Abstract
Background:
Recent studies on Leishmaniasis treatment have confirmed the antiparasitic effects of flavonols and organic antimony pentavalent [(Sb(V)] complexes.Objectives:
This study aimed to identify new Sb(V) complexes by combining the benefits of antimonials and flavonols as well as by optimizing their properties.Methods:
Kaempferol and quercetin peracetate were prepared using acetic anhydride in pyridine. By performing regioselective synthesis, 7-O-paramethylbenzyl as an electron-donating group and 7-O-paranitrobenzyl as an electron-withdrawing group were added to quercetin, and, then, the synthesis of Sb(V) kaempferol and quercetin derivative complexes were performed using SbCl5 solution in glacial acetic acid. The structures were confirmed by UV, ESI mass, IR, 1H-, and 13C-NMR spectral data, and the Stoichiometry of the ligand-metal complex by the mole ratio method. Computational molecular modeling was conducted using the Gaussian program.Results:
The structures were confirmed based on the results from UV, nuclear magnetic resonance (NMR), and electrospray ionization (ESI) mass analyses (3-12). Among the produced compounds, 11 and 12 as newly described, and other compounds as pre-defined compounds were identified. According to the results from biological test, kaempferol triacetate with more lipophilicity showed the highest anti-promastigote activity with an IC50 value of 14.93 ± 2.21 µM. As for anti-amastigote activity, despite the differences, all antimony complexes showed anti-amastigote effects in vitro with IC50 values of 0.52 to 14.50 µM.Conclusions:
All flavonol Sb(V) complexes showed higher activity compared to meglumine antimonate in anti-amastigote effect. Inside the host macrophages, by breaking down the complex into antimony and quercetin or kaempferol analogs, the observed antiparasitic effects may have been related to both Sb(V)/Sb(III) conversion and flavonoid antileishmanial activities.Keywords
Antileishmania Antimony Pentavalent Flavonoid Synthesis Kaempferol Quercetin
1. Background
Leishmaniasis is caused by an intracellular parasite of the genus Leishmania, which is found in two manifestations: visceral and cutaneous. It is the third most important arthropod-borne disease that has been widely reported in more than 89 countries where more than 350 million people are at risk. The estimated number of infected people is 12 to 15 million, with approximately 2 million new cases annually, of which approximately 0.5 million have visceral leishmaniasis, and 1.5 million have cutaneous leishmaniasis. As for country distribution, more than two-thirds of new leishmaniasis cases have been reported in six countries, including Brazil, Colombia, Afghanistan, and Iran (1, 2).
Effective drugs against Leishmania have been already developed; however, it is currently a neglected parasitic disease whose current treatments are facing serious problems such as the toxicity of its drugs, the need for injectable administration, high failure rate, and the emergence of resistance against its drugs. Therefore, a sustained effort is required to develop new drugs that have no high toxicity or cause no failure in treatment if an effective disease control is really desired.
Leishmaniasis is mainly treated with pentavalent antimonial, miltefosine, amphotericin B, and paromomycin. However, drug resistance is a persistent threat to the integrity of treatments, which has increased the requisition of new drug development in pharmaceutics (3, 4). Sodium antimony gluconate is one of the victims of drug resistance which is most prevalent in the Indian subcontinent after decades of its application (3, 5).
Previous studies investigating treatments for Leishmania have suggested that organic Sb(V) functions as a prodrug which is converted into the Sb (III) as a more toxic form of the antimony for amastigotes (6-8), even though some evidence has supported the direct involvement of pentavalent (6, 9).
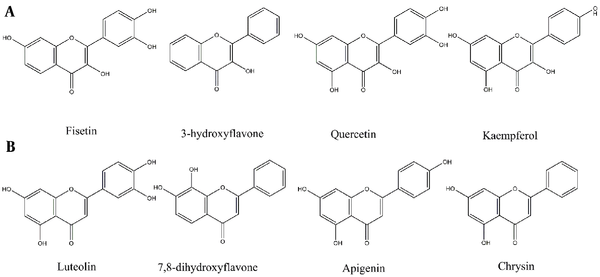
The antiparasitic effects of flavonols have been highlighted by several recent studies (Figure 1) (10, 11). Quercetin, Kaempferol, Rutin, and Taxifolin, for instance, have been recognized as potential anti-leishmanial agents (10-12). In a study by Tasdemir on 105 phenolic compounds, majority of the flavones and flavon-3-ols showed remarkable antileishmanial activities. In flavon-3-ols, 3,7,3’,4’-flavone (fisetin), 3-hydroxyflavone and quercetin, as well as in flavones, luteolin showed potent activities with IC50 values of 0.6 - 1.0 μg/mL (13), respectively. Lesser activities of flavans with saturated (Δ2,3) derivatives like naringenin implicitly demonstrated the significance of the C-2 and C-3 double bond (13). However, this change may lead to the enhancement of other effects of naringenin (14). Flavonoids also exhibit other biological activities like antioxidant, immunomodulatory, cytotoxic, or synergic activities that affect other protozoa or cancer cells (14-16).
Polyamine biosynthesis is one of the targets in anti-leishmanial drug therapy. Quercetin and its glucosides have shown their ability to inhibit arginase, which is essential in polyamine biosynthesis (10). In another study, quercetin, dihydroquercetin, and quercetin glucuronide showed inhibitory activity against L. amazonensis arginase (11). Cataneo et al showed that quercetin increased the ROS production in the promastigotes. They reported that quercetin exerted anti-amastigote effect on infected macrophages by upregulating nuclear factor erythroid 2-related factor 2/heme oxygenase 1 (Nrf2/HO-1), signaling axis, and modulating iron availability (12).
2. Objectives
This study aimed to identify a new class of potential drugs in the field by combining the benefits of antimony and flavonols through examining their complex formation as well as by optimizing their properties.
Flavonoids with Leishmanicidal activity by order of activities. (A) Flavon-3-ols, (B) Flavones (13).
3. Methods
3.1. Materials
Kaempferol, Quercetin, Antimony pentachloride (SbCl5), and Giemsa stain were purchased from Sigma Aldrich (USA). Promastigotes of Leishmania major and macrophage cell line J774 were obtained from Pasteur Institute (Tehran, Iran). Fetal Bovine Serum (FBS) and Novy-MacNeal-Nicolle medium (NNN) were obtained from Gibco (Waltham, Massachusetts, USA) and MERCK (Darmstadt, Germany), respectively.
3.2. General Procedures
The progression of all synthetic reactions was controlled undertaking thin-layer chromatography (TLC), and the results were visualized using a natural product reagent. The reaction mixture was purified using a polyamide SC6 column with the solvent system of hexane: acetone in a gradient manner to increase polarity. NMR spectra were taken by a Bruker 400 using DMSO-d6 at 400.13 MHz for 1H-NMR and 100.61 MHz for 13C-NMR. ESI Mass spectra were done on Shimadzu 2010EV (Shimadzu, Japan). The Fourier transform infrared (FTIR) spectra were taken on a Rayleigh WQF-510 in KBr films.
3.3. Synthesis of Kaempferol Tetra-, and Triacetate
Kaempferol (1, 1.72g, 6.0 mmol) was stirred in 20 mL of pyridine at 75°C under N2. Acetic anhydride (Ac2O) was gradually added dropwise (2.84 mL, 30 mmol) and refluxed gently overnight. After cooling, water was added to the sample and filtered through a C-18 Sep-Pak 12 cc Vac (H2O, Fr.1; MeOH: H2O, 3:7, Fr.2; MeOH: H2O, 5:5; Fr.3; MeOH: H2O, 7:3, Fr.4; MeOH, Fr.5; each 200 mL). Fr.3 and 4 yielded kaempferol-3,7, 4′-triacetate (3, 1.50 g) with a reaction yield of 60.54% as the main compound, and kaempferol peracetate (4) in small quantity as biproduct.
3.4. Synthesis of Quercetin Pentaacetate
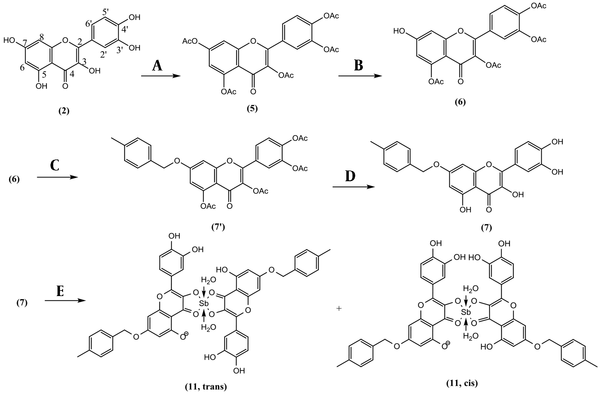
Quercetin (2, 1.813 g, 6.00 mmol) and N-Methyl-2-Pyrrolidone (NMP, 15 mL) were heated at 140°C using a reflux apparatus under nitrogen. Acetic anhydride was gradually added dropwise (2.84 mL, 30 mmol) and refluxed for 8 hr. The reaction mixture was cooled and diluted with 200 mL deionized water and submitted on a C-18 Sep-Pak 12 cc Vac (H2O, Fr.1; MeOH: H2O, 3:7, Fr.2; MeOH: H2O, 5:5; Fr.3; MeOH: H2O, 7:3, Fr.4; MeOH, Fr.5; each 200 mL). Fr.1 contained NMP and acetic acid, Fr.2, quercetin as starting material, Fr. 3, and Fr.4 contained quercetin di-, three- and tetraacetate. Fr. 4 yielded quercetin-3,5,7,3′,4′-pentacetate (5, 2.51 g) in a pure state with a reaction yield of 81.7% (Figure 2) (2).
3.5. Synthesis of Quercetin 7-O-paramethyl and 7-O-paranitro Derivatives
Quercetin pentaacetate (5, 1.1 g, 2.15 mmol) and imidazole (50 mg, 0.74 mmol) were added together in 25 mL of NMP at -5°C on a magnet stirrer. Thiophenol (0.22 mL, 2.13 mmol) was added slowly. After completion of the reaction (each 15 min checked by TLC), the reaction was quenched by adding 5 mL HCl 1.0 N and 30 mL brine. The resulting solution was extracted with 20 mL of dichloromethane in a separating funnel. It was washed with a small HCl 1.0 N and dehydrated with sodium sulfate. The dried organic phase was loaded on a polyamide column (hexanes; hexanes: acetone, 9:1; hexanes: acetone, 8:2; hexanes: acetone, 7:3; hexanes: acetone, 6:4; each 100 mL) and yielded quercetin 3,5,3′,4′-tetraacetate (6, 41.1%) as the main compound. Quercetin 3,5,3′,4′-tetraacetate (6, 100 mg, 0.21 mmol), P-methyl benzyl chloride (44.9 mg, 0.32 mmol), and K2CO3 (88 mg, 0.64 mmol) were added together in 15 mL anhydrous acetone at room temperature for about 3 h and yielded 7-O-paramethylbenzyl quercetin 3,5,3′,4′-tetraacetate. For removing the acetate groups, the reaction mixture was filtered, concentrated, and dissolved in a solution of NH3/MeOH (2M, 10 mL) while stirring for 5 to 10 min until deacetylation was completed (Checked by TLC). Then reaction mixture (RM) was neutralized by 0.5 N HCl and purified on a C-18 Sep-Pak 12 cc Vac (H2O; MeOH: H2O, 3:7, MeOH: H2O, 5:5; MeOH: H2O, 7:3; each 200 mL). The fraction eluted by MeOH: H2O, 30:70, yielded 7-O-paramethylbenzyl quercetin (7, 43 mg) in a pure state with a production yield of 35.2 %. Quercetin 3,5,3′,4′-tetraacetate (6, 100 mg, 0.21 mmol), 4-nitrobenzyl chloride (54.7 mg, 0.32 mmol), and K2CO3 (88 mg, 0.64 mmol) were added together in acetone (20 mL) for 3 h. It was deacetylated in the same way as compound 7 and yielded 7-O-paranitrobenzyl quercetin (8, 51 mg) with a production yield of 39.6 % (17, 18).
3.6. Synthesis of Sb(V) Kaempferol and Quercetin Derivative Complexes
A solution of 198 mg (0.662 mmol) SbCl5 in 10 mL glacial acetic acid was prepared. Kaempferol (2, 19 mg, 0.0662 mmol) in 100 mL anhydrous methanol was the subject of adding 100 µL of SbCl5 (6.62 µmol) solution for 5 consecutive times and the time gap of 5 minutes in order to let the complexation complete. The complex formation could also be witnessed by the instant gradient change in the color of the reaction medium, from light yellow to dark orange. The UV spectrum was recorded at room temperature at the end of each given time gap. The maximum absorbance (λmax) was determined using UV spectra to determine the end of the reaction. At the end of the series, the reaction medium was air-dried for 24 hours to a fine orange powder and washed with deionized water to remove residue of free SbCl5 to obtain Sb(V) kaempferol complex (9). Quercetin (2, 20 mg, 0.0662 mmol) in 100 mL anhydrous methanol was the subject of adding SbCl5 solution (0.033 mmol) in the same way as 9 to obtain Sb(V) quercetin complex (10). Also, 7-O-paramethylbenzyl quercetin (7, 27 mg, 0.0663 mmol) and 7-O-paranitrobenzyl quercetin (8, 29 mg, 0.0663 mmol) each in 100 mL anhydrous methanol was the subject of adding SbCl5 solution (0.033 mmol), separately as mentioned for 9 to obtain Sb(V) 7-O-paramethylbenzyl quercetin complex (11), and Sb(V) 7-O-paranitrobenzyl quercetin complex (12) as two new compounds.
Reagents and conditions for the synthesis of Sb(V) 7-O-paramethylbenzyl Quercetin complex: (A) Anhydride acetic (Ac2O), N-Methyl-2-pyrrolidone (NMP), 140°C, 8 h, Yield: 81.7 % (B) Thiophenol, imidazole, NMP, -10°C, 1 h, yield: 41.1 % (C) 4-methylbenzyl chloride, Na2CO3, acetone, room temperature, 3 h. (D) NH3/MeOH (2M), 0°C, 5 min, yield: 35.2 % (e) SbCl5, glacial acetic acid, MeOH, 30 min.
3.7. Stoichiometry of Ligand-Metal Complex
A stock solution of each flavonoid (66.17 µmol) in HPLC-grade methanol (100 mL) was prepared and diluted to 3.31 µmol in 10 mL. A solution of 19.8 mg (66.2 µmol) SbCl5 in 5 mL glacial acetic acid was also prepared. This solution was added 10 consecutive times in the amount of 5 µL (0.066 µmol) each time, to 2 mL (0.662 µmol) of the diluted flavonoid solution, in a Quartz cuvette with a cap. A time gap of 5 minutes was considered between every two consecutive additions to let the complexation complete. At the end of each given time gap, the UV spectrum in the range of 200 to 600 nm was recorded at room temperature (19-21). The maximum absorbances of flavonoid (λmax: 370 nm) and complex (λmax: 429 nm) were determined using UV spectra. The absorbance of each concentration at 370 and 429 nm was plotted against metal ion/ ligand (M/L) molar ratios in a range of 0.1 to 1 to determine the composition of complexes concerning the proportional concentration of metal and ligand (21).
3.8. Computational Molecular Modeling
The semi-empirical Parametric Method 3 (PM3) calculations were performed using the Gaussian program to obtain the stabilized geometries of the models. Next, the density functional theory (DFT) B3LYP/3-21G* calculations were performed to evaluate the values of enthalpy of the models. The 3D molecular structures were prepared to implement in the optimization calculations to reach the minimized energy structures. Then, the thermochemical frequency calculations were performed on the already optimized molecules to evaluate the values of enthalpy (H) for each of the investigated models (22).
3.9. Anti-promastigote Activity
Promastigotes of L. major were cultured in NNN medium and stored in RPMI enriched with 10% Fetal Bovine Serum (FBS) at 25°C, Penicillin (100 IU/mL), and Streptomycin (100 µg/mL) as antibacterial agents. Serially diluted complexes of samples were added to 96-well plates seeded with metacyclic Promastigotes (7.5 × 105 parasites/well) to obtain final concentrations of 1, 10, 50, 100, and 200 µM, along with wells without parasites as blank, untreated parasites as negative controls, and Amphotericin B as the positive control. The results were counted with a Neubauer chamber after 24 and 48 hours of incubation at 25°C (19).
3.10. Anti-amastigote Activity
J-774 macrophage cells were dissociated from cell culture flasks using the Trypsin protocol (18). The obtained cells were counted via Neubauer chamber, and 25 × 104 were seeded in each well of 6-wells plates with 24 × 24 mm cover glasses covering the interior bottoms of the wells. After adding 2 mL of enriched RPMI-1640 medium, the plates were incubated for 48 h at 37°C. Then, metacyclic Promastigotes were added to manually depleted wells with the count of 223.5 × 104/well (8.8 promastigotes/ macrophage) along with enriched RPMI-1640 medium up to 2mL. Plates were incubated again in a 37°C CO2 incubator for another 24 hours. After the given time, macrophages were inspected for being infected with parasites. By confirmation of that, wells were washed out with phosphate-buffered saline (PBS). Then two series of samples and Glucantime (as positive control), in concentrations of 1, 10, 50, 100, and 200 µM, were added to the respective wells, along with maintaining untreated wells as negative controls. Plates were incubated for 24 and 48 h. After the respective periods, covering glasses were gathered from the bottom of the wells, fixated with methanol, and stained using the Giemsa staining protocol (21).
4. Results
Kaempferol (1) was gently acetylated in acetic anhydride at 70°C for overnight and yielded kaempferol-3,7, 4′-triacetate (KTA, 60.5%). KTA was selectively deacetylated in C-7 to yield kaempferol-3, 4′-diacetate (KDA, 44.3%). Quercetin (2) in the same procedure was hyperacetylated to Quercetin-3,5,7,3′,4′-pentacetate (QPA, 81.7%). QPA was regioselectivity deacetylated in the C-7 position to Quercetin 3,5,3′,4′-tetraacetate (QTA, 41.1%). QTA was arylated in a nucleophilic substitution process by paramethylbenzyl or paranitrobenzyl chloride, separately followed by deacetylation in ammonia to yield 7-O-paramethylbenzyl quercetin (QPMB, 35.2%), and 7-O-paranitro benzyl quercetin (QPNB,39.6%). Among synthesized compounds, compounds 11 and 12 were newly described, and other compounds as pre-defined compounds were identified. NMR data of compounds 7, and 8 were assigned using one- and two-dimensional NMR for the first time. NMR, IR, and mass spectra are available in the supplementary file (appendices 1 - 28 in Supplementary File).
4.1. Kaempferol (K, 1)
UV (MeOH) λmax: 268, 367 nm. 1H-NMR: δ 12.54 (s, 1H, 5-OH), 10.84 (s, 1H, 7-OH), 10.17 (s, 1H, 4′-OH), 9.46 (s, 1H, 3-OH), 8.04 (d, 2H, J = 8.9, H-2’,6’,), 6.99 (d, 2H, J = 8.9, 2H H-3’,5’), 6.50 (d, 1H, J = 2.0, H-8), 6.25 (d, J = 2.0, 1H, H-6). 13C-NMR: 176.4(C-4), 164.4 (C-7), 161.2 (C-5), 159.7(C-4′), 156.6 (C-9), 147.3 (C-2), 136.1 (C-3), 130.0 (C-2′,6′), 122.1 (C-1′), 115.9 (C-3′,5′), 103.5 (C-10), 98.7 (C-6), 93.9 (C-8). IR (KBr); νmax: 3316, 1654, 1616, 1598, 1560, 1515, 1508, 1444, 1317, 1166 cm-1 (23).
4.2. Quercetin (Q, 2)
UV (MeOH) λmax: 257, 371 nm. 1H NMR δ 12.49 (s, 1H, 5-OH), 10.78 (s, 1H, 7-OH), 9.59 (s, 1H, 4′-OH), 9.36 (s, 1H, 3-OH), 9.30 (s, 1H, 3′-OH), 7.68 (d, 1H, J = 2.2, H-2′), 7.54 (dd, 1H, J = 8.5, 2.2, H-6′), 6.89 (d, 1H, J = 8.5, H-5′), 6.41 (d, 1H, J = 2.1, H-8), 6.19 (d, 1H, J = 2.1, H-6). 13C-NMR: 176.4(C-4), 164.4 (C-7), 161.2 (C-5), 148.2(C-4′), 145.5 (C-3′), 157.0 (C-9), 147.4 (C-2), 136.2 (C-3), 122.1 (C-1′), 116.1 (C4′), 115.7 (C-2′), 115.6 (C-6′), 103.5 (C-10), 98.7 (C-6), 93.8 (C-8). IR (KBr); νmax: 3401, 3305, 3122, 1662, 1608, 1521, 1448, 1382, 1317, 1263, 1199, 1168 cm−1 (17, 18).
4.3. Kaempferol-3,7, 4′-triacetate (KTA, 3)
1H-NMR δ: 12.06 (s, 1H, 5-OH), 7.98 (dd, 2H, J = 8.5, H-2’,6’), 7.40 (dd, 2H, J = 8.5, H-3′,5′), 7.14 (bs, 1H, H-8), 6.76 (bs, 1H, H-6), 2.36 (s, 3H, one acetate methyl), 2.32 (s, 6H, two acetate methyls). 13C-NMR δ: 174.0 (C-4), 167.4 (Acetate), 166.9 (Acetate), 166.3 (Acetate), 158.7 (C-5), 154.7(C-7), 154.6 (C-2), 154.0 (C-9), 151.4 (C-4′), 129.7 (C-3), 128.2 (C-2′,5′), 124.5 (C-1′), 121.2 (C-3′,5′), 106.5 (C-10), 104.1 (C-6), 100.5 (C-8), 19.3 (Acetate), 19.2 (Acetate), 18.6 (Acetate). ESI-MS (m/z): 411 [M-H]- (23).
4.4. 7-O-Paramethylbenzyl Quercetin (QPMB, 7)
UV (MeOH) λmax: 257, 377 nm;1H-NMR δH: 12.49 (5-OH), 7.72(bs, 1H, H-2′), 7.57 (bd, 1H, J = 8.4, H-6′), 7.36 (d, 2H, J = 8.1, H-3",5"), 7.21 (d, 2H, J = 8.0, H-2",6"), 6.89 (dd, 1H, J = 2.2, J = 8.1, H-5′), 6.77 (bs, 1H, H-8), 6.41 (bs, 1H, H-6), 5.18 (s, 2H, H-7"), 2.21 (s, 3H, paramethylbenzyl-Me); 13C-NMR δC: 176.0 (C-4), 164.0 (C-7), 160.4 (C-5), 156.0 (C-9), 147.9 (C-2), 147.4 (C-4′), 145.1 (C-3′), 137.5 (C-3), 137.5 (C-1"), 136.1 (C-3), 133.2 (C-4"), 129.2 (C-3", C-5"), 128.0 (C-2", C6"), 121.9 (C-1′), 120.1 (C-6′), 115.6 (C-5′), 115.3 (C-2′), 104.1 (C-10), 98.1 (C-6), 92.9 (C-8), 69.9 (C-7"), 20.9 (paramethylbenzyl-Me); IR (KBr); νmax: 3408, 3253, 2922, 2850, 1655,1610, 1589, 1498, 1460, 1350,1323, 1207, 1165, 1095, 1003, 933 cm−1; ESI-MS (m/z): 405.15 [M-H]− (17).
4.5. 7-O-Paranitrobenzyl Quercetin (QPNB, 8)
UV (MeOH) λmax: 266, 371 nm;1H-NMR δH: 8.36 (d, 2H, J = 8.8, H-3",5"), 7.82 (d, 2H, J = 8.8, H-2",6"), 7.80 (d, 1H, J = 2.0, H-2′), 7.64 (dd, 1H, J = 2.0, 8.8, H-6′), 7.01 (d, 1H, J = 8.4, H-5′), 6.91 (d, 1H, J = 2.0, H-8), 6.56 (d, 1H, J = 2.0, H-6), 5.51 (s, 2H, H-7"). 13C-NMR δC: 176.0 (C-4), 163.4 (C-7), 160.5 (C-5), 156.0 (C-9), 148.0 (C-2), 147.5 (C-4"), 147.2 (C-4′), 145.2 (C-3′), 144.2 (C-1"), 136.2 (C-3), 128.4 (C-3", C-5"), 123.8 (C-2", C-6"), 121.8 (C-1′), 120.1 (C-6′), 115.7 (C-5′), 115.3 (C-2′), 104.4 (C-10), 98.1 (C-6), 92.9 (C-8), 68.7 (C-7"); IR (KBr) νmax: 3404, 2958, 2922, 2850, 1655, 1595, 1522, 1500, 1448, 1419, 1346, 1261, 1165, 1107, 1093, 1003 cm−1; ESI-MS (m/z): 436.10 [M-H]− (18).
4.6. Sb(V) Kaempferol Complex (K-Sb, 9)
UV (MeOH) λmax: 268, 413; IR (KBr); νmax: 3446, 3178, 1652, 1635, 1558, 1540, 1506, 1457, 1396, 1172 cm−1; ESI-MS (m/z): 726[C30H20O14Sb, 2Kaempferol+Sb+2H2O]+, 690 [C30H16O12Sb+, 2Kaempferol+Sb]+.
4.7. Sb(V) Quercetin Complex (Q-Sb, 10)
UV (MeOH) λmax: 267, 429; IR (KBr); νmax: 3421, 3178, 1652, 1646, 1637, 1590, 1508, 1484, 1430, 1411, 1351, 1319, 1267, 1201, 1162 cm−1; ESI-MS (m/z): 758[C30H20O16Sb, 2Q+Sb+2H2O]+, 722[C30H16O14Sb, 2Q+Sb]+.
4.8. Sb(V) 7-O-Paramethylbenzyl Quercetin Complex (QPMB-Sb, 11)
UV (MeOH) λmax: 268, 429; IR (KBr); νmax: 3421, 3212, 1652, 1646, 1635, 1558, 1540, 1506, 1488, 1457, 1338, 1267, 1159 cm−1; ESI-MS (m/z): 964 [C46H34O16Sb, 2QPMB+Sb+2H2O] -, 928 [C46H30O14Sb, 2QPMB +Sb] -.
4.9. Sb(V) 7-O-Paranitrobenzyl Quercetin Complex (QPNB-Sb, 12)
UV (MeOH) λmax: 269, 423; IR (KBr); νmax: 3446, 1652, 1346, 1635, 1558, 1540, 1506, 1338, 1267, 1201, 1160 cm−1; ESI-MS (m/z): 1026 [C44H28N2O20Sb, 2QPNB +Sb+2H2O]-.
4.10. Stoichiometry of Metal-ligand Complexes
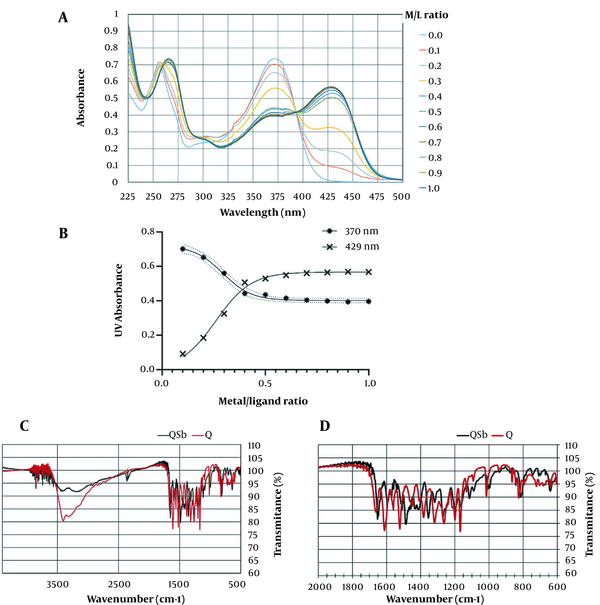
Quercetin showed two UV maxima absorption at 370 nm named band I, and 259 nm, named band II (Figure 3A). A series of 10 complex samples with the metal/ligand (M/L) proportion range of 0.1 - 1 were prepared using SbCl5 solution. All samples were initially characterized using UV spectra, as shown in Figure 3A. The red shift of band I at 370 nm to 429 nm with 59 nm, as well as FTR shifts of carbonyl absorptions and the reduction of free hydroxy absorptions at 3421 cm-1 compared to quercetin, were related to the formation of the Q-Sb complex (Figure 3C, D). In the molar ratios' plot, as shown in Figure 3B, the nonlinear fitted curve crossing the points corresponded to the molar ratio of the ligand/metal complex, with the stoichiometry ratio of 1:2. In the same Amastigote form, considering its manner, K-Sb (9), QPMB-Sb (11), and QPNB-Sb (12) complexes were analyzed with an M/L ratio of 1:2, respectively.
(A) UV spectra of Sb(V) 7 quercetin complex (Q-Sb, 10) with M/L in the ranges of 0.1 - 1. (B) Molar ratio of the metal ion in ligand-metal complex, at 370 nm and 429 nm. (C, D) Broad and expanded IR Spectra Q-Sb.
4.11. Computational Molecular Modeling
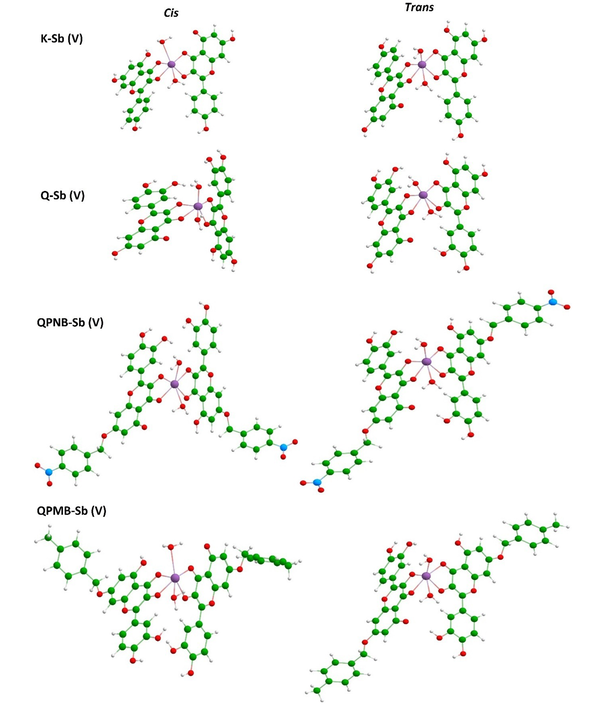
The stabilized models are shown in Figure 4, and their evaluated enthalpy values are summarized in Table 1. As could be seen by the visualized models, different configurations of Cis and Trans were obtained for each model, and, accordingly, the enthalpy values were changed for those configurations. The trans configuration was found more suitable than the Cis configuration based on the obtained enthalpy values in a brief notation. But all models were achievable by the negative sign of enthalpy (Figure 4).
Optimized representations of Cis and Trans configurations of Sb(V) complex of kaempferol and quercetin derivatives (9-12).
The Evaluated Enthalpy (H) Values for the Proposed Sb(V) Complexes Using the B3LYP/3-21G* Calculations
| Sb(V) Flavonoid Complex | Enthalpy, H (kcal/mol) | |
|---|---|---|
| Cis Isomer | Trans Isomer | |
| K-Sb (9) | -5320203 | -5320208 |
| Q-Sb (10) | -5413998 | -5414008 |
| QPMB-Sb (11) | -5800026 | -5800035 |
| QPNB-Sb (12) | -6005994 | -6006003 |
4.12. Anti-promastigote Leishmanial Activity
Inhibitory data were analyzed using IBM SPSS Statistics 26. IC50 values of each complex were calculated after 48 hours, as shown in Table 2.
IC50 Values Against the Promastigote form of Leishmania major After 48 h
| Compound | Promastigote L. major IC50 (µM) |
|---|---|
| KTA (3) | 14.93 ± 2.21 |
| K-Sb (9) | 113.09 ± 9.04 |
| Q-Sb (10) | 160.3 ± 12.03 |
| QPMB-Sb (11) | > 200 |
| QPNB-Sb (12) | > 200 |
| Amphotericin B | 3.68 ± 0.74 |
4.13. Anti-amastigote Leishmanial Activity
Even though flavonol Sb(V) complexes showed weak inhibition against promastigotes, they showed remarkable dose-dependent activity against amastigotes of L. major with IC50 values in the range of 0.5 to 15 μM after 48 h (Table 3).
IC50 Values Against the Amastigote form of Leishmania major
| Sb(V) Complex | Amastigote L. major IC50 (µM) | Normal J774 Murine Macrophages CC50 (µM) | Selectivity Index 48 h (CC50/IC50) | |
|---|---|---|---|---|
| 24 h | 48 h | 48 h | 48 h | |
| K-Sb (9) | 3.55 ± 0.61 | 0.52 ± 0.010 | 11.81 ± 1.6 | 22.71 |
| Q-Sb (10) | 13.85 ± 2.34 | 1.48 ± 0.25 | 30.65 ± 0.46 | 20.71 |
| QPMB-Sb (11) | 18.41 ± 2.22 | 1.10 ± 0.29 | 22.41 ± 0.83 | 20.37 |
| QPNB-Sb (12) | 23.45 ± 2.74 | 14.50 ± 1.62 | 33.61 ± 2.67 | 2.32 |
| Meglumine antimonate | 57.30 ± 16.64 | 40.3 ± 5.46 | 1613 ± 112.06 | 40.03 |
5. Discussion
In non-aqueous conditions, glacial acetic acid works as the solvent in order to form Sb5+ from SbCl5. Sb5+ with H2O molecules as its ligands to form primary complexes. With the addition of Kaempferol or Quercetin analogs, the flavonoid compounds would remain unionized in an acidic solution. In excess flavonoid addition, the chemical equilibrium of the primary complex tends to shift towards forming the secondary complex by replacing H2O (a monodentate ligand) with quercetin or kaempferol analogs (bidentate ligands). Due to the availability of enough vacant orbitals of Sb5+, two molecules of Kaempferol or Quercetin analogs and two molecules of water could bind through the Sb5+ bridging atom making the complex systems. Additionally, such a secondary complex is more stable than the primary one because Kaempferol or Quercetin analogs as bidentate ligands can fix properly within the central surface of the complex, whereas H2O as a monodentate ligand which has more spatial liberation, causes transitional instability.
There are two possible bidentate positions in Kaempferol and Quercetin analogs at 3-OH and 4-oxo in the C ring or 5-OH and 4-oxo in the A ring. The 5-OH in the A ring is relatively inactive because of the intermolecular hydrogen bond. Therefore, complexation occurs at C-4 and C-3. Quercetin and Kaempferol can relax almost in the planar position to form each of the Cis or Trans configurations. H2O molecules are instead suitable to be dispositioned in two axial positions.
These results are in agreement with those from Tasdemir study on the leishmanicidal activity of phenolic compounds. He reported that flavone-3-ol showed antileishmanial activity against L. donovani seven times more than simple flavone. Hydroxylation at other positions at C-5, C-6, or C-7 was not as active as flavone-3-ol. Covering 3-OH by methylation or glycosylation led to a remarkable activity reduction (13).
In the biological test, among samples, kaempferol triacetate with more lipophilicity showed more anti-promastigote activity with an IC50 value of 14.93 ± 2.21 µM, compared to amphotericin B with an IC50 value of 3.68 ± 0.74 µM while antimony complexes showed weak activity. In anti-amastigote activity, despite differences, all antimony complexes showed anti-amastigote effects in vitro with IC50 values of 0.52 to 14.50 µM with potency more than meglumine antimonate with IC50 value of 40.3 ± 5.46 µM.
Even though organic antimony complexes have been used for over half a century to treat leishmaniasis, their exact mechanism is not yet fully discovered. However, there is some evidence that in a procedure mediated by macrophages in mildly acidic conditions, pentavalent antimony in amastigotes is reduced to the trivalent form and activates specific endonucleases that cause fragmentation of the oligonucleosomal DNA of amastigote forms and stimulate antiparasitic activity (7). In Leishmania species, glutathione tripeptide and spermidine polyamine bind together as a dimer to form trypanothione. Rapid reduction of Sb(V) to Sb (III) occurs with the parasite-specific trypanothione in amastigotes under acidic macrophage conditions, while it is slow or negligible alone in other mammalian cells in the presence of glutathione alone in amastigotes in the culture medium (24). In this research, flavonoid Sb(V) complexes showed better activity in vitro but with less selectivity than meglumine antimonate. In quercetin analogs, QPMB-Sb (11) with 7-O-paramethylbenzyl substitution as an electron-donating group showed more potency with an IC50 value of 1.10 ± 0.29 µM, than QPNB-Sb (12) with 7-O-paranitrobenzyl substitution as an electron-withdrawing group with IC50 value of 14.50 ± 1.62 µM.
Inside the host macrophages, by breaking down the complex into antimony and quercetin or kaempferol analogs, the observed antiparasitic effects may have been related to both Sb(V)/Sb (III) conversion and flavonoid antileishmanial activities. However, it was recommended further studies should be carried out to explore the antiparasitic effects of flavonol antimony (V) complexes and explain their exact mechanism of action.
5.1. Conclusions
In this study, new potential Sb(V) complexes were identified by combining the benefits of antimony (V) and flavonols and optimizing their properties. The UV, NMR, and ESI analysis confirmed the structure of synthesized compounds (3-12). Among them, QPMB-Sb (11) and QPNB-Sb (12) as newly described, and other compounds as pre-defined compounds were identified. In the biological test, kaempferol triacetate with more lipophilicity showed more anti-promastigote activity, while flavonol antimony complexes showed weak activity. As for anti-amastigote activity, despite differences, all antimony complexes showed anti-amastigote effects with potency more than those of meglumine antimonate.
References
-
1.
Akhlagh A, Salehzadeh A, Zahirnia AH, Davari B. 10-Year Trends in Epidemiology, Diagnosis, and Treatment of Cutaneous Leishmaniasis in Hamadan Province, West of Iran (2007-2016). Front Public Health. 2019;7:27. [PubMed ID: 30891438]. [PubMed Central ID: PMC6413056]. https://doi.org/10.3389/fpubh.2019.00027.
-
2.
Mohajeri M, Saghaei L, Ghanadian M, Saberi S, Pestechian N, Ostadhusseini E. Synthesis and In vitro Leishmanicidal Activities of Six Quercetin Derivatives. Adv Biomed Res. 2018;7:64. [PubMed ID: 29862213]. [PubMed Central ID: PMC5952540]. https://doi.org/10.4103/abr.abr_76_17.
-
3.
Hefnawy A, Berg M, Dujardin JC, De Muylder G. Exploiting Knowledge on Leishmania Drug Resistance to Support the Quest for New Drugs. Trends Parasitol. 2017;33(3):162-74. [PubMed ID: 27993477]. https://doi.org/10.1016/j.pt.2016.11.003.
-
4.
Phumee A, Jariyapan N, Chusri S, Hortiwakul T, Mouri O, Gay F, et al. Determination of anti-leishmanial drugs efficacy against Leishmania martiniquensis using a colorimetric assay. Parasite Epidemiol Control. 2020;9. e00143. [PubMed ID: 32300665]. [PubMed Central ID: PMC7153290]. https://doi.org/10.1016/j.parepi.2020.e00143.
-
5.
Jones CM, Welburn SC, Jones JD. Treatment failure of pentavalent antimonial therapy for human visceral leishmaniasis: a meta-analysis. J Glob Health Rep. 2019;3. https://doi.org/10.29392/joghr.3.e201948.
-
6.
Tunç T, Ertabaklar H, Karacan N. In vitro anti-leishmanial activities and structure-activity relationship analysis of new antimony (III) complexes. Indian J Chem A. 2020;59(11):1609-17.
-
7.
Boreiko CJ, Rossman TG. Antimony and its compounds: Health impacts related to pulmonary toxicity, cancer, and genotoxicity. Toxicol Appl Pharmacol. 2020;403:115156. [PubMed ID: 32710957]. https://doi.org/10.1016/j.taap.2020.115156.
-
8.
Moreira VR, de Jesus LCL, Soares RP, Silva LDM, Pinto BAS, Melo MN, et al. Meglumine Antimoniate (Glucantime) Causes Oxidative Stress-Derived DNA Damage in BALB/c Mice Infected by Leishmania (Leishmania) infantum. Antimicrob Agents Chemother. 2017;61(6). [PubMed ID: 28320726]. [PubMed Central ID: PMC5444157]. https://doi.org/10.1128/AAC.02360-16.
-
9.
da Silva SS, Mizokami SS, Fanti JR, Costa IN, Bordignon J, Felipe I, et al. Glucantime reduces mechanical hyperalgesia in cutaneous leishmaniasis and complete Freund's adjuvant models of chronic inflammatory pain. J Pharm Pharmacol. 2018;70(6):768-77. [PubMed ID: 29532470]. https://doi.org/10.1111/jphp.12896.
-
10.
Carter NS, Stamper BD, Elbarbry F, Nguyen V, Lopez S, Kawasaki Y, et al. Natural Products That Target the Arginase in Leishmania Parasites Hold Therapeutic Promise. Microorganisms. 2021;9(2). [PubMed ID: 33525448]. [PubMed Central ID: PMC7911663]. https://doi.org/10.3390/microorganisms9020267.
-
11.
da Silva ER, Brogi S, Lucon-Junior JF, Campiani G, Gemma S, Maquiaveli CDC. Dietary polyphenols rutin, taxifolin and quercetin related compounds target Leishmania amazonensis arginase. Food Funct. 2019;10(6):3172-80. [PubMed ID: 31134235]. https://doi.org/10.1039/c9fo00265k.
-
12.
Cataneo AHD, Tomiotto-Pellissier F, Miranda-Sapla MM, Assolini JP, Panis C, Kian D, et al. Quercetin promotes antipromastigote effect by increasing the ROS production and anti-amastigote by upregulating Nrf2/HO-1 expression, affecting iron availability. Biomed Pharmacother. 2019;113:108745. [PubMed ID: 30836276]. https://doi.org/10.1016/j.biopha.2019.108745.
-
13.
Tasdemir D, Kaiser M, Brun R, Yardley V, Schmidt TJ, Tosun F, et al. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: in vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob Agents Chemother. 2006;50(4):1352-64. [PubMed ID: 16569852]. [PubMed Central ID: PMC1426963]. https://doi.org/10.1128/AAC.50.4.1352-1364.2006.
-
14.
Noori S, Rezaei Tavirani M, Deravi N, Mahboobi Rabbani MI, Zarghi A. Naringenin Enhances the Anti-Cancer Effect of Cyclophosphamide against MDA-MB-231 Breast Cancer Cells Via Targeting the STAT3 Signaling Pathway. Iran J Pharm Res. 2020;19(3):122-33. [PubMed ID: 33680016]. [PubMed Central ID: PMC7757999]. https://doi.org/10.22037/ijpr.2020.113103.14112.
-
15.
Ortiz S, Vasquez-Ocmin PG, Cojean S, Bouzidi C, Michel S, Figadere B, et al. Correlation study on methoxylation pattern of flavonoids and their heme-targeted antiplasmodial activity. Bioorg Chem. 2020;104:104243. [PubMed ID: 32920360]. https://doi.org/10.1016/j.bioorg.2020.104243.
-
16.
Yang B, Liu H, Yang J, Gupta VK, Jiang Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci Technol. 2018;79:116-24. https://doi.org/10.1016/j.tifs.2018.07.006.
-
17.
Zhong D, Liu M, Cao Y, Zhu Y, Bian S, Zhou J, et al. Discovery of Metal Ions Chelator Quercetin Derivatives with Potent Anti-HCV Activities. Molecules. 2015;20(4):6978-99. [PubMed ID: 25913935]. [PubMed Central ID: PMC6272327]. https://doi.org/10.3390/molecules20046978.
-
18.
Yamauchi K, Fujieda A, Mitsunaga T. Selective synthesis of 7-O-substituted luteolin derivatives and their melanonenesis and proliferation inhibitory activity in B16 melanoma cells. Bioorg Med Chem Lett. 2018;28(14):2518-22. [PubMed ID: 29887353]. https://doi.org/10.1016/j.bmcl.2018.05.051.
-
19.
Varshosaz J, Arbabi B, Pestehchian N, Saberi S, Delavari M. Chitosan-titanium dioxide-glucantime nanoassemblies effects on promastigote and amastigote of Leishmania major. Int J Biol Macromol. 2018;107(Pt A):212-21. [PubMed ID: 28867228]. https://doi.org/10.1016/j.ijbiomac.2017.08.177.
-
20.
Barbosa VT, de Menezes JB, Santos JCC, Bastos MLA, de Araujo-Junior JX, do Nascimento TG, et al. Characterization and Stability of the Antimony-Quercetin Complex. Adv Pharm Bull. 2019;9(3):432-8. [PubMed ID: 31592433]. [PubMed Central ID: PMC6773938]. https://doi.org/10.15171/apb.2019.051.
-
21.
Tanui HK, Nkabyo HA, Pearce BH, Hussein AA, Lopis AS, Luckay RC. Iron(III) and copper(II) complexes derived from the flavonoids morin and quercetin: Chelation, crystal structure and DFT studies. J Mol Struc. 2022;1257:132591. https://doi.org/10.1016/j.molstruc.2022.132591.
-
22.
Nguyen QNN, Xia KT, Zhang Y, Chen N, Morimoto M, Pei X, et al. Source of Rate Acceleration for Carbocation Cyclization in Biomimetic Supramolecular Cages. J Am Chem Soc. 2022;144(25):11413-24. [PubMed ID: 35699585]. https://doi.org/10.1021/jacs.2c04179.
-
23.
Mai LH, Chabot GG, Grellier P, Quentin L, Dumontet V, Poulain C, et al. Antivascular and anti-parasite activities of natural and hemisynthetic flavonoids from New Caledonian Gardenia species (Rubiaceae). Eur J Med Chem. 2015;93:93-100. [PubMed ID: 25659770]. https://doi.org/10.1016/j.ejmech.2015.01.012.
-
24.
Duffin RN, Stephens LJ, Blair VL, Kedzierski L, Andrews PC. The thiol-based reduction of Bi(V) and Sb(V) anti-leishmanial complexes. J Inorg Biochem. 2021;221:111470. [PubMed ID: 33971522]. https://doi.org/10.1016/j.jinorgbio.2021.111470.