Abstract
Background:
Insomnia and emotional disorders share common factors that underlie and perpetuate these disorders. The Unified Protocol (UP) for transdiagnostic treatment of emotional disorders is a new treatment approach designed to target core processes of emotional disorders.Objectives:
The present study examined the effects of UP on behavioral inhibition/behavioral activation, anxiety sensitivity, and emotion dysregulation as transdiagnostic factors, as well as cognition and behaviors specific to insomnia, in a small sample of patients (N = 6) with comorbid insomnia and emotional disorders.Methods:
A multiple-baseline across subjects single-case experimental design with a 3-month follow-up was used. Participants were allocated to 2, 4, and 6 baseline periods and then received 14 weekly individual sessions of UP. The outcome measures were Behavioral Inhibition/Behavioral Activation Scales (BIS/BAS), Anxiety Sensitivity Index-3 (ASI-3), Difficulties in Emotion Regulation Scale (DERS), Dysfunctional Beliefs and Attitudes about Sleep Scale-10 (DBAS-10), and Sleep-Related Behaviors Questionnaire (SRBQ). Data were analyzed using visual inspection, mixed model analysis, and reliable change index (RCI).Results:
Mixed model analysis showed significant changes in BIS/BAS, ASI-3, DERS, DBAS-10, and SRBQ from pre-treatment to post-treatment. Most participants achieved a reliable change in BIS, ASI-3, DERS, DBAS-10, and SRBQ at a 3-month follow-up.Conclusions:
This preliminary study provides empirical evidence on the utility of UP for reducing common vulnerability and sleep-specific factors in chronic insomnia comorbid with emotional disorders. Further research is needed to test these findings in randomized controlled studies.Keywords
Anxiety Disorders Depressive Disorders Psychotherapy Sleep Initiation and Maintenance Disorders
1. Background
Insomnia, as a syndrome or symptom, is a widespread and persistent problem (1). People with insomnia experience a wide range of problems such as fatigue, irritability, difficulties in memorization and concentration, impairment of daily functions, and reduced quality of life (2).
Comorbidity of psychiatric disorders is a rule rather than an exception (3). It is estimated that insomnia is comorbid with other psychiatric disorders in 41 - 53% of cases (4). Depression and anxiety are among the most common psychiatric disorders that accompany insomnia (5, 6). It seems that there is a bilateral relationship between insomnia and these emotional disorders to an extent that insomnia contributes to depressive and anxiety symptoms and these symptoms can lead to sleep disturbances, too (7). Conceptual similarities between the new models of insomnia and emotional disorders indicate that the key contributing factors in insomnia are similar to the proposed cognitive and behavioral factors in emotional disorders (8).
Anxiety sensitivity (AS) or faulty belief about the dangers of anxious sensations (9) is a common factor in emotional disorders and insomnia. Research indicates that AS is a risk factor for anxiety (10) and depression (11). In addition, AS mediates the relationship between sleep anticipatory anxiety and sleep onset latency (12). Thus, AS may be a common factor accounting for the comorbidity of emotional disorders and insomnia (8).
Emotion dysregulation is another common factor that seems to play a role in both emotional disorders and insomnia. Results of a meta-analysis showed that anxiety and depression are associated with increased use of avoidance, suppression, and rumination and reduced use of problem-solving (13). Reliance on maladaptive emotion regulation strategies such as rumination and worry can lead to sleep disturbance, too (14).
Temperament is another factor predisposes individuals to emotional disorders and insomnia. Two dimensions of temperament, i.e. behavioral inhibition and behavioral activation, play a significant role in the development and maintenance of depression and anxiety disorders (15). Research has shown that insomnia, like emotional disorders, is associated with some dimensions of temperament. The severity of insomnia has a positive relationship with harm avoidance and self-transcendence, and a negative relationship with novelty seeking, rewards dependence, and cooperativeness (16). In addition to these transdiagnostic factors, maladaptive cognitions and behaviors specific to insomnia are related to the severity of insomnia in samples with comorbid insomnia and emotional disorders (8).
Non-transdiagnostic evidence-based treatments target only one disorder at a time, while the majority of people who seek psychological treatments have many comorbid disorders (17). In the cases where clinicians need to make a decision about various comorbid disorders, targeting underlying or common factors may simplify treatment offering and reduce the complexity of clinical decision making (17).
The majority of previous research studies on patients with both insomnia and emotional problems have only focused on one problem. Cognitive behavioral therapy for Insomnia (CBT-I) is the first therapeutic option for primary insomnia (18). However, 20 to 30% of patients with insomnia do not respond to CBT-I (19). The findings of a meta-analysis have shown that CBT-I has moderate effects on anxiety in patients suffering from insomnia with or without anxiety disorder (20). Another meta-analysis has indicated that cognitive behavioral therapy for anxiety disorders have moderate effects on sleep problems (21). Thus, it seems that targeting one disorder has only a moderate effect on another disorder. Moreover, it is determined that high levels of depressive symptoms at baseline are associated with early dropouts in CBT-I (22).
Transdiagnostic treatments apply similar underlying therapeutic principles across mental disorders (23). Unified Protocol (UP) is a transdiagnostic treatment derived from key principles of evidenced-based cognitive-behavioral treatments, as well as advances in the emotion regulation field (24). Most studies that have thus far investigated the efficacy of UP have been limited to emotional disorders. The results of these studies support the efficacy of UP in the treatment of emotional disorders in Iran and other countries (25-28). However, no study has examined its efficacy in patients with chronic insomnia comorbid with emotional disorders.
2. Objectives
The current study aimed to contribute to the literature by investigating the effects of UP, which was originally designed to be used across mood and anxiety disorders and possibly somatoform and dissociative disorders (25) in a sample with comorbid insomnia and emotional disorders. Thus, based on the high co-occurrence rate of insomnia and emotional disorders and common contributing factors in both disorders, the present study aimed to test the effects of UP on transdiagnostic and specific factors in patients with comorbid insomnia and emotional disorders using a multiple baseline experimental design. It was expected that the treatment through UP targeting both problems in sessions would result in the reduction of anxiety sensitivity, behavioral inhibition, emotion dysregulation, and improvement of behavioral activation and specific sleep-related cognitive and behavioral factors.
3. Materials and Methods
3.1. Participants
Six Iranian subjects with chronic insomnia disorder comorbid with emotional disorders who were referred to the Danesh sleep disorders clinic in Tehran participated in this study. Eligibility criteria for the study included an age requirement of 18 years or older, fluency in Persian, having at least completed high school education, a diagnosis of chronic insomnia disorder according to the DSM-IV-TR criteria, meeting research diagnostic criteria (29) and quantitative criteria for insomnia (30), diagnosis of an anxiety or depressive disorder or both of them according to Structured Clinical Interview for DSM-IV Disorders-I (SCID-I), stable medication use for 6 month prior to the study and not starting, stopping or changing medication during the baseline or treatment phase. Exclusion criteria included a current or recent history of substance abuse or dependence, presence of psychotic disorder or bipolar disorder, presence of another sleep disorder, past history of CBT for at least 8 weeks without any improvement in symptoms, being implicated in any concurrent psychological treatments, experiencing active suicidal ideation, presence of any major medical disease or disability.
Of the 8 subjects who enrolled to participate in the study, 6 subjects attended in all treatment sessions. One subject stopped the study at session two of the baseline phase and one subject at the first session of the treatment phase. Socio-demographic and clinical characteristics of the study population are presented in Table 1.
3.2. Design
A multiple-baseline across subjects single-case experimental design with a 3-month follow-up was applied (31). Subjects were assigned to three baseline periods including two, four, and six-week durations. During this period, no treatment was provided. Following the baseline phase, UP was conducted on a weekly basis and the participants were asked to complete all questionnaires at the end of each week. All subjects participated in 14 sessions of UP. On termination of the treatment, the subjects were followed up for 3 months in order to evaluate the maintenance of treatment gains. No further treatment was administered during the follow-up phase. In order to identify changes in study measures, visual inspection method, which is a common method in single-case experimental designs, was applied (31). This method was used for all participants with common factors related measures. During modules, which spanned over one week, scores were averaged over those modules. In addition, data were analyzed using a mixed model analysis to investigate whether the intervention works as compared with the baseline period (32). This method can provide information about differences in within-subject symptom changes between two distinct phases. The parameter b2 is the rate of change at the baseline and the parameter b3 is the difference in the rate of changes between baseline and treatment. Moreover, reliable change index (RCI) (33) was utilized to determine whether the magnitude of changes for a specific client is statistically reliable and is greater than the difference that could be attributed to the random measurement error alone.
Participants’ Socio-Demographic and Clinical Characteristics
| Variable | P1 | P2 | P3 | P4 | P5 | P6 |
|---|---|---|---|---|---|---|
| Age | 40 | 38 | 52 | 49 | 25 | 28 |
| Gender | F | M | F | F | F | M |
| BMI | 23 | 28 | 27 | 24 | 25 | 33 |
| Diagnosis | OCD, MDD | MDD, SAD | MDD, SAD | MDD, GAD | MDD, PD | MDD, specific phobia |
| Marital status | Married | Divorced | Married | Married | Single | Single |
| Education | Bachelor | Bachelor | Associate Degree | Medical degree | Bachelor | Bachelor |
| Religion | Islam | Islam | Islam | Islam | Islam | Islam |
| Medication | None | None | Citalopram, trazodone, gabapentin | None | None | Clonazepam |
3.3. Procedure
Data for this study were derived from a preliminary study investigating the effects of UP on insomnia, as well as emotional symptoms with the current sample. Subjects were recruited from different psychiatric and psychological centers in Tehran. Each subject who had volunteered to participate in the study completed a brief screening telephone interview to ascertain the presence of primary inclusion criteria for the study. Individuals who were not excluded during this primary screening were visited by a sleep clinician to rule out other sleep disorders. Then, eligible subjects were visited by a trained doctoral student during which detailed information about the study was given to them and they were asked to complete a form about medical and demographic information. Then, they underwent SCID-I and completed the Global Sleep assessment questionnaire (GASQ). After this stage, potential patients were asked to complete an informed consent form to participate in the study. They were also provided with a sleep diary to complete during two future weeks. After two weeks, sleep diary data were reviewed to verify the presence of quantitative and research diagnostic criteria for insomnia. Individuals, who passed this stage, were assigned to 2-, 4- and 6-week baseline periods. After termination of the baseline phase, the subjects underwent UP on a weekly basis. Post-treatment evaluation included the completion of 2 weeks of sleep diary and all battery questionnaires. The study was approved by the Ethics committee of Iran University of Medical Sciences (IR.IUMS.rec.1394.2295162793).
3.4. Treatment
Treatment consisted of 14 face-to-face individual therapy sessions. All therapy sessions were delivered according to the UP published manual (34). The main therapist (the first author) was a Ph.D. student with more than 5 years of experience in CBT protocols. In every session, the authors tried to use the concepts of UP with regard to both insomnia and emotional disorders. Adapting UP concepts for insomnia was according to a chapter regarding the application of UP for insomnia disorder (35).
3.5. Measures
3.5.1. Global Sleep Assessment Questionnaire (GASQ)
GASQ is an 11-item questionnaire that is used as a brief screening tool for identifying sleep disorders. GASQ assesses each sleep disorder separately and the sum of responses to items indicates the necessity for more evaluation. The test-retest reliability of the GASQ in an adult sample with different sleep disorders was reported as 0.51 to 0.92. Sensitivity and specificity of the scale for various sleep disorders have been adequate (36). A Persian version of GASQ was used in this study.
3.5.2. Structured Clinical Interview for DSM-IV Disorders-I (SCID-I)
SCID-I is a semi-structured interview, which is used to diagnose common axis I disorders according to the DSM-IV criteria (37). The Persian version of SCID-I was used in the current study. Previous results have indicated that the Persian version of SCID-I is of good specificity (more than 0.85) and sensitivity (0.60 - 0.80) in Iranian populations (38).
3.5.3. Consensus Sleep diary (CSD)
Core CSD (39) was used as a standardized measure to tracking prospective sleep data. Previous studies have supported its validity and clinical utility (40). This scale was translated into Persian for the purpose of the study.
3.5.4. Behavioral Inhibition and Behavioral Activation Scales
BIS/BAS is a 20-item self-report measure, which evaluates the sensitivity of behavioral inhibition and behavioral activation systems. The BIS subscale includes seven items that refer to potential negative events while BAS subscale contains 13 items and refers to potentially positive events. The scale has demonstrated good internal consistency (α = 0.74) and test-retest reliability for BIS (0.66) and drive (0.66), fun-seeking (0.69), and rewards responsiveness (0.59) subscales of BAS in an eight-week interval (41). Internal consistency of the Persian Version was reported as 0.69 for BIS and 0.78 for BAS and the test-retest reliability with a 2-week interval was reported as 0.68 and 0.71 for BIS and BAS, respectively (42).
3.5.5. Anxiety Sensitivity Index- 3(ASI-3)
ASI-3 is an 18-item self-report questionnaire, which includes three subscales: physical, cognitive, and social concerns. Participants are asked to determine their agreement on a 5-point Likert scale (0 = very little to 4 = very much). Structural, convergent, and discriminant validity of the scale has been acceptable. Internal consistency of the scale was reported as 0.76 to 0.86 for physical concerns, 0.79 to 0.91 for cognitive concerns, and 0.73 to 0.86 for social concerns (43). This scale was translated into Persian for the purpose of the study. The results supported the internal consistency (α = 0.87) and test-retest reliability of the entire scale in a 4-week interval (r = 0.81).
3.5.6. Difficulties in Emotion Regulation Scale (DERS)
DERS is a comprehensive 36-item scale designed to assess difficulties in different dimensions of emotion regulation. Participants are asked to rate each item on a 5-point Likert scale from 1 = “almost never” to 5 = “almost always.” Higher scores indicate greater difficulties with emotion regulation. DERS has adequate internal consistency (α = 0.93) and test-retest reliability (r = 0.88) in 4-8-week intervals (44). The Persian version of DERS includes 33 items, in which 3 items (17, 22, and 34) have been deleted. The internal consistency of the subscales in a sample of college students ranged between 0.86 and 0.88 and test-retest reliability of the subscales at a one-week interval was reported as 0.79 to 0.91 (45).
3.5.7. Dysfunctional Beliefs and Attitudes about Sleep Scale (DBAS-10)
This inventory evaluates sleep-related cognitions (46) and is the short version of DBAS-30 (47). The items are responded based on a 10-point visual analog scale (0 = completely disagree, 10 = completely agree). The DBAS-10 total score is calculated using the average score of all items, which ranges from zero to 10. Studies have supported the validity and reliability of DBAS-10 (46). The internal consistency and test-retest reliability of the Persian version of DBAS-10 (2-week interval) has been reported as 0.84 and 0.83, respectively (48).
3.5.8. Sleep-Related Behaviors Questionnaire (SRBQ)
This 32-item questionnaire assesses the strategies people use to prevent a feared outcome related to insomnia. Respondents should rate each item on a 5-point Likert scale. The total score ranges from zero to 160. SRBQ is of a good internal consistency (α = 0.92) and can discriminate between individuals with and without insomnia. Moreover, it correlates significantly with ISI (49). The Persian version of SRBQ has adequate internal consistency (α = 0.90) and test-retest reliability in a 2-week interval (r = 0.73) (50).
4. Results
4.1. Visual Inspection
Figures 1 - 3 show the data obtained from BIS/BAS, ASI-3, and DERS during the study phases. The illustration of changes in the study measures over each phase is presented in detail.
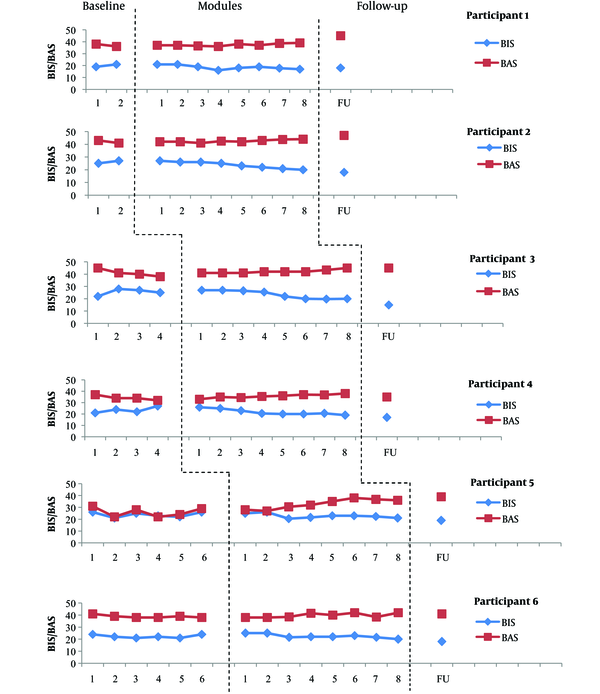
Scores obtained on the BIS/BAS by each participant at each phase. Data obtained from BIS/BAS at the baseline indicated relatively no trend with moderate variability for participant 5. The scores of participant 6 showed relatively no trend with low variability and the scores of participant 3 indicated an increasing trend with low variability on BIS. However, participants 3 and 6 showed a decreasing trend with low variability on BAS. The scores of participant 1, 2, and 4 showed an increasing trend with low variability on BIS and a decreasing trend with low variability on BAS. Given that these changes are in opposite direction of what is expected in treatment, they pose no problem for the interpretation of the results. UP was associated with a reduction in BIS and an improvement in BAS for most participants. The BIS scores reached a lower level relative to the baseline data at the end of module 8 for participants 5 and 6 but this reduction occurred at the end of module 4 (participants 1 and 4), 5 (participant 2), and 6 (participant 3). The BAS scores reached a higher level relative to the baseline data at the end of module 4 (participants 5 and 6), module 7 (participants 1 and 2), and module 8 (participant 4). Only participants 1, 2, and 5 obtained a higher BAS score relative to the baseline data at follow-up. The BIS scores continued to decrease at follow-up for all participants, except for participant 1, who showed a minor increase on BIS. Participants 1, 2, and 5 showed an increase and participants 4 and 6 indicated a reduction in the BAS score at follow-up.
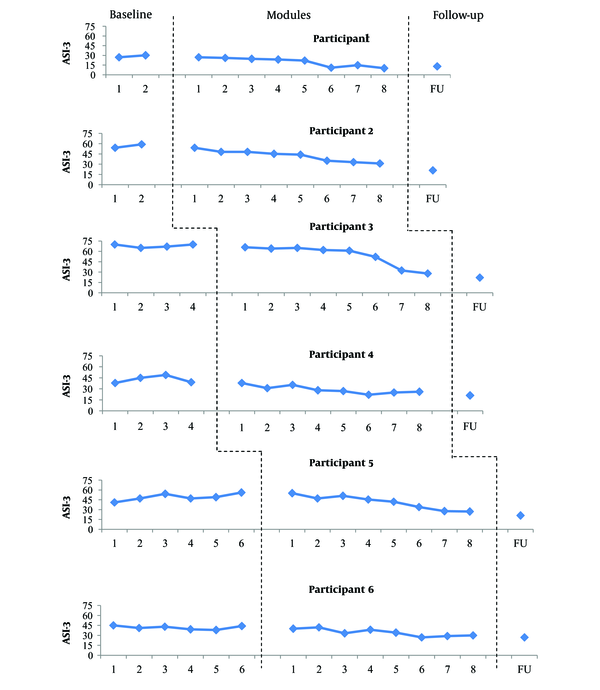
Scores obtained on the ASI-3 through the study. Data obtained at the baseline generally showed no trend with low variability for participants 3 and 6 and moderate variability for participant 4. Data of participant 5 revealed an increasing trend with moderate variability. Participants 1 and 2 showed an increasing trend with low variability at the baseline. The introduction of UP was accompanied by a progressive reduction in the ASI-3 scores in most participants. The decrease in the ASI scores was most obvious at the end of module 2 and 4 for participant 4, module 3 for participant 6, module 6 for participants 1, 2, and 5, and at the end of module 7 for participant 3. The observed improvement in the ASI-3 scores was maintained at follow-up for all participants.
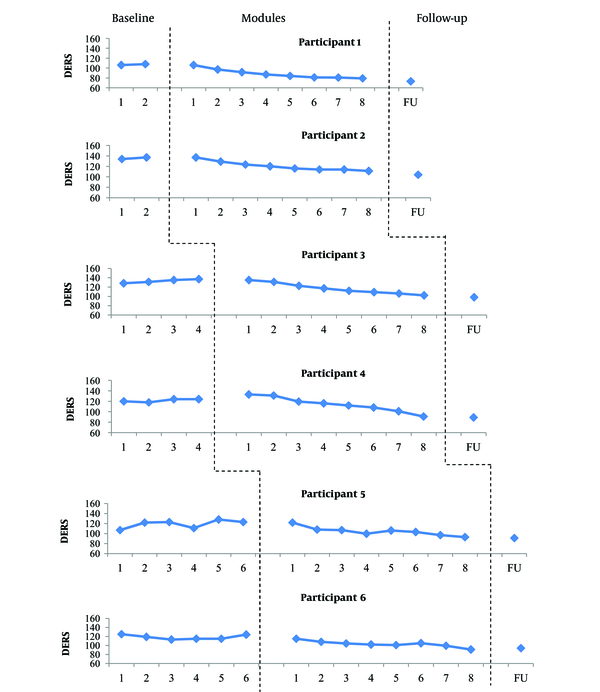
Scores obtained on the DERS through the study. Visual inspection of DERS scores during the baseline period showed relatively stable data for participants 1, 2, 4 and 6. The data of participants 3 and 5 revealed an increasing trend with low and moderate variability, respectively. However, the observed trends in the scores of participants 3 and 5 are in opposite direction of changes expected in treatment, and this does not interfere with the interpretation of the results. With the introduction of treatment, the DERS scores began to decrease gradually for most participants. The most obvious reduction occurred at the end of module 2 (participants 1, 2, 5, and 6) and module 3 (participants 3 and 4). Although the DERS scores increased slightly at 3-month follow-up for participant 6, it remained below the pre-treatment score. The DERS score decreased at 3-month follow-up in other participants.
4.2. Comparisons Across Study Phases
Pre-treatment to post-treatment changes in the questionnaire scores were analyzed using a mixed model analysis (32). All observation points of the treatment (14 sessions) and baseline phases were included in this analysis. Cohen’s effect sizes were utilized to compare the magnitude of the differences in pre- and post-treatment. Effect sizes were interpreted as small (d = 0.2), medium (d = 0.5), and large (d = 0.8) (51). The results of the weekly assessments indicated that after the UP, the end point of BIS (P = 0.001), ASI-3 (P < 0.001), DERS (P < 0.001), DBAS (P < 0.001), and SRBQ (P < 0.001) was lower and the end point of BAS (P = 0.04) was higher as compared with the baseline. Regarding differences in trends between the treatment and baseline phases, BIS (P = 0.04), ASI-3 (P = 0.03), DERS (P < 0.001), DBAS (P = 0.03), and SRBQ (P = 0.04) decreased at a higher rate and BAS (P = 0.01) increased at a higher rate during UP as compared with the baseline. The results of the mixed model analysis and effect sizes for the outcome measures are included in Table 2 and 3, respectively.
4.3. RCI
The RCI for BIS/BAS, ASI-3, DERS, DBAS-10, and SRBQ scores was calculated for all participants at post-treatment and follow-up. The results of RCI are presented in Table 4. All participants showed reliable changes in ASI-3, DERS, and DBAS-10 at follow-up. However, 5 out of 6 participants indicated a reliable change in BIS and SRBQ and only one participant had a reliable change in BAS at follow-up.
Treatment Efficacy Results Using Mixed Models
| Estimate | SE | p | 95% Confidence Interval | ||
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Results Concerning BIS | |||||
| Intercept (b0) | 2.41 | 0.911 | 0.000 | 22.25 | 25.97 |
| Phase (b1) | - 4.88 | 1.32 | 0.001 | -7.60 | -2.15 |
| Time_in_phase (b2) | -0.37 | 0.33 | 0.26 | -1.03 | 0.29 |
| Time_in_phase*phase (b3) | 0.75 | 0.37 | 0.046 | 0.01 | 1.50 |
| Results Concerning BAS | |||||
| Intercept (b0) | 3.55 | 1.98 | 0.000 | 31.24 | 39.93 |
| Phase (b1) | 5.07 | 2.42 | 0.04 | 0.18 | 9.97 |
| Time_in_phase (b2) | 0.97 | 0.44 | 0.03 | 0.08 | 1.86 |
| Time_in_phase*phase (b3) | -1.30 | 0.51 | 0.01 | -2.32 | -0.27 |
| Results Concerning ASI-3 | |||||
| Intercept (b0) | 4.92 | 4.84 | 0.000 | 38.65 | 59.77 |
| Phase (b1) | -2.46 | 5.99 | 0.000 | -36.70 | -12.50 |
| Time_in_phase (b2) | -1.10 | 1.11 | 0.32 | -3.32 | 1.11 |
| Time_in_phase*phase (b3) | 2.76 | 1.28 | 0.03 | 0.21 | 5.31 |
| Results Concerning DERS | |||||
| Intercept (b0) | 1.24 | 4.72 | 0.000 | 113.61 | 134.51 |
| Phase (b1) | -3.07 | 5.30 | 0.000 | -41.39 | -20.11 |
| Time_in_phase (b2) | -1.70 | 0.94 | 0.07 | -3.57 | 0.16 |
| Time_in_phase*phase (b3) | 4.00 | 1.08 | 0.000 | 1.85 | 6.14 |
| Results Concerning DBAS-10 | |||||
| Intercept (b0) | 8.49 | 0.400 | 0.000 | 7.64 | 9.34 |
| Phase (b1) | -3.42 | 0.54 | 0.000 | -4.54 | -2.30 |
| Time_in_phase (b2) | -0.03 | 0.11 | 0.73 | -0.26 | 0.18 |
| Time_in_phase*phase (b3) | 0.28 | 0.12 | 0.03 | 0.02 | 0.53 |
| Results Concerning SRBQ | |||||
| Intercept (b0) | 7.22 | 5.09 | 0.000 | 61.42 | 83.08 |
| Phase (b1) | -3.61 | 6.82 | 0.000 | -49.97 | -22.28 |
| Time_in_phase (b2) | -0.76 | 1.35 | 0.57 | -3.45 | 1.93 |
| Time_in_phase*phase (b3) | 3.20 | 1.55 | 0.04 | 0.10 | 6.30 |
Pre-Teatment, Post-Treatment, and Follow-Up Means, Standard Deviations, and Effect Sizes of Dependent Variablesa
| Measure | Pre-Treatment | Post-Treatment | Follow-Up | d |
|---|---|---|---|---|
| BIS | 23.52 (2.18) | 19.50 (1.37) | 17.50 (1.37) | -0.70 |
| BAS | 36.54 (5.87) | 40.67 (3.55) | 42 (4.51) | 0.63 |
| ASI-3 | 47.73 (13.57) | 25.33 (7.73) | 20.83 (4.49) | -1.07 |
| DERS | 122.38 (10.42) | 94.50 (10.91) | 91.50 (10.52) | -1.39 |
| DBAS-10 | 8.40 (0.72) | 5.25 (1.05) | 5.33 (0.92) | -1.65 |
| SRBQ | 71.02 (15.04) | 37 (7.97) | 36 (7.23) | -1.20 |
Reliable Changes in Independent Measures
| Reliable Change | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | |||||||
| Post | FU | Post | FU | Post | FU | Post | FU | Post | FU | Post | FU | |
| BIS | -1.52 | -1.01 | -3.04 | -4.05 | -2.78 | -5.32 | -2.28 | -3.29 | -1.43 | -2.44 | -1.18 | -2.19 |
| BAS | 0.40 | 1.63 | 0.40 | 1.02 | 0.81 | 0.81 | 0.76 | 0.15 | 2.04 | 2.65 | 0.61 | 0.40 |
| ASI-3 | -2.98 | -2.49 | -4.11 | -5.72 | -6.44 | -7.41 | -2.69 | -3.50 | -3.54 | -4.51 | -1.88 | -2.36 |
| DERS | -6.21 | -7.54 | -5.43 | -6.99 | -6.82 | -7.71 | -6.77 | -7.21 | -5.77 | -6.21 | -6.10 | -5.43 |
| DBAS-10 | -5.16 | -4.54 | -6.60 | -7.01 | -5.57 | -6.60 | -5.98 | -5.57 | -8.01 | -7.80 | -7.74 | -6.50 |
| SRBQ | -3.19 | -3.01 | -2.15 | -2.33 | -5.48 | -6.02 | -3.46 | -3.37 | -2.24 | -2.51 | -1.81 | -1.63 |
5. Discussion
This study examined the effects of UP on three common factors and specific cognitive and behavioral sleep-related variables in a sample with mixed chronic insomnia and emotional disorders, using a single-case experimental across-subjects design.
The results obtained using the mixed model analysis showed a significant reduction in BIS and a significant increase in BAS from pre-treatment to post-treatment. These results indicate that treatment with UP improved both dimensions of temperament. Targeting underlying temperamental processes of emotional disorders is one of the goals of UP (52). One previous study demonstrated that although UP has a modest to moderate effect on BIS/BAS, these changes were not maintained at a 6-month follow-up (52). Another study (53) on an Iranian population investigated the effects of UP on BIS in 3 women with GAD using a single-case experimental design, showed overall improvement percentage of 21.75 for 3 patients. In general, the findings regarding the effects of psychological treatments on temperament are mixed. Some studies have shown that even though temperamental variables reduce during treatment, the difference between treatment and control groups increases over time (54). Moreover, previous studies have indicated that changes in temperamental variables, such as positive affect, are related to the dosage of medication (55). Thus, in contrast with traditional concepts, various studies have shown that temperament may change over time and in response to treatment. However, it seems that further research is needed to determine specific factors affecting temperament (52). The results of the current study regarding changes in temperamental dimensions (especially the improvement of BIS scores from post-treatment to follow-up) are congruent with the studies that have proven that changes in temperament increase over time.
Another result of this study was that anxiety sensitivity reduced significantly from baseline to post-treatment. Although, according to visual inspection, different modules of UP were important in reducing AS, the most level of reduction occurred after module 6 (i.e. interoceptive exposure) for a half of the participants. Interoceptive exposure is a behavioral intervention that is known as a strategy to reduce anxiety sensitivity by targeting anxiety associated with physical sensations (56). Studies have indicated that interoceptive exposure is effective in reducing anxiety and depressive symptoms (57). In addition, interoceptive exposure has an indirect effect on insomnia symptoms via reducing anxiety sensitivity (58). In general, the effects of UP on reducing anxiety sensitivity in the current sample may be attributed to different modules, especially module 6 which is specifically designed to target physical sensations.
Significant reductions in emotion dysregulation were observed from baseline to post-treatment. The results of the current study are congruent with those of the previous studies that have proven the efficacy of UP on improving emotion regulation difficulties (27, 53, 59, 60). Emotion regulation difficulties are a risk factor for insomnia even after controlling anxiety and depression (61). UP targets highly negative reactivity to emotions via identification of maladaptive responses to emotions and development of more effective strategies regarding the management of emotional experiences (25). With regard to visual inspection data, two modules of UP may be more accountable for the observed changes. It seems that module 2 via increasing patients’ awareness of functional nature of emotions and components of emotional experiences and module 3 through educating mindfulness skills to accept emotions (34) play a more significant role in emotion regulation. Given that difficulties in emotion regulation may moderate the relationship between insomnia and psychiatric problems, interventions (such as UP) that target emotion dysregulation may enhance current treatments of insomnia (61).
Regarding dysfunctional sleep-related beliefs and behaviors, the results obtained by the present study showed that DBAS and SRBQ scores reduced significantly from baseline to post-treatment. Previous research has demonstrated that insomniac patients have more catastrophes about the consequences of sleep loss and probability overestimation of the occurrence of these consequences in comparison with good sleepers (62). In the present study, the most obvious reduction in DBAS for most participants occurred after module 4 (i.e. cognitive restructuring). Cognitive restructuring as a major module of UP increases thought flexibility in response to emotional experiences. In this module, automatic appraisals and two common cognitive errors are discussed (34). The efficacy of cognitive restructuring as a standalone therapeutic component in improving sleep difficulties has been demonstrated previously (63). Although cognitive restructuring may be a more important module in reducing DBAS scores in the present study, previous studies have indicated that behavioral interventions also have a significant effect on dysfunctional beliefs about sleep (64). Thus, the effects of UP on reducing dysfunctional beliefs specific to sleep may be attributed to both cognitive and behavioral modules of UP.
Insomniac patients, similar to patients with emotional disorders, may be involved in specific safety behaviors (65). Studies have shown that sleep-related safety behaviors are associated with insomnia severity even in patients with depressive and anxiety disorders (8). Many modules of UP may contribute to the reduction of sleep-related safety behaviors. In the current study, the most reduction in SRBQ scores was observed after modules 5 and 7. In insomniac patients, going to bed earlier, staying in bed despite inability to sleep, napping during the day, avoidance of, or reduction in, physical activity during the day, and using alcohol to facilitate sleeping can be conceptualized as specific sleep-related safety behaviors that disrupt sleep hemostat and perpetuate insomnia (66). Like avoidances and emotion-driven behaviors specific to emotional disorders, these sleep-related safety behaviors can also be targeted in the fifth module. Moreover, avoidances and emotion-driven behaviors related to sleep can be graded in a hierarchy according to their difficulty, and then be exposed similar to those in emotional disorders in module 7. However, similar to cognitions, different modules of UP are important in reducing safety behaviors, which is not unexpected considering the interaction between cognition, emotion, and behavior.
The current study had a number of limitations that should be addressed in future studies. This preliminary study included a sample of only six patients with mixed insomnia and emotional disorders. The small sample size makes it difficult to generalize the study results to a larger group of the population. Given the study’s small sample size, it is important for future studies to extend these findings to larger samples to obtain more precise conclusions about the effects of UP on transdiagnostic and specific variables. In addition, the treatment sessions were delivered by only one therapist that limits the generalizability of the results to different therapists. Thus, replication of the current study with different therapists would also be beneficial. Although the multiple baseline design controls factors such as time, it is not possible to specify with confidence whether the improvements were due to identified UP components. Future research would benefit from the inclusion of a control group to facilitate comparison of the effects of treatment with those of nonspecific factors. Evaluation of the intervention with a randomized control design to compare the efficacy of treatment with other common treatments for patients with mixed insomnia and emotional disorders is a necessary next step. This study focused on the evaluation of temperament using BIS/BAS. Although there is an empirical overlap between different temperamental constructs, the results of the current study on temperamental dimensions may be limited to this specific measure. Future work might replicate the current study by administering varied measures to assess and include different temperamental constructs. Finally, longer-term follow-up assessment with 6 or 12 months intervals may be beneficial for assessing the durability and stability of the therapeutic effects.
5.1. Conclusions
The current study marks the first evaluation of the effects of the UP on transdiagnostic and specific factors in a sample with mixed insomnia and emotional disorders and highlights a promising way to deliver UP to reduce vulnerability among this specific population. The findings of the present study have important implications for the clinicians seeking to provide cost-effective interventions for patients suffering from both insomnia and emotional disorders. These results indicate that even in a small sample, UP produced durable and meaningful changes in underlying variables contributing to comorbid insomnia and emotional disorders. Further studies with larger sample sizes and controlled clinical design are needed to evaluate the effects of the UP on transdiagnostic and specific variables in patients with primary and comorbid insomnia.
Acknowledgements
References
-
1.
Morin CM, Jarrin DC. Epidemiology of Insomnia. Sleep Medicine Clinics. 2013;8(3):281-97. https://doi.org/10.1016/j.jsmc.2013.05.002.
-
2.
Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I Sleep. 1999;22(Suppl 2):347-53.
-
3.
Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. Journal of Abnormal Psychology. 2001;110(4):585-99. https://doi.org/10.1037/0021-843x.110.4.585.
-
4.
Harvey AG. Insomnia: Symptom or Diagnosis? Clinical Psychology Review. 2001;21(7):1037-59. https://doi.org/10.1016/s0272-7358(00)00083-0.
-
5.
Ohayon MM. Insomnia: A ticking clock for depression? Journal of Psychiatric Research. 2007;41(11):893-4. https://doi.org/10.1016/j.jpsychires.2007.07.008.
-
6.
Ohayon MM. Observation of the Natural Evolution of Insomnia in the American General Population Cohort. Sleep Medicine Clinics. 2009;4(1):87-92. https://doi.org/10.1016/j.jsmc.2008.12.002.
-
7.
Jansson-Fröjmark M, Lindblom K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. Journal of Psychosomatic Research. 2008;64(4):443-9. https://doi.org/10.1016/j.jpsychores.2007.10.016.
-
8.
Fairholme CP, Carl JR, Farchione TJ, Schonwetter SW. Transdiagnostic processes in emotional disorders and insomnia: Results from a sample of adult outpatients with anxiety and mood disorders. Behaviour Research and Therapy. 2012;50(7-8):522-8. https://doi.org/10.1016/j.brat.2012.03.011.
-
9.
McNally RJ. Anxiety sensitivity and information-processing biases for threat. In: Taylor S, editor. Anxiety sensitivity: Theory, research, and the treatment of the fear of anxiety. Mahwah, NJ: Lawrence Erlbaum Associates Inc; 1999. p. 183-97.
-
10.
Taylor S, Koch WJ, McNally RJ. How does anxiety sensitivity vary across the anxiety disorders? Journal of Anxiety Disorders. 1992;6(3):249-59. https://doi.org/10.1016/0887-6185(92)90037-8.
-
11.
Cox BJ, Enns MW, Taylor S. The Effect of Rumination as a Mediator of Elevated Anxiety Sensitivity in Major Depression. Cognitive Therapy and Research. 2001;25(5):525-34. https://doi.org/10.1023/a:1005580518671.
-
12.
Babson KA, Trainor CD, Bunaciu L, Feldner MT. An Examination of Anxiety Sensitivity as a Moderator of the Relation Between Sleep Anticipatory Anxiety and Sleep Onset Latency. Journal of Cognitive Psychotherapy. 2008;22(3):258-70. https://doi.org/10.1891/0889-8391.22.3.258.
-
13.
Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review. 2010;30(2):217-37. https://doi.org/10.1016/j.cpr.2009.11.004.
-
14.
Gruber J, Eidelman P, Harvey AG. Transdiagnostic emotion regulation processes in bipolar disorder and insomnia. Behaviour Research and Therapy. 2008;46(9):1096-100. https://doi.org/10.1016/j.brat.2008.05.004.
-
15.
Brown TA, Barlow DH. A proposal for a dimensional classification system based on the shared features of the DSM-IV anxiety and mood disorders: Implications for assessment and treatment. Psychological Assessment. 2009;21(3):256-71. https://doi.org/10.1037/a0016608.
-
16.
An H, Park J, Jang E, Chung S. The impact of temperament and character on the efficacy of nonpharmacologic treatment of primary insomnia. Comprehensive Psychiatry. 2012;53(2):201-7. https://doi.org/10.1016/j.comppsych.2011.02.008.
-
17.
Farchione TJ, Bullis JR. Addressing the Global Burden of Mental Illness: Why Transdiagnostic and Common Elements Approaches to Evidence-Based Practice Might Be Our Best Bet. Cognitive and Behavioral Practice. 2014;21(2):124-6. https://doi.org/10.1016/j.cbpra.2013.12.003.
-
18.
Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Family Practice. 2012;13(1). https://doi.org/10.1186/1471-2296-13-40.
-
19.
Morin CM, Vallières A, Guay B, Ivers H, Savard J, Mérette C, et al. Cognitive Behavioral Therapy, Singly and Combined With Medication, for Persistent Insomnia. Jama. 2009;301(19):2005. https://doi.org/10.1001/jama.2009.682.
-
20.
Belleville G, Cousineau H, Levrier K, St-Pierre-Delorme M. Meta-analytic review of the impact of cognitive-behavior therapy for insomnia on concomitant anxiety. Clinical Psychology Review. 2011;31(4):638-52. https://doi.org/10.1016/j.cpr.2011.02.004.
-
21.
Belleville G, Cousineau H, Levrier K, St-Pierre-Delorme ME, Marchand A. The Impact of Cognitive-Behavior Therapy for Anxiety Disorders on Concomitant Sleep Disturbances: A Meta-Analysis. Journal of Anxiety Disorders. 2010;24(4):379-86. https://doi.org/10.1016/j.janxdis.2010.02.010.
-
22.
Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clinical Psychology Review. 2005;25(5):559-92. https://doi.org/10.1016/j.cpr.2005.04.004.
-
23.
McEvoy PM, Nathan P, Norton PJ. Efficacy of Transdiagnostic Treatments: A Review of Published Outcome Studies and Future Research Directions. Journal of Cognitive Psychotherapy. 2009;23(1):20-33. https://doi.org/10.1891/0889-8391.23.1.20.
-
24.
Wilamowska ZA, Thompson-Hollands J, Fairholme CP, Ellard KK, Farchione TJ, Barlow DH. Conceptual background, development, and preliminary data from the unified protocol for transdiagnostic treatment of emotional disorders. Depression and Anxiety. 2010;27(10):882-90. https://doi.org/10.1002/da.20735.
-
25.
Ellard KK, Fairholme CP, Boisseau CL, Farchione TJ, Barlow DH. Unified Protocol for the Transdiagnostic Treatment of Emotional Disorders: Protocol Development and Initial Outcome Data. Cognitive and Behavioral Practice. 2010;17(1):88-101. https://doi.org/10.1016/j.cbpra.2009.06.002.
-
26.
Farchione TJ, Fairholme CP, Ellard KK, Boisseau CL, Thompson-Hollands J, Carl JR, et al. Unified Protocol for Transdiagnostic Treatment of Emotional Disorders: A Randomized Controlled Trial. Behavior Therapy. 2012;43(3):666-78. https://doi.org/10.1016/j.beth.2012.01.001.
-
27.
Mohammadi A, Birashk B, Gharaie B. Comparison of the effect of group transdiagnostic therapy and group cognitive therapy on anxiety and depressive symptoms. Iran J Public Health. 2013;42(1):48-55. [PubMed ID: 23514901]. [PubMed Central ID: PMC3595628].
-
28.
Akbari M, Roshan R, Shabani A, Fata L, Shairi MR, Zarghami F. The Comparison of the Efficacy of Transdiagnostic Therapy Based on Repetitive Negative Thoughts with Unified Transdiagnostic Therapy in Treatment of Patients with Co-occurrence Anxiety and Depressive Disorders: A Randomized Clinical Trial. IJPCP. 2015;21(2):88-107. Persian.
-
29.
Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, et al. Derivation of Research Diagnostic Criteria for Insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567-96. https://doi.org/10.1093/sleep/27.8.1567.
-
30.
Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003;41(4):427-45.
-
31.
Kazdin AE. Research design in clinical psychology. Boston, MA: Allyn & Bacon; 2003.
-
32.
Maric M, de Haan E, Hogendoorn SM, Wolters LH, Huizenga HM. Evaluating Statistical and Clinical Significance of Intervention Effects in Single-Case Experimental Designs: An SPSS Method to Analyze Univariate Data. Behavior Therapy. 2015;46(2):230-41. https://doi.org/10.1016/j.beth.2014.09.005.
-
33.
Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59(1):12-9. https://doi.org/10.1037/0022-006x.59.1.12.
-
34.
Barlow DH, Farchione TJ, Fairholme CP, Ellard KK, Boisseau CL, Allen LB. The unified protocol for transdiagnostic treatment of emotional disorders: Therapist guide. New York: NY: Oxford University Press; 2011. p. 666-78.
-
35.
Bullis JR, Sauer-Zavala S. The Unified Protocol for insomnia disorder. In: Farchione TJ, Barlow DH, editors. Applications of the Unified Protocol for Transdiagnostic Treatment of Emotional Disorders. New York: Oxford University Press; 2017. https://doi.org/10.1093/med-psych/9780190255541.003.0010.
-
36.
Roth T, Zammit G, Kushida C, Doghramji K, Mathias SD, Wong JM, et al. A new questionnaire to detect sleep disorders. Sleep Medicine. 2002;3(2):99-108. https://doi.org/10.1016/s1389-9457(01)00131-9.
-
37.
First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders, clinician version (SCID-CV). Washington, D.C: American Psychiatric Press; 1996.
-
38.
Amini H, Sharifi V, Asadi SM, Mohammadi MR, Kaviani H, Semnani Y. Validity of the Iranian Version of the Structured Clinical Interview for DSM-IV (SCID-I) In the Diagnosis of Psychiatric disorders. Payesh. 2008;7(1):49-57. Persian.
-
39.
Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287-302. [PubMed ID: 22294820]. [PubMed Central ID: PMC3250369]. https://doi.org/10.5665/sleep.1642.
-
40.
Maich KH, Lachowski AM, Carney CE. Psychometric Properties of the Consensus Sleep Diary in Those With Insomnia Disorder. Behavioral Sleep Medicine. 2016;16(2):117-34. https://doi.org/10.1080/15402002.2016.1173556.
-
41.
Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67(2):319-33. https://doi.org/10.1037/0022-3514.67.2.319.
-
42.
Mohammadi N. The Psychometric Properties of the Behavioral Inhibition System (BIS) and Behavioral Activation System (BAS) scales Among Students of Shiraz University. Clinical Psychology & Personality. 2008;1(28):61-8. Persian.
-
43.
Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, et al. Robust dimensions of anxiety sensitivity: Development and initial validation of the Anxiety Sensitivity Index-3. Psychological Assessment. 2007;19(2):176-88. https://doi.org/10.1037/1040-3590.19.2.176.
-
44.
Gratz KL, Roemer L. Multidimensional Assessment of Emotion Regulation and Dysregulation: Development, Factor Structure, and Initial Validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology and Behavioral Assessment. 2004;26(1):41-54. https://doi.org/10.1023/b:joba.0000007455.08539.94.
-
45.
Khanzadeh M, Saeediyan M, Hosseinchari M, Edrissi F. Factor structure and psychometric properties of difficulties in emotion regulation scale. JBS. 2012;6(1):23-4.
-
46.
Espie CA, Inglis SJ, Harvey L, Tessier S. Insomniacs' attributions: psychometric properties of the dysfunctional beliefs and attitudes about sleep scale and the sleep disturbance questionnaire. Journal of Psychosomatic Research. 2000;48(2):141-8. https://doi.org/10.1016/s0022-3999(99)00090-2.
-
47.
Morin CM, Stone J, Trinkle D, Mercer J, Remsberg S. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychology and Aging. 1993;8(3):463-7. https://doi.org/10.1037/0882-7974.8.3.463.
-
48.
Doos Ali Vand H, Gharraee B, Asgharnejad Farid AA, Rezvanifar S. Psychometric Properties of Short Form Persian Version of the Dysfunctional Beliefs and Attitudes about Sleep Scale. IJPCP. 2014;20(3):264-75. Persian.
-
49.
Ree MJ, Harvey AG. Investigating Safety Behaviours in Insomnia: The Development of the Sleep-related Behaviours Questionnaire (SRBQ). Behaviour Change. 2004;21(1):26-36. https://doi.org/10.1375/bech.21.1.26.35971.
-
50.
Doos Ali Vand H, Gharraee B, Asgharnejad Farid AA, Ghalehbandi MF. Predicting Safety Behaviors Related To Insomnia According to Cognitive,Meta-Cognitive and Emotional Variables in College Students. Res Psychologic Health. 2010;4(1):1-11. Persian.
-
51.
Cohen J. Statistical power analysis for the behavioral sciences. New Jersey: Erlbaum Associates; 1988.
-
52.
Carl JR, Gallagher MW, Sauer-Zavala SE, Bentley KH, Barlow DH. A preliminary investigation of the effects of the unified protocol on temperament. Comprehensive Psychiatry. 2014;55(6):1426-34. https://doi.org/10.1016/j.comppsych.2014.04.015.
-
53.
Abdi R, Bakhshipour A, Mahmoud Alilu M. Efficacy of Unified Transdiagnostic Treatment on Reduction of Transdiagnostic Factors and Symptoms Severity in Emotional Disorders. J Psychologic Model Method. 2013;3(13):1-27. Persian.
-
54.
Kennedy SJ, Rapee RM, Edwards SL. A Selective Intervention Program for Inhibited Preschool-Aged Children of Parents With an Anxiety Disorder: Effects on Current Anxiety Disorders and Temperament. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(6):602-9. https://doi.org/10.1097/CHI.0b013e31819f6fa9.
-
55.
Mata J, Thompson RJ, Jaeggi SM, Buschkuehl M, Jonides J, Gotlib IH. Walk on the bright side: Physical activity and affect in major depressive disorder. Journal of Abnormal Psychology. 2012;121(2):297-308. https://doi.org/10.1037/a0023533.
-
56.
Boettcher H, Brake C, Barlow DH. Origins and outlook of interoceptive exposure. Journal of Behavior Therapy and Experimental Psychiatry. 2016;53:41-51. https://doi.org/10.1016/j.jbtep.2015.10.009.
-
57.
Mitchell MA, Capron DW, Raines AM, Schmidt NB. Reduction of cognitive concerns of anxiety sensitivity is uniquely associated with reduction of PTSD and depressive symptoms: A comparison of civilians and veterans. Journal of Psychiatric Research. 2014;48(1):25-31. https://doi.org/10.1016/j.jpsychires.2013.10.013.
-
58.
Short NA, Allan NP, Raines AM, Schmidt NB. The effects of an anxiety sensitivity intervention on insomnia symptoms. Sleep Medicine. 2015;16(1):152-9. https://doi.org/10.1016/j.sleep.2014.11.004.
-
59.
Bullis JR, Fortune MR, Farchione TJ, Barlow DH. A preliminary investigation of the long-term outcome of the Unified Protocol for Transdiagnostic Treatment of Emotional Disorders. Comprehensive Psychiatry. 2014;55(8):1920-7. https://doi.org/10.1016/j.comppsych.2014.07.016.
-
60.
Boswell JF, Anderson LM, Barlow DH. An idiographic analysis of change processes in the unified transdiagnostic treatment of depression. Journal of Consulting and Clinical Psychology. 2014;82(6):1060-71. https://doi.org/10.1037/a0037403.
-
61.
Jansson-Fröjmark M, Norell-Clarke A, Linton SJ. The role of emotion dysregulation in insomnia: Longitudinal findings from a large community sample. British Journal of Health Psychology. 2016;21(1):93-113. https://doi.org/10.1111/bjhp.12147.
-
62.
Harvey AG, Greenall E. Catastrophic worry in primary insomnia. Journal of Behavior Therapy and Experimental Psychiatry. 2003;34(1):11-23. https://doi.org/10.1016/s0005-7916(03)00003-x.
-
63.
Harvey AG, Sharpley AL, Ree MJ, Stinson K, Clark DM. An open trial of cognitive therapy for chronic insomnia. Behaviour Research and Therapy. 2007;45(10):2491-501. https://doi.org/10.1016/j.brat.2007.04.007.
-
64.
Eidelman P, Talbot L, Ivers H, Bélanger L, Morin CM, Harvey AG. Change in Dysfunctional Beliefs About Sleep in Behavior Therapy, Cognitive Therapy, and Cognitive-Behavioral Therapy for Insomnia. Behavior Therapy. 2016;47(1):102-15. https://doi.org/10.1016/j.beth.2015.10.002.
-
65.
Harvey AG. A cognitive model of insomnia. Behaviour Research and Therapy. 2002;40(8):869-93. https://doi.org/10.1016/s0005-7967(01)00061-4.
-
66.
Perlis ML JCSMPD;2. Cognitive Behavioral Treatment of Insomnia: A Session-by-Session Guide. New York: Springer; 2005. p. 1439-44.
