Abstract
Keywords
1. Background
Breast cancer is one of the most prevalent cancers worldwide, and over 60% of breast cancers are estrogen receptor (ER)-positive, which is currently the most common type of breast cancer (1). Endocrine therapy using tamoxifen (TAM) has been successfully used to treat breast cancer for about 40 years. However, despite the apparent anticancer effect of TAM, patients often develop resistance to TAM, limiting its therapeutic effectiveness (2-4). The mechanism by which breast tumors lose their response to TAM remains unclear. A growing body of evidence over recent years has indicated that high expression of the epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) in breast cancer cells is associated with TAM resistance (5-8). Researchers have shown that inhibitors targeting EGFR and/or HER2 can effectively prevent TAM resistance in breast cancer cells (9). Considering these inhibitors’ toxicity and side effects, less toxic therapeutic agents to treat TAM resistance in ER-positive breast cancer are urgently needed.
In 2005, Wang et al. reported a novel subtype of ER-α, ER-α36, with a molecular weight of 36 kDa (10, 11). Different from classic ER-α66, ER-α36, which is mainly located in the plasma membrane, is a mediator of nongenomic estrogen signaling. Clinical research demonstrated that patients with high levels of ER-α36 expression in breast cancer tissues have a worse prognosis in TAM therapy than those with low levels of ER-α36 in cancers (12). TAM treatment was reported to induce ER-α36 expression in TAM-sensitive MCF7 breast cancer cells (13); however, ER-α36 knockdown restored TAM sensitivity in these cells (8). The aforementioned results indicate that ER-α36 plays an essential role in TAM-resistant breast cancer. Further mechanistic studies verified that ER-α36 could interact with the EGFR or HER2 in breast cancer cells, creating a positive regulatory loop between ER-α36 and EGFR/HER2 (14, 15). Yin et al. demonstrated that this regulatory loop is one important mechanism involved in TAM resistance and that disruption of this loop restores TAM sensitivity in TAM-resistant breast cancer cells (13).
Berberine (Figure 1A), an isoquinoline alkaloid purified from Chinese herbs, has been reported to have a wide variety of bioactivities, such as its antidiarrheal, anticancer, and anti-inflammatory properties. Berberine exhibits cancer therapy-sensitizing activity and assists chemotherapy by affecting several signaling pathways. Wen et al. reported that berberine enhanced the anticancer effect of TAM in TAM-resistant MCF7 (MCF7-TAMR) cells (16). Therefore, berberine is a potential TAM-sensitizing agent that might re-sensitize breast cancers to TAM therapy. This study investigated the TAM-sensitizing effect and underlying mechanism of berberine in MCF7-TAMR and BT-474 breast cancer cells.
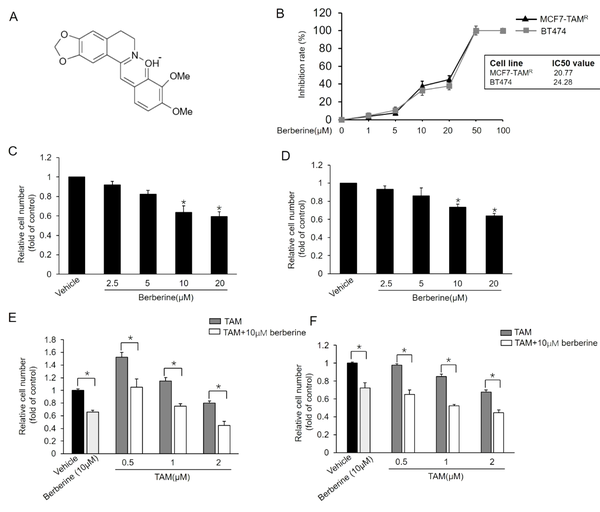
Sensitization of MCF7-TAMR and BT-474 breast cancer cells to TAM by berberine; A, Chemical structure of berberine; B, Inhibitory effects of different doses of berberine on the viability of MCF7-TAMR and BT-474 cells; C and D, Effect of different doses of berberine on the growth of MCF7-TAMR (A) and BT-474 (B) Cells; *P < 0.05 vs. vehicle; E and F, Effects of combined treatment with berberine and TAM on the growth of MCF7-TAMR (E) and BT-474 (F). Cells were treated with various concentrations of TAM with or without berberine for 7 days, and the numbers of surviving cells were counted. *P < 0.05 Each point represents the mean ± standard error of the mean of three independent experiments.
2. Objectives
The current study revealed that berberine destroyed the positive regulating loop between ER-α36 and EGFR/HER2, which sensitized breast cancer cells to TAM. The findings of this study suggested that berberine might be an adjuvant agent for the treatment of TAM-resistant breast cancer.
3. Methods
3.1. Chemicals and Antibodies
Berberine (≥ 98% pure) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-EGFR and anti-HER2 antibodies were obtained from cell signaling technology (Boston, USA). An anti-ER-α36 primary antibody was kindly provided by Dr. Zhao-Yi Wang (Creighton University, Nebraska, USA). An anti-β-actin antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, USA). Dulbecco’s modified eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific (Sunnyvale, CA, USA).
3.2. Cell Culture and Treatments
BT-474 cells were obtained from the China Center for Type Culture Collection (Wuhan, China). MCF7-TAMR cells were kindly provided by Dr. Zhao-Yi Wang (Creighton University). Breast cancer cells were maintained in DMEM containing 10% FBS and 1% penicillin/streptomycin (Thermo Fisher Scientific, Sunnyvale, CA, USA) at 37°C in a 5% CO2 incubator. Before berberine treatment, the cells were cultured in phenol red-free DMEM (Thermo Fisher Scientific, Sunnyvale, CA, USA) containing 2.5% charcoal-stripped fetal calf serum (HyClone, Logan, UT, USA) for 24 hours.
3.3. Cell Viability Assay
The MTT cell viability assay was applied to determine the inhibitory effect of berberine or lapatinib on breast cancer cells as previously described (17). Briefly, the cells seeded in 96-well culture plates were exposed to different concentrations of berberine or lapatinib for 72 hours. Then, cell viability was analyzed using the MTT assay. The cells were treated with 5 mg/mL MTT for 4 hours at 37ºC and resuspended in dimethyl sulfoxide (DMSO). The resultant MTT formazan was dissolved in 100 μL of DMSO, and the absorbance was measured at the wavelength of 490 nM with a microplate reader.
3.4. Cell Growth Assay
The breast cancer cells were harvested at a final concentration of 50,000 cells per dish (60 mm). Berberine and/or TAM at the indicated concentrations were added after 24 hours. The TAM and/or berberine were usually added to the cell culture medium on days 0, 3, and 6 to maintain TAM and/or berberine dosage level. After the 7-day treatment of berberine and/or TAM, the cell numbers were determined as previously described (18). Each concentration was tested in three dishes, and all of the experiments were conducted in triplicate.
3.5. Establishment of Stable Cell Lines
The ER-α36 knockdown cells were established using the short hairpin ribonucleic acid (shRNA) method as previously described (19). Briefly, ER-α36-specific shRNA expression vector or negative control vector (both vectors kindly provided by Dr. Zhao-Yi Wang) were transfected in MCF7-TAMR or BT-474 cells, respectively, and stable cell lines were selected in a medium containing 300 μg/mL G418 for at least 3 weeks. The MCF7-TAMR cells stably transfected with negative control vector and ER-α36-specific shRNA expression vector were named MCF7-TAMR/shNC and MCF7-TAMR/sh36 cells, respectively. The BT-474 cells stably transfected with negative control vector and ER-α36-specific shRNA expression vector were named BT-474/shNC and BT-474/sh36 cells, respectively. The extent of the ER-α36 knockdown was verified using the western blot analysis.
3.6. Western Blot
The total proteins were extracted from cells with RIPA buffer (Beyotime, China) containing 1% phenylmethylsulfonyl fluoride (PMSF) and a 1% phosphatase inhibitor cocktail solution (Beyotime, China). The BCA protein assay kit (Bio-Rad, USA) was used to determine protein quantities. The proteins were isolated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, which were then transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore Corp., Billerica, MA, USA). Then, the membrane was incubated with a primary antibody overnight at 4°C. Subsequently, the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody and developed using an ECL Western blotting detection system (GE Healthcare, Piscataway, NJ, USA). Data analysis was performed using ImageJ computer software (version 1.8.0; National Institutes of Health, USA).
3.7. Statistical Analysis
All the experiments in this study were repeated at least three times. All the data are presented as the mean ± standard error of mean and analyzed by GraphPad InStat software (version 3.06). Some comparison data were analyzed by the Tukey-Kramer multiple comparisons test. Differences with a p-value less than 0.05 were considered statistically significant.
4. Results
4.1. Sensitization of Breast Cancer Cells to TAM by Berberine
Berberine at certain concentrations was reported to inhibit the growth of TAM-sensitive MCF7 and MCF7-TAMR cells (16). BT-474, a breast cancer cell line overexpressing HER2, also exhibits resistance to TAM treatment (20). This study first examined the inhibitory effect of berberine on MCF7-TAMR and BT-474 cells using MTT assays. As shown in Figure 1B, berberine inhibited breast cancer MCF7-TAMR cell growth with a half-maximal inhibitory concentration (IC50 value of 20.77 µM, and its IC50 value in BT-474 was 24.28 µM. We then examined whether berberine sensitizes MCF7-TAMR and BT-474 cells to TAM treatment. The MCF7-TAMR and BT-474 cells were treated with different concentrations of berberine for 7 days, and the cell numbers were counted on day 7, respectively. As the results depicted in Figure 1C and D, berberine markedly suppressed the growth of both cell lines. Then, this study tested the effect of TAM together with berberine on the growth of MCF7-TAMR and BT-474 cells and showed that TAM and berberine synergistically inhibited the growth of both cell lines (Figure 1E and F). The aforementioned results indicated that berberine sensitized MCF7-TAMR and BT-474 cells to TAM treatment.
4.2. Downregulation of ER-α36 Expression in TAM-Resistant Breast Cancer Cells by Berberine
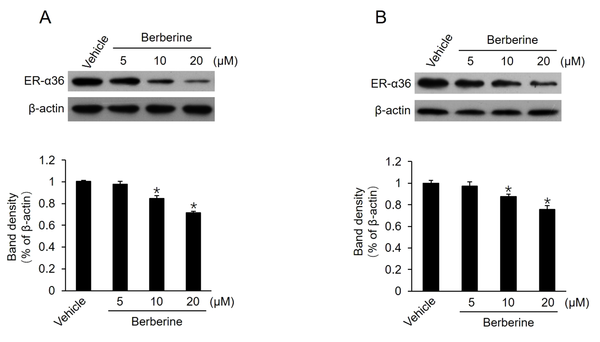
It has been reported that TAM therapy has a poorer curative effect on patients with breast cancers expressing a high level of ER-α36, compared to those with cancers expressing a low level of ER-α36 (12). Previous studies also confirmed that TAM-resistant breast cancer cells, such as BT-474 cells and cultured MCF7-TAMR cells, express high levels of ER-α36 (8, 18). Moreover, ER-α36 knockdown can restore TAM sensitivity in TAM-resistant breast cancer cells, implying that a high level of ER-α36 expression plays a key role in TAM resistance. Then, this study determined whether berberine inhibits ER-α36 expression. It was found that ER-α36 expression was downregulated by berberine in a dose-dependent manner in both MCF7-TAMR and BT-474 cells (Figure 2A and B). The aforementioned results revealed that ER-α36 downregulation is one possible reason for the TAM sensitization induced by berberine in breast cancer cells.
Downregulation of estrogen receptor (ER)-α36 expression in MCF7-TAMR and BT-474 cells by berberine; cells maintained in phenol red-free medium with 2.5% charcoal-stripped fetal calf serum treated with vehicle dimethyl sulfoxide and the indicated concentrations of berberine for 24 hours; performing western blot analysis to examine the expression of ER-α36 in MCF7-TAMR cells (A) and BT-474 cells (B). All membranes were stripped and reprobed with a β-actin antibody to ensure equal loading. The columns and bars represent the means of three experiments and the standard error of the mean, respectively (*P < 0.05 vs. control cells treated with vehicle).
4.3. Disruption of Regulatory Loop Between ER-α36 and EGFR/HER2 by Berberine
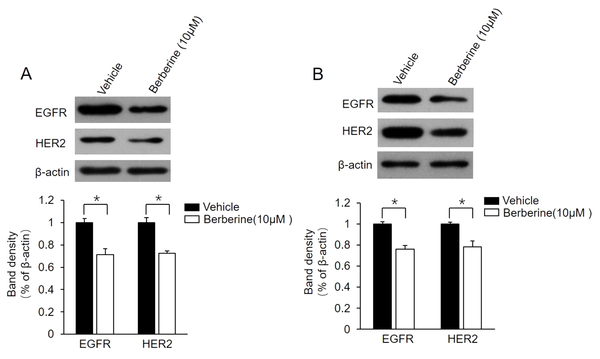
It has been confirmed that the positive regulatory loop between ER-α36 and EGFR/HER2 confers TAM resistance in breast cancer cells (13, 21). For the determination of whether this loop is also involved in berberine-mediated TAM resistance, the present study examined the change of expression level of EGFR/HER2 in MCF7-TAMR and BT-474 cells following berberine treatment. The obtained data showed that berberine treatment suppressed both EGFR and HER2 expression in these two cell lines (Figure 3A and B). The aforementioned findings demonstrated that berberine disrupts the regulatory loop between ER-α36 and EGFR/HER2 in MCF7-TAMR and BT-474 cells.
Suppression of epidermal growth factor receptor (EGFR)/human epidermal growth factor receptor 2 (HER2) expression in MCF7-TAMR and BT-474 cells by berberine; MCF7-TAMR (A) and BT-474 (B) cells treated with 10 μM berberine for 48 hours. Cell lysates were subjected to western blot analysis with an antibody against EGFR or HER2. The membrane was stripped and reprobed with a β-actin antibody to ensure equal loading. The columns and bars represent the means of three experiments and the standard error of the mean, respectively (*P < 0.05 vs. control cells treated with vehicle dimethyl sulfoxide).
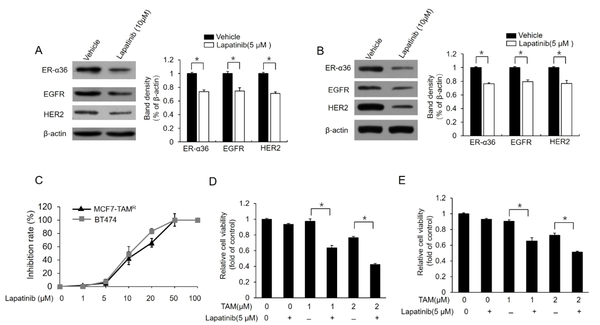
For the further confirmation of the relationship between ER-α36-EGFR/HER2 loop and berberine-induced TAM sensitization, MCF7-TAMR and BT-474 cells were treated with different concentrations of lapatinib, a dual tyrosine kinase inhibitor. Lapatinib treatment inhibited the expression of both EGFR/HER2 and ER-α36 in these cell lines (Figure 4A and B). Meanwhile, lapatinib treatment improved TAM sensitivity in MCF7-TAMR and BT-474 cells (Figure 4C and D). In total, the aforementioned results demonstrated that berberine could disrupt the loop between ER-α36 and EGFR/HER2, which consequently restores TAM sensitivity in breast cancer cells.
Downregulation of dual kinase inhibitor lapatinib estrogen receptor (ER)-α36 expression and sensitization of TAM-resistant cells to TAM; (A) and (B) western blot analysis of the expression of ER-a36, epidermal growth factor receptor (EGFR), and human epidermal growth factor receptor 2 (HER2) in MCF7-TAMR (A) and BT-474 (B) cells treated with 5 μM lapatinib for 48 hours; (C) inhibitory effects of different doses of lapatinib on the viability of MCF7-TAMR and BT-474 cells; (D) and (E) MCF7-TAMR (D) and BT-474 (E) cells treated with the indicated concentrations of TAM (2 μM) with a vehicle or 5 μM lapatinib for 7 days. The numbers of surviving cells were counted. The columns and bars represent the means of three experiments and the standard error of the mean, respectively (*P < 0.05).
4.4. Reduction of Sensitivity to Berberine in Breast Cancer Cells by ER-α36 Knockdown
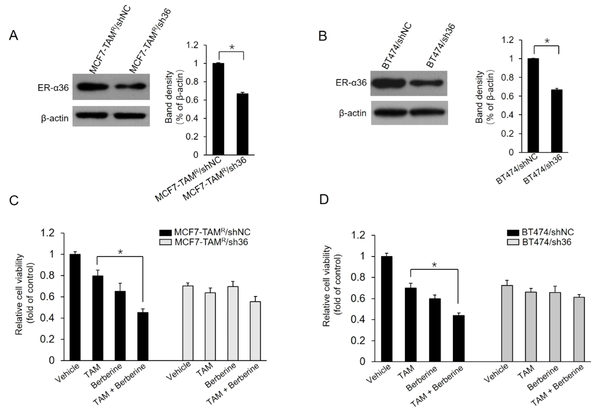
The current study further characterized whether the ER-α36 expression level alters the effects of berberine on MCF7-TAMR and BT-474 cells. Two stable ER-α36 knockdown cell lines, MCF7-TAMR/sh36 and BT-474/sh36, were established. Western blot results demonstrated that ER-α36 was significantly suppressed in ER-α36 knockdown MCF7-TAMR/sh36 and BT-474/sh36 cells, compared to control cells, MCF7-TAMR/shNC and BT-474/shNC cells, respectively (Figure 5A and B). Then, the effect of ER-α36 knockdown on berberine sensitization activity was examined in breast cancer cells. As the results shown in Figure 5C, MCF7-TAMR/sh36 cells did not exhibit significantly increased sensitization to TAM, compared to control cells transfected with shNC vectors. Similar results were also obtained in BT-474/sh36 cells (Figure 5D). Therefore, the obtained data from this study suggested that ER-α36 mediates the TAM sensitization induced by berberine in breast cancer cells.
Sensitization of TAM-resistant cells to TAM treatment by estrogen receptor (ER)-α36 knockdown; A and B, ER-α36 expression levels measured in ER-α36 knockdown MCF7-TAMR/sh36 (A) and BT-474/sh36 (B) cells using western blot assays; C and D, MCF7-TAMR/shNC, MCF7-TAMR/sh36, BT-474/shNC, and BT-474/sh36 cells treated with 2 μM TAM together with a vehicle or 5 μM lapatinib for 7 days. The numbers of surviving cells were counted. The columns and bars represent the means of three experiments and the standard error of the mean, respectively (*P < 0.05).
5. Discussion
TAM is the most widely used endocrine drug to treat ER-positive breast tumors. However, the high rates of de novo resistance and acquired resistance to TAM have often limited its effectiveness. Therefore, novel TAM-sensitizing agents to enhance the efficacy of TAM are needed for current ER-positive breast cancer therapy. The present study focused on the TAM-sensitizing potential of berberine in TAM-resistant breast cancer cells. This study demonstrated that berberine potently sensitized MCF7-TAMR and BT-474 cells to TAM, presumably through the inhibition of ER-α36 expression and disruption of the regulatory loop between ER-α36 and EGFR/HER2.
Berberine is a nitrogenous cyclic compound extracted from the roots and stem bark of numerous plants belonging to Berberis species, including barberry (Berberis vulgaris) and golden thread (Coptis chinensis) (22). Berberine, a monomer of traditional Chinese medicine, has long been used to treat intestinal problems and inflammation (23) and has increasingly been reported as a promising anticancer agent in recent years (24). Berberine has also been demonstrated to inhibit cell proliferation, induce apoptosis, and restrain tumor invasion (25). The underlying mechanisms are multifaceted and include its antioxidant activity, apoptosis induction, interaction with nucleic acids, and modulation of cancer-relevant signaling pathways. Interestingly, berberine has been shown to have synergistic effects with other anticancer drugs (25). Wang et al. reported that the cotreatment of hepatic carcinoma cell lines with vincristine and berberine significantly inhibited cell growth and induced apoptosis (26). Previous studies also showed that berberine enhanced chemosensitivity to chemotherapy drugs, such as 5-fluorouracil (27) and irinotecan (CPT-11) (28), in cancer cells by downregulating nuclear factor kappa-light-chain-enhancer of B cells (NF-κB)-mediated activation of antiapoptotic genes.
A recent study showed the dose- and time-dependent antiproliferative effects of berberine treatment on MCF7-TAMR cells (16). The aforementioned study also demonstrated that combined berberine and TAM treatment inhibited cell growth more effectively than TAM alone (16). The present study reported that berberine treatment effectively inhibited the growth of MCF7-TAMR and BT-474 cell lines and that berberine can promote the growth-suppressing effects of TAM in both cell lines. The current study’s results verified that berberine could enhance the antitumor effect of TAM in TAM-resistant breast cancer cells, which means berberine sensitized breast cancer cells to TAM.
The further investigation of the mechanism by which berberine sensitized breast cancer cells to TAM revealed that berberine treatment downregulated ER-α36 expression in MCF7-TAMR and BT-474 cells. The ER-α36, a new isoform of ER-α, has been detected in approximately 40% of ER-positive breast cancer cases (12). The promotor of ER-α36, which contains AP-1 and NF-κB binding sites, is located in the first intron of the ER-α66 gene (29). It has been reported that berberine prohibits p65 expression and inhibits NF-κB levels in several types of tumors, including breast cancer (30, 31). Berberine has been shown to downregulate EGFR expression (32, 33); however, EGFR-mediated signaling can induce ER-α36 expression via the AP-1 binding site of its promotor (14). Therefore, it was speculated that berberine might suppress ER-α36 expression through the regulation of its transcription. Since ER-α36 has a significant role in TAM resistance, this study investigated the character of ER-α36 in berberine-induced TAM sensitization. The present study analyzed two pairs of breast cancer cell lines, MCF7-TAMR/sh36 and MCF7-TAMR/shNC cells, in addition to BT-474/sh36 and BT-474/shNC cells. It was shown that ER-α36 knockdown significantly decreases TAM sensitivity induced by berberine in breast cancer cells, indicating that ER-α36 plays a key role in berberine-induced TAM sensitization.
It has been verified that the expression level of ER-α36 is highly correlated with HER2 expression level (12, 15), and a study conducted on the underlying mechanism revealed the positive regulating loop between them (15). In addition, ER-α36 has been demonstrated to regulate the HER2 protein level through the proteasome system (18). Zhang et al. reported that EGFR signaling increases the promoter activity of ER-α36 via an AP-1 binding site, and ER-α36 can also stabilize EGFR protein (14). In the present study, EGFR and HER2 expression were inhibited in berberine-treated cells, which was consistent with the inhibition of ER-α36 expression. The current study also showed that EGFR, HER2, and ER-α36 expression was suppressed by lapatinib treatment in both BT-474 and MCF7-TAMR cells. Therefore, the obtained results further verified the presence of a regulatory loop between ER-α36 and EGFR/HER2. The results of the present study also showed that lapatinib could restore the sensitivity of breast cancer cells to TAM, which is in good agreement with the results of previous reports (34, 35).
In summary, berberine potently sensitizes the response to TAM in TAM-resistant breast cancer cells, presumably through suppressing the expression of ER-α36 and disrupting the positive regulatory loop between ER-α36 and EGFR/HER2. The present study also provided evidence to support the need for specific ER-α36 inhibitors in adjuvant therapy for TAM-resistant breast cancer.
Acknowledgements
References
-
1.
Montemurro F, Aglietta M. Hormone receptor-positive early breast cancer: controversies in the use of adjuvant chemotherapy. Endocr Relat Cancer. 2009;16(4):1091-102. [PubMed ID: 19726539]. https://doi.org/10.1677/ERC-09-0033.
-
2.
Clarke R, Leonessa F, Welch JN, Skaar TC. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol Rev. 2001;53(1):25-71. [PubMed ID: 11171938].
-
3.
Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY, Zhu Y, et al. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22(47):7316-39. [PubMed ID: 14576841]. https://doi.org/10.1038/sj.onc.1206937.
-
4.
MacGregor JI, Jordan VC. Basic guide to the mechanisms of antiestrogen action. Pharmacol Rev. 1998;50(2):151-96. [PubMed ID: 9647865].
-
5.
Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29(2):217-33. [PubMed ID: 18216219]. [PubMed Central ID: PMC2528847]. https://doi.org/10.1210/er.2006-0045.
-
6.
Chung YL, Sheu ML, Yang SC, Lin CH, Yen SH. Resistance to tamoxifen-induced apoptosis is associated with direct interaction between Her2/neu and cell membrane estrogen receptor in breast cancer. Int J Cancer. 2002;97(3):306-12. [PubMed ID: 11774281]. https://doi.org/10.1002/ijc.1614.
-
7.
Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96(12):926-35. [PubMed ID: 15199112]. https://doi.org/10.1093/jnci/djh166.
-
8.
Zhang X, Wang ZY. Estrogen receptor-alpha variant, ER-alpha36, is involved in tamoxifen resistance and estrogen hypersensitivity. Endocrinology. 2013;154(6):1990-8. [PubMed ID: 23546601]. https://doi.org/10.1210/en.2013-1116.
-
9.
Gee JM, Harper ME, Hutcheson IR, Madden TA, Barrow D, Knowlden JM, et al. The antiepidermal growth factor receptor agent gefitinib (ZD1839/Iressa) improves antihormone response and prevents development of resistance in breast cancer in vitro. Endocrinology. 2003;144(11):5105-17. [PubMed ID: 12960029]. https://doi.org/10.1210/en.2003-0705.
-
10.
Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun. 2005;336(4):1023-7. [PubMed ID: 16165085]. https://doi.org/10.1016/j.bbrc.2005.08.226.
-
11.
Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci U S A. 2006;103(24):9063-8. [PubMed ID: 16754886]. [PubMed Central ID: PMC1482566]. https://doi.org/10.1073/pnas.0603339103.
-
12.
Shi L, Dong B, Li Z, Lu Y, Ouyang T, Li J, et al. Expression of ER-{alpha}36, a novel variant of estrogen receptor {alpha}, and resistance to tamoxifen treatment in breast cancer. J Clin Oncol. 2009;27(21):3423-9. [PubMed ID: 19487384]. [PubMed Central ID: PMC2717750]. https://doi.org/10.1200/JCO.2008.17.2254.
-
13.
Yin L, Zhang XT, Bian XW, Guo YM, Wang ZY. Disruption of the ER-alpha36-EGFR/HER2 positive regulatory loops restores tamoxifen sensitivity in tamoxifen resistance breast cancer cells. PLoS One. 2014;9(9). e107369. [PubMed ID: 25203051]. [PubMed Central ID: PMC4159333]. https://doi.org/10.1371/journal.pone.0107369.
-
14.
Zhang XT, Kang LG, Ding L, Vranic S, Gatalica Z, Wang ZY. A positive feedback loop of ER-alpha36/EGFR promotes malignant growth of ER-negative breast cancer cells. Oncogene. 2011;30(7):770-80. [PubMed ID: 20935677]. [PubMed Central ID: PMC3020987]. https://doi.org/10.1038/onc.2010.458.
-
15.
Kang L, Guo Y, Zhang X, Meng J, Wang ZY. A positive cross-regulation of HER2 and ER-alpha36 controls ALDH1 positive breast cancer cells. J Steroid Biochem Mol Biol. 2011;127(3-5):262-8. [PubMed ID: 21907803]. [PubMed Central ID: PMC3220752]. https://doi.org/10.1016/j.jsbmb.2011.08.011.
-
16.
Wen C, Wu L, Fu L, Zhang X, Zhou H. Berberine enhances the antitumor activity of tamoxifen in drugsensitive MCF7 and drugresistant MCF7/TAM cells. Mol Med Rep. 2016;14(3):2250-6. [PubMed ID: 27432642]. https://doi.org/10.3892/mmr.2016.5490.
-
17.
Song Z, Han S, Pan X, Gong Y, Wang M. Pterostilbene mediates neuroprotection against oxidative toxicity via oestrogen receptor alpha signalling pathways. J Pharm Pharmacol. 2015;67(5):720-30. [PubMed ID: 25644078]. https://doi.org/10.1111/jphp.12360.
-
18.
Yin L, Pan X, Zhang XT, Guo YM, Wang ZY, Gong Y, et al. Downregulation of ER-alpha36 expression sensitizes HER2 overexpressing breast cancer cells to tamoxifen. Am J Cancer Res. 2015;5(2):530-44. [PubMed ID: 25973295]. [PubMed Central ID: PMC4396031].
-
19.
Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, et al. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24(4):709-21. [PubMed ID: 20197310]. [PubMed Central ID: PMC2852353]. https://doi.org/10.1210/me.2009-0317.
-
20.
Chan JC, Ong PS, Lim P, Teng PX, Chan EC. Synergistic disruption of ERalpha/HER2 crosstalk by endoxifen and lapatinib in breast cancer cells. Cancer Chemother Pharmacol. 2017;79(1):117-30. [PubMed ID: 27942916]. https://doi.org/10.1007/s00280-016-3211-7.
-
21.
Yin L, Wang ZY. Roles of the ER-alpha36-EGFR/HER2 positive regulatory loops in tamoxifen resistance. Steroids. 2016;111:95-9. [PubMed ID: 26884313]. https://doi.org/10.1016/j.steroids.2016.01.019.
-
22.
Jin Y, Khadka DB, Cho WJ. Pharmacological effects of berberine and its derivatives: a patent update. Expert Opin Ther Pat. 2016;26(2):229-43. [PubMed ID: 26610159]. https://doi.org/10.1517/13543776.2016.1118060.
-
23.
Rabbani GH, Butler T, Knight J, Sanyal SC, Alam K. Randomized controlled trial of berberine sulfate therapy for diarrhea due to enterotoxigenic Escherichia coli and Vibrio cholerae. J Infect Dis. 1987;155(5):979-84. [PubMed ID: 3549923]. https://doi.org/10.1093/infdis/155.5.979.
-
24.
Wang N, Tan HY, Li L, Yuen MF, Feng Y. Berberine and Coptidis Rhizoma as potential anticancer agents: Recent updates and future perspectives. J Ethnopharmacol. 2015;176:35-48. [PubMed ID: 26494507]. https://doi.org/10.1016/j.jep.2015.10.028.
-
25.
Jabbarzadeh Kaboli P, Rahmat A, Ismail P, Ling KH. Targets and mechanisms of berberine, a natural drug with potential to treat cancer with special focus on breast cancer. Eur J Pharmacol. 2014;740:584-95. [PubMed ID: 24973693]. https://doi.org/10.1016/j.ejphar.2014.06.025.
-
26.
Wang L, Wei D, Han X, Zhang W, Fan C, Zhang J, et al. The combinational effect of vincristine and berberine on growth inhibition and apoptosis induction in hepatoma cells. J Cell Biochem. 2014;115(4):721-30. [PubMed ID: 24243568]. https://doi.org/10.1002/jcb.24715.
-
27.
Pandey MK, Sung B, Kunnumakkara AB, Sethi G, Chaturvedi MM, Aggarwal BB. Berberine modifies cysteine 179 of IkappaBalpha kinase, suppresses nuclear factor-kappaB-regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008;68(13):5370-9. [PubMed ID: 18593939]. https://doi.org/10.1158/0008-5472.CAN-08-0511.
-
28.
Yu M, Tong X, Qi B, Qu H, Dong S, Yu B, et al. Berberine enhances chemosensitivity to irinotecan in colon cancer via inhibition of NFkappaB. Mol Med Rep. 2014;9(1):249-54. [PubMed ID: 24173769]. https://doi.org/10.3892/mmr.2013.1762.
-
29.
Zou Y, Ding L, Coleman M, Wang Z. Estrogen receptor-alpha (ER-alpha) suppresses expression of its variant ER-alpha 36. FEBS Lett. 2009;583(8):1368-74. [PubMed ID: 19328202]. [PubMed Central ID: PMC2737280]. https://doi.org/10.1016/j.febslet.2009.03.047.
-
30.
Liu J, Zhang X, Liu A, Liu S, Zhang L, Wu B, et al. Berberine induces apoptosis in p53-null leukemia cells by down-regulating XIAP at the post-transcriptional level. Cell Physiol Biochem. 2013;32(5):1213-24. [PubMed ID: 24335171]. https://doi.org/10.1159/000354520.
-
31.
Kuo HP, Chuang TC, Tsai SC, Tseng HH, Hsu SC, Chen YC, et al. Berberine, an isoquinoline alkaloid, inhibits the metastatic potential of breast cancer cells via Akt pathway modulation. J Agric Food Chem. 2012;60(38):9649-58. [PubMed ID: 22950834]. https://doi.org/10.1021/jf302832n.
-
32.
Wang L, Cao H, Lu N, Liu L, Wang B, Hu T, et al. Berberine inhibits proliferation and down-regulates epidermal growth factor receptor through activation of Cbl in colon tumor cells. PLoS One. 2013;8(2). e56666. [PubMed ID: 23457600]. [PubMed Central ID: PMC3573001]. https://doi.org/10.1371/journal.pone.0056666.
-
33.
Liu Y, Hao H, Xie H, Lv H, Liu C, Wang G. Oxidative demethylenation and subsequent glucuronidation are the major metabolic pathways of berberine in rats. J Pharm Sci. 2009;98(11):4391-401. [PubMed ID: 19283771]. https://doi.org/10.1002/jps.21721.
-
34.
Chu I, Blackwell K, Chen S, Slingerland J. The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res. 2005;65(1):18-25. [PubMed ID: 15665275].
-
35.
Leary AF, Drury S, Detre S, Pancholi S, Lykkesfeldt AE, Martin LA, et al. Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res. 2010;16(5):1486-97. [PubMed ID: 20179226]. https://doi.org/10.1158/1078-0432.CCR-09-1764.