47.6%) cases were female. The most common EA type was type C (proximal esophageal atresia with distal fistula), with a prevalence of 94.4%. In this cohort, 23 (9.9%) cases had vertebral anomalies, anal atresia, cardiovascular malformations, trachea-esophageal fistula, renal and limb anomalies (VACTERL) association, and 29 (12.4%) cases died during the in-hospital course. Moreover, neonates with lower birth weight, gestational birth weight < 37 weeks, other coexisting anomalies, cardiovascular defects, and non-VACTERL anomalies were at higher risk of in-hospital mortality. In contrast, EA types and transmission distance did not increase the mortality risk. Furthermore, we measured a cut-off value of < 2575.0 g for birth weight to predict in-hospital mortality with 65.5% sensitivity and 61.3% specificity.Conclusions:
Lower birth weight, prematurity (< 37 weeks), and coexisting anomalies, especially cardiovascular defects, were associated with an increased risk of in-hospital mortality in neonates after EA/TEF repair surgery.Keywords
Esophageal Atresia Low Birth Weight Prematurity Tracheoesophageal Fistula
1. Background
Congenital esophageal atresia and tracheoesophageal fistula (EA/TEF) is one of the most frequent developmental anomalies of the esophagus, which occurs in 2.9 per 10,000 live births in different geographical areas of the world (1, 2). It is a life-threatening issue, and the prognosis depends on several preventable and unpreventable factors (2). In 1697, T. Gibson documented the first case of EA with distal fistula, and the first attempt to correct esophageal atresia was reported in 1936 (3). It has been discovered that EA/TEF subtypes, prenatal diagnosis, pre-/post-surgical intensive care, and concomitant congenital anomalies have an important impact on prognosis in such patients (4-7). It is found that neonates with EA/TEF are 50% more likely to have coexisting anomalies, whereas vertebral anomalies, anal atresia, cardiovascular malformations, trachea-esophageal fistula, renal and limb anomalies (VACTERL) are the most prevalent association (8, 9).
There are few studies investigating the prognosis of neonates with EA/TEA among the Iranian population; however, they are limited to small sample sizes and previous treatment strategies (10-). These studies reported a varying range of associated anomalies (13.3% to 37.8%) and mortality rate (4.7 to 29.7%) in neonates with EA/TEF (10-14). Nevertheless, the current prevalence of coexisting anomalies and predictors of poor outcomes after EA/TEF surgery in the Iranian population is still unclear.
2. Objectives
In this observational study, we aim to evaluate predictors of in-hospital mortality in neonates with EA/TEF within a 12-year period from a tertiary referral center in Tehran, Iran.
3. Methods
3.1. Ethical Considerations
The study protocol corresponded with the 2013 Helsinki declaration and was approved by the ethics committee of the Shahid Beheshti University of Medical Sciences, Tehran, Iran (ethical code: IR.SBMU.RICH.REC.1398.039). Signed informed consent was obtained from parents before enrollment in the study.
3.2. Study Population and Design
In this retrospective cohort study, we investigated neonates with EA/TEF admitted/referred to the Mofid Children's hospital in Tehran, Iran, from March 2008 to April 2020. The inclusion criteria were neonates aged less than 10 days at operation date, confirmed diagnosis of any type of EA/TEF, and no prior history of EA/TEF surgery. Neonates with chromosomal anomalies and those lacking key information in their medical records were excluded from the study. Mofid Children's hospital is a tertiary teaching hospital affiliated with the Shahid Beheshti University of Medical Sciences. It is a referral center for patients from other hospitals in Tehran and other provinces. Most patients were transported by ground using a coded ambulance based on standard protocols.
All infants were evaluated for different types of esophageal atresia and other associated anomalies. The VACTERL association was defined as the presence of at least three anomalies, including vertebral defects, anorectal malformations, cardiovascular defects, EA/TEF, renal or radial anomalies, and limb defects (15, 16). The demographics and clinical data of infants were extracted from patients' electronic medical records by trained staff. We compared neonates based on baseline characteristics, including age, sex, birth weight, gestational age, kind of delivery, hospital length of stay, and in-hospital mortality. We also considered the distance between each patient's residential location and our center by categorizing transmission distance into three groups: (A) transmission < 100 km, (B) transmission between 100 - 249 km, and (C) transmission ≥ 250 km.
3.3. Statistical Analysis
We presented categorical variables as numbers (percentage) and compared them using the chi-square or Fisher’s exact test. The distribution of numerical variables was evaluated by using the Kolmogorov-Smirnov tests. Eventually, numerical variables with normal distribution were reported as mean ± standard deviation and compared using the independent samples T-test; otherwise, they were reported as median [interquartile range] and compared using the Mann-Whitney U-test. The receiver operating characteristic (ROC) curve of birth weight for predicting in-hospital mortality was depicted, and the area under the curve (AUC) was calculated. To determine the optimal cut-off value, the point on the ROC curve with maximum Youden index [sensitivity - (1-specificity)] was identified (17). All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 23, and P-value < 0.05 was considered statistically significant.
4. Results
In this study, we excluded 12 infants with unknown types of atresia and three neonates with trisomy 18 and 21; ultimately, 233 neonates with EA/TEF were enrolled in the final analysis. Overall mean age at operation was 3.1 ± 1.7 days, and 111 (47.6%) cases were female. The mean gestational age was 37.5 ± 2.34 weeks, and 24.3% had a gestational age < 37 weeks. The mode of delivery was C-section in 65.7% of cases with a mean birth weight of 2653.4 ± 584.8 g, while 94 (40.3%) neonates had a birth weight ≤ 2500 g. The prevalence of the VACTERL association was 9.9% (n = 23). Regarding EA/TEF types, 94.4% were type C (proximal atresia with distal fistula), 3.9% were type A (atresia but no fistula), 1.3% were type B (proximal fistula and distal atresia), and 0.4% were type D (proximal and distal fistula). We did not have any patients with type E atresia. The median hospital length of stay was 16.0 [interquartile range (IQR): 12.0 - 22.0] days, and in-hospital mortality occurred in 29 (12.4%) neonates. Details of baseline and clinical characteristics are shown in (Table 1).
| Characteristics* | Total (N = 233) | Deceased (N = 29) | Survived (N = 204) | P-Value |
|---|---|---|---|---|
| Birth weight (g) | 2750.0 [2270.0 - 3100.0] | 2470.0 [1775.0 - 2800.0] | 2800.0 [2300.0 - 3115.0] | 0.009 |
| Birth weight ≤ 2500 (g) | 94 (40.3) | 18 (62.1) | 76 (37.3) | 0.011 |
| Sex | 0.471 | |||
| Female | 111(47.6) | 12 (41.4) | 99 (48.5) | |
| Male | 122(52.4) | 17 (58.6) | 105 (51.5) | |
| Gestational age (week) | 38.0 [37.0 - 39.0] | 37.5 [34.75 - 39.0] | 38.0 [37.0 - 39.0] | 0.061 |
| Gestational age < 37 (week) | 51 (24.3) | 12 (46.2) | 39 (21.2) | 0.005 |
| Kind of delivery | 0.393 | |||
| NVD | 80 (34.3) | 12 (41.4) | 68 (33.3) | |
| C-section | 153 (65.7) | 17 (58.6) | 136 (66.7) | |
| Transmission distance (km) | 148.0 [0 - 320.0] | 52.0 [0 - 279.0] | 148.0 [0 - 332.25] | 0.246 |
| Transmission distance (km) | 0.308 | |||
| < 100 | 109 (48.4) | 16 (55.2) | 93 (47.5) | |
| 100 - 249 | 59 (26.2) | 9 (31.0) | 50 (25.5) | |
| ≥ 250 | 57 (25.3) | 4 (13.8) | 53 (27.0) | |
| Type of esophageal atresia | 0.530 | |||
| Type A | 9 (3.9) | 2 (6.9) | 7 (3.4) | |
| Type B | 3 (1.3) | 1 (3.4) | 2 (1.0) | |
| Type C | 220 (94.4) | 26 (89.7) | 194 (95.1) | |
| Type D | 1 (0.4) | 0 | 1 (0.5) | |
| Type E | 0 | 0 | 0 | |
| Associated congenital anomalies | ||||
| Any anomaly | 108 (46.4) | 19 (65.5) | 89 (43.6) | 0.027 |
| VACTERL association | 23 (9.9) | 3 (10.3) | 20 (9.8) | 0.927 |
| Vertebral defects | 14 (6.0) | 0 | 14 (6.9) | 0.146 |
| Anorectal malformations | 24 (10.3) | 4 (13.8) | 20 (9.8) | 0.508 |
| Cardiovascular defects | 59 (25.3) | 13 (44.8) | 46 (22.5) | 0.010 |
| Renal or radial anomalies | 11 (4.7) | 1 (3.4) | 10 (4.9) | 0.730 |
| Limb defects | 12 (5.2) | 2 (6.9) | 10 (4.9) | 0.649 |
| Non-VACTERL anomalies | 85 (36.5) | 16 (55.2) | 69 (33.8) | 0.025 |
| Clinical variables | ||||
| Age at operation (day) | 3.0 [2.0 - 4.0] | 2.0 [2.0 - 4.0] | 3.0 [2.0 - 4.0] | 0.425 |
| Hospital length of stay (day) | 16.0 [12.0 - 22.0] | 16.0 [4.0 - 31.0] | 16.0 [12.0 - 21.0] | 0.795 |
4.1. Predictors of In-hospital Mortality
We observed that lower birth weight and gestational age < 37 weeks were associated with a higher risk of in-hospital mortality in neonates with EA/TEF (Table 2). Furthermore, the presence of any congenital anomaly, cardiovascular defects, and non-VACTERL anomalies were associated with an increased risk of in-hospital mortality. Results showed that the prevalence of VACTERL association was similar among neonates who deceased compared to those who survived (10.3% vs. 9.8%, P-value: 0.927). Age at operation, sex, kind of delivery, type of EA, and other anomalies did not increase the risk of mortality in neonates with EA/TEF.
Baseline and Clinical Characteristics of Neonates with EA/TEF Based on Associated Congenital Anomalies a
| Characteristics | Anomaly (N = 108) | No Anomaly (N = 125) | P-Value |
|---|---|---|---|
| Birth weight (g) | 2800.0 [2200.0 - 3095.0] | 2700.0 [2325.0 - 3140.0] | 0.922 |
| Birth weight ≤ 2500 (g) | 41 (38.0) | 52 (42.4) | 0.491 |
| Sex | 0.519 | ||
| Female | 49 (45.4) | 62 (49.6) | |
| Male | 59 (54.6) | 63 (50.4) | |
| Gestational age (week) | 38.0 [36.75 - 39.0] | 38.0 [37.0 - 39.0] | 0.968 |
| Gestational age < 37 (week) | 24 (24.5) | 27 (24.1) | 0.949 |
| Kind of delivery | 0.765 | ||
| NVD | 36 (33.3) | 44 (35.2) | |
| C-section | 72 (66.7) | 81 (64.8) | |
| Transmission distance (km) | 148.0 [0 - 334.0] | 148.0 [0 - 279.0] | 0.556 |
| Transmission distance (km) | 0.415 | ||
| < 100 | 52 (49.1) | 57 (47.9) | |
| 100 - 249 | 31 (29.2) | 28 (23.5) | |
| ≥ 250 | 23 (21.7) | 34 (28.6) | |
| Type of esophageal atresia | 0.461 | ||
| Type A | 6 (5.6) | 3 (2.4) | |
| Type B | 1 (0.9) | 2 (1.6) | |
| Type C | 101 (93.5) | 119 (95.2) | |
| Type D | 0 | 1 (0.8) | |
| Type E | 0 | 0 | |
| Clinical variables | |||
| Age at operation (day) | 3.0 [2.0 - 4.0] | 3.0 [2.0 - 4.0] | 0.493 |
| Hospital length of stay (day) | 14.0 [11.0 - 19.0] | 18.0 [13.0 - 26.0] | 0.001 |
| In-hospital mortality | 19 (17.6) | 10 (8.0) | 0.027 |
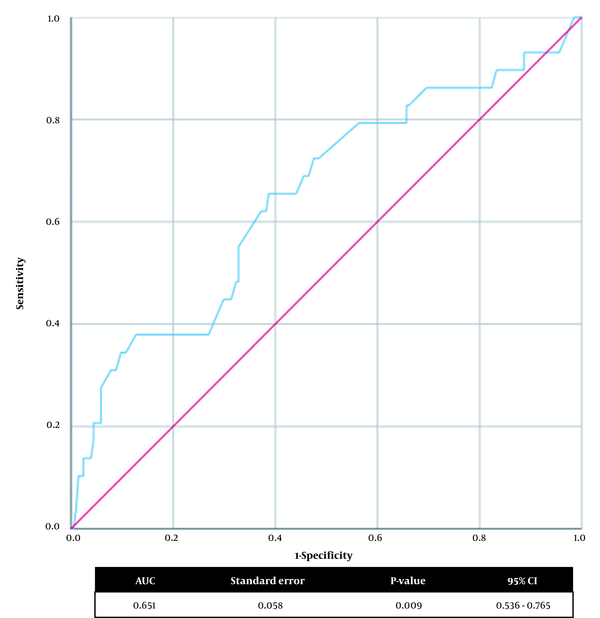
We investigated the optimum birth weight cut-off point for predicting in-hospital mortality (Figure 1). In the ROC curve analysis, the cut-off value of the birth weight was < 2575.0 g, with 65.5% sensitivity and 61.3% specificity (AUC: 0.651, 95% CI: 0.536 - 0.765, P-value: 0.009) for predicting in-hospital mortality in neonates with EA/TEF.
Receiver operating characteristic (ROC) curves for birth weight and in-hospital mortality. AUC, area under the curve; CI, confidence interval.
4.2. Associated Congenital Anomalies
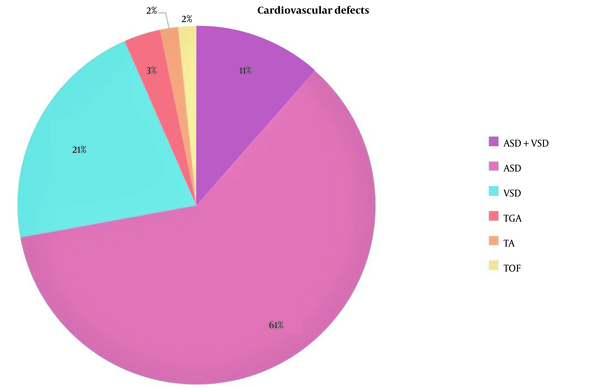
Among all neonates, 108 (46.4%) had at least one congenital anomaly. The most prevalent VACTERL anomalies were cardiovascular defects (25.3%), anorectal malformations (10.3%), vertebral defects (9.9%), limb defects (5.2%), and renal/radial anomalies (4.7%). Besides, the prevalence of non-VACTERL anomalies was 85 (36.5%) in the cohort population. Baseline characteristics, including age, sex, gestational age, kind of delivery, birth weight, and types of EA were similar in neonates with and without anomaly (Table 2). Concomitant anomaly significantly increased the risk of in-hospital mortality. In subgroup analysis, the most prevalent cardiovascular defects were atrial septal defect (ASD) with 61%, ventricular septal defect (VSD) with 21%, and a combination of both ASD and VSD with 11% (Figure 2).
A pie chart presents the prevalence of different cardiovascular defects in the study. ASD, atrial septal defect; TA, truncus arteriosus; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; VSD, ventricular septal defect.
4.3. Transmission Distance
We determined the distance between each patient's residential location and our center. The median transmission distance was 148.0 [IQR: 0 - 320.0] km. Most patients were transmitted < 100 km (48.4%), while 26.2% were transmitted 100 - 249 km and 25.3% transmitted ≥ 250 km to our center from other country regions (Table 1). There was no association between transmission distance and in-hospital mortality (P-value: 0.308). Moreover, we observed no association between other coexisting anomalies and transmission distance among neonates with EA/TEF (P-value: 0.415, Table 2).
5. Discussion
In this cohort study, we evaluated 233 neonates with EA/TEF admitted/referred to our tertiary referral center within a 12-year period. We observed that neonates with lower birth weight, gestational age < 37 weeks, coexisted anomaly, cardiovascular defects, and non-VACTERL anomalies are at higher risk of in-hospital mortality. Furthermore, EA types and transmission distance did not affect mortality risk in neonates with EA/TEF.
The EA is a known correctable anomaly of the esophagus, which can be accompanied by TEF. It can cause polyhydramnios during the prenatal period, and routine fetal ultrasound scans after 20 weeks' gestation can detect EA/TEF (9). But most patients are diagnosed after birth since prenatal ultrasound scan has only a 26% sensitivity to diagnose EA/TEF (18). The clinical presentation includes excessive salivation, coughing, and choking following oral feeding, cyanosis, or even respiratory distress; once EA/TEF is suspected, confirmatory radiography tests are warranted (19). In this study, type C (94.4%) was the most common type of EA/TEF followed by type A, type B, and type D, respectively, which is in line with previous findings (9, 12, 13). Type E fistula accounts for 4 - 5% of all congenital esophageal anomalies and may not be immediately diagnosed after birth (20). We included only neonates aged less than 10 days at the operation date, which may explain why we had no cases of type E fistula in our study.
Term infants undergoing EA/TEF repair surgery have a better prognosis than preterm infants with very low birth weight (21). Furthermore, concomitant anomalies play a significant role in dictating neonatal prognosis when compared with weight alone (9, 22). In 1994, Spitz proposed a prognostic classification to predict neonatal survival after EA/TEF surgery and categorized patients into three groups based on birth weight and major cardiovascular anomalies (23). Spitz predicted that infants with birth weight < 1500 g and major cardiovascular anomaly have the lowest survival rate (50%) (23). In this study, we found that cardiovascular defects significantly increased the risk of in-hospital mortality. Also, the optimal cut-off value of birth weight for predicting in-hospital mortality was 2575 g, which is 1070 g higher than the cut-off point suggested by Spitz. There can be several explanations for this difference. Over the last decades, improved prenatal care has reduced preterm delivery and low birth weight (24). In addition, developments in neonatal surgical techniques, equipment, and better post-operative care lead to a higher survival rate (25). Moreover, different study populations and ethnicities may have a great influence on the study results and associated congenital anomalies (26). Such explanations can elucidate the difference in cut-off values, and previous predicting risk scores can be expected to change in the future.
Previous studies in the Iranian population have reported different mortality rates in neonates with EA/TEF varying from 4.7 to 29.7%. In a study by Osia et al. on 37 neonates who underwent EA/TEF surgery, the mean gestational age was 37.2 ± 1.7 weeks, the mean birth weight was 2601 ± 504 g, 37.8% had associated anomaly, and 29.7% deceased following the surgery (10). On the other hand, the mortality rate in our study was 12.4%, which is less than half compared to their findings (10). We found slightly higher mean gestational age (mean difference: 0.3 weeks), birth weight (mean difference: 52 g), and concomitant anomaly (46.4% vs. 37.8%). These differences underline that there are other attributable risk factors for post-operative prognosis in neonates undergoing EA/TEA surgery. Another recent study among 95 neonates with EA/TEA in Tehran identified low birth weight, major cardiac anomaly, and the need for prolonged mechanical ventilation as predictors of poor outcomes (13). The researchers observed a 15.75% mortality rate in their study population, which may be explained by both lower gestational age and birth weight compared to our results. We believe that more experienced staff, better post-operative care, and different ethnicities may explain significantly different mortality rates among the studies. Further multicentral studies with larger sample sizes in different ethnicities are warranted to provide more details on other prognostic risk factors.
This study evaluated 233 neonates with EA/TEF within a 12-year period. Despite several strengths of this study, including a large sample size from a tertiary referral center with experienced staff, we might mention several limitations. First, our research was an observational study with potential inherent biases. Second, it is a single-center study on the Iranian population, which may have influenced our baseline characteristics. Third, we lacked information regarding surgical techniques, post-operative complications, and cause of death. Fourth, due to the lack of follow-up, we could not assess predictors of long-term outcomes.
5.1. Conclusions
We found that lower birth weight, prematurity (< 37 weeks), and coexisting anomalies, especially cardiovascular defects, are associated with an increased risk of in-hospital mortality in neonates after EA/TEF repair surgery. Multicentral studies with longer follow-ups among different ethnicities are needed to further investigate the risk factors associated with post-operative outcomes in neonates undergoing EA/TEF repair surgery.
Acknowledgements
References
-
1.
Nassar N, Leoncini E, Amar E, Arteaga-Vazquez J, Bakker MK, Bower C, et al. Prevalence of esophageal atresia among 18 international birth defects surveillance programs. Birth Defects Res A Clin Mol Teratol. 2012;94(11):893-9. [PubMed ID: 22945024]. [PubMed Central ID: PMC4467200]. https://doi.org/10.1002/bdra.23067.
-
2.
Stoll C, Alembik Y, Dott B, Roth MP. Associated anomalies in cases with esophageal atresia. Am J Med Genet A. 2017;173(8):2139-57. [PubMed ID: 28577344]. https://doi.org/10.1002/ajmg.a.38303.
-
3.
Spitz L. Esophageal atresia. Lessons I have learned in a 40-year experience. J Pediatr Surg. 2006;41(10):1635-40. [PubMed ID: 17011260]. https://doi.org/10.1016/j.jpedsurg.2006.07.004.
-
4.
Pardy C, D'Antonio F, Khalil A, Giuliani S. Prenatal detection of esophageal atresia: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2019;98(6):689-99. [PubMed ID: 30659586]. https://doi.org/10.1111/aogs.13536.
-
5.
Folaranmi SE, Jawaid WB, Gavin L, Jones MO, Losty PD. Influence of birth weight on primary surgical management of newborns with esophageal atresia. J Pediatr Surg. 2021;56(5):929-32. [PubMed ID: 33276972]. https://doi.org/10.1016/j.jpedsurg.2020.11.023.
-
6.
van Hoorn CE, Costerus SA, Lau J, Wijnen RMH, Vlot J, Tibboel D, et al. Perioperative management of esophageal atresia/tracheo-esophageal fistula: An analysis of data of 101 consecutive patients. Paediatr Anaesth. 2019;29(10):1024-32. [PubMed ID: 31343794]. https://doi.org/10.1111/pan.13711.
-
7.
Cassina M, Ruol M, Pertile R, Midrio P, Piffer S, Vicenzi V, et al. Prevalence, characteristics, and survival of children with esophageal atresia: A 32-year population-based study including 1,417,724 consecutive newborns. Birth Defects Res A Clin Mol Teratol. 2016;106(7):542-8. [PubMed ID: 26931365]. https://doi.org/10.1002/bdra.23493.
-
8.
Guptha S, Shumate C, Scheuerle AE. Likelihood of meeting defined VATER/VACTERL phenotype in infants with esophageal atresia with or without tracheoesophageal fistula. Am J Med Genet A. 2019;179(11):2202-6. [PubMed ID: 31436871]. https://doi.org/10.1002/ajmg.a.61337.
-
9.
Lee S. Basic Knowledge of Tracheoesophageal Fistula and Esophageal Atresia. Adv Neonatal Care. 2018;18(1):14-21. [PubMed ID: 29373345]. https://doi.org/10.1097/ANC.0000000000000464.
-
10.
Osia S, Hadipour A, Moshrefi M, Mirzapour M. Esophageal Atresia: 13 Years'experience in Amirkola Children’s Hospital, North of Iran. Caspian J Pediatr. 2015;1(1):22-4.
-
11.
Peyvasteh M, Askarpour S, Sarmast MH, Javaherizadeh H, Mehrabi V, Ahmadi J, et al. Esophageal atresia: comparison between survivors and mortality cases who underwent surgery over a 2-year period in two referral hospitals, Tehran, Iran. Ann Pediatr Surg. 2012;8(2):42-4. https://doi.org/10.1097/01.XPS.0000412346.91254.0e.
-
12.
Goodarzi M, Khazaei HA, Ashjaei B, Ghavami M, Mollaeian M, Bigdeli N, et al. Esophageal atresia: Recent five years’ mortality and morbidity. Acta Medica Iranica. 2018:660-4.
-
13.
Kamrani K, Eftekhari K, Malekiantaghi A, Kaveh M, Hosseinali Beigi E. The Some Predictive Factors for Survival of Newborns with Esophageal Atresia. Int J Pediatr. 2019;7(12):10565-72. https://doi.org/10.22038/ijp.2019.42627.3573.
-
14.
Tanasan A, Jiryaee N, Sabzehei MK, Amiraadi M. VACTERL Association in Neonates Hospitalized with Esophageal Atresia and Imperforate Anus. Int J Pediatr. 2021;9(9):14413-20. https://doi.org/10.22038/ijp.2020.
-
15.
Solomon BD, Baker LA, Bear KA, Cunningham BK, Giampietro PF, Hadigan C, et al. An approach to the identification of anomalies and etiologies in neonates with identified or suspected VACTERL (vertebral defects, anal atresia, tracheo-esophageal fistula with esophageal atresia, cardiac anomalies, renal anomalies, and limb anomalies) association. J Pediatr. 2014;164(3):451-7 e1. [PubMed ID: 24332453]. [PubMed Central ID: PMC3943871]. https://doi.org/10.1016/j.jpeds.2013.10.086.
-
16.
Lautz TB, Mandelia A, Radhakrishnan J. VACTERL associations in children undergoing surgery for esophageal atresia and anorectal malformations: Implications for pediatric surgeons. J Pediatr Surg. 2015;50(8):1245-50. [PubMed ID: 25913268]. https://doi.org/10.1016/j.jpedsurg.2015.02.049.
-
17.
Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J. 2008;50(3):419-30. [PubMed ID: 18435502]. [PubMed Central ID: PMC2515362]. https://doi.org/10.1002/bimj.200710415.
-
18.
Bradshaw CJ, Thakkar H, Knutzen L, Marsh R, Pacilli M, Impey L, et al. Accuracy of prenatal detection of tracheoesophageal fistula and oesophageal atresia. J Pediatr Surg. 2016;51(8):1268-72. [PubMed ID: 26932255]. https://doi.org/10.1016/j.jpedsurg.2016.02.001.
-
19.
Morini F, Conforti A, Zani A, Sindjic-Antunovic S, Koivusalo A, Friedmacher F, et al. Diagnostic Workup of Neonates With Esophageal Atresia: Results From the EUPSA Esophageal Atresia Registry. Front Pediatr. 2020;8:489. [PubMed ID: 32984205]. [PubMed Central ID: PMC7477332]. https://doi.org/10.3389/fped.2020.00489.
-
20.
Sampat K, Losty PD. Diagnostic and management strategies for congenital H-type tracheoesophageal fistula: a systematic review. Pediatr Surg Int. 2021;37(5):539-47. [PubMed ID: 33474597]. [PubMed Central ID: PMC8026411]. https://doi.org/10.1007/s00383-020-04853-3.
-
21.
Spitz L. Oesophageal atresia. Orphanet J Rare Dis. 2007;2:24. [PubMed ID: 17498283]. [PubMed Central ID: PMC1884133]. https://doi.org/10.1186/1750-1172-2-24.
-
22.
Schmidt A, Obermayr F, Lieber J, Gille C, Fideler F, Fuchs J. Outcome of primary repair in extremely and very low-birth-weight infants with esophageal atresia/distal tracheoesophageal fistula. J Pediatr Surg. 2017;52(10):1567-70. [PubMed ID: 28554817]. https://doi.org/10.1016/j.jpedsurg.2017.05.011.
-
23.
Spitz L, Kiely EM, Morecroft JA, Drake DP. Oesophageal atresia: at-risk groups for the 1990s. J Pediatr Surg. 1994;29(6):723-5. [PubMed ID: 8078005]. https://doi.org/10.1016/0022-3468(94)90354-9.
-
24.
Abu-Ghanem S, Sheiner E, Sherf M, Wiznitzer A, Sergienko R, Shoham-Vardi I. Lack of prenatal care in a traditional community: trends and perinatal outcomes. Arch Gynecol Obstet. 2012;285(5):1237-42. [PubMed ID: 22124534]. https://doi.org/10.1007/s00404-011-2153-x.
-
25.
Taguchi T. Current progress in neonatal surgery. Surg Today. 2008;38(5):379-89. [PubMed ID: 18560958]. https://doi.org/10.1007/s00595-007-3657-7.
-
26.
Quiroz HJ, Turpin A, Willobee BA, Ferrantella A, Parreco J, Lasko D, et al. Nationwide analysis of mortality and hospital readmissions in esophageal atresia. J Pediatr Surg. 2020;55(5):824-9. [PubMed ID: 32061361]. https://doi.org/10.1016/j.jpedsurg.2020.01.025.
