Abstract
Background:
Transradial approach (TRA) is widely used all around the world in coronary angiography and percutaneous coronary interventions. Ulnar artery (UA) access can be used as an alternative to TRA in coronary catheterization. Transulnar approach (TUA) is as effective and safe as TRA in coronary angiography and angioplasty.Objectives:
The aim of the present study was to evaluate the effects of unilateral reciprocal compression of radial artery (RA) and ulnar artery (UA) on arterial diameter, flow velocity, and volume flow in healthy individuals.Patients and Methods:
A total of 210 extremity arteries of 105 consecutive cases were evaluated using Doppler ultrasonography. At the wrist level, UA and RA were sequentially compressed for 60 seconds. The diameter, peak systolic velocity (PSV), and volume flow (VF) were evaluated during UA and RA compressions.Results:
The mean baseline RA diameter was 2.04 ± 0.33 mm and the mean baseline UA diameter was 1.92 ± 0.38 mm, indicating a significantly greater RA diameter than the UA diameter (P = 0.005). Baseline diameters, PSV, and VF of the RA and UA significantly increased during reciprocal compression (P < 0.001). The increase in the diameter, PSV, and VF of UA in percent during reciprocal compression was higher, compared to the increase in the RA.Conclusion:
The present study results showed significant increases in the diameters, PSV, and VF values of both RA and UA during reciprocal compression, compared to baseline values. The increases in the diameter, velocity and volume flow of the UA from baseline were more prominent compared to the RA. Our study results showed that an ultrasonographic measurement of diameter, PSV and VF would still be necessary in preoperative period.Keywords
Radial Artery Ulnar Artery Doppler Ultrasonography Hemodynamics
1. Background
Transradial approach (TRA) is widely used all around the world in coronary angiography and percutaneous coronary interventions (PCI) (1, 2). Compared to femoral artery, radial artery has a more superficial course and it is of lower caliber. Therefore, access site complications such as bleeding, hematoma, and pseudoaneurysms are less commonly seen and vascular hemostasis can be more easily achieved, allowing early discharge (3, 4). Patients also prefer TRA due to the shorter length of hospital stay, less need for supine position during the procedure, and improved and early physical functioning after the procedure (5). Hand circulation is supplied by two arterial systems, radial artery (RA) and ulnar artery (UA). Transradial approach is used in coronary angiography in conditions where there is sufficient ulnar circulation (6). However, approximately 5% - 15% of patients undergoing coronary angiography or angioplasty are not suitable for TRA due to various reasons such as abnormal Allen’s test (AT), significant anatomic variations, arterial stenosis or hypoplasia, aberrant vessel origin, and arterial vasospasm (7). In addition, TRA may not be possible in patients with low RA diameter, even if there is sufficient ulnar collateral circulation (7). UA is one of the two terminal branches of the brachial artery and has a greater diameter than RA, and UA access can be used as an alternative to TRA in coronary catheterization (2). There are reports in the literature suggesting that TUA is as effective and safe as TRA in coronary angiography and angioplasty (2).
There are anastomotic connections between the RA and UA at the level of wrist and had through superficial and deep palmar arches (3). Hand and forearm therefore receive double blood supply with these anastomotic connections, and these anastomotic connections protect hands against ischemia (3). Ischemia of the hand can occur after TRA in the presence of occlusive atherosclerotic lesions in the upper extremity arteries and underdevelopment of the superficial palmar arch, malformations of UA, dominant RA in vascularization of the hand, and anatomic variations (3). RA cannulation can result in a decrease in blood flow and sometimes occlusion in the early or late periods (7, 8). Radial artery occlusion (RAO) occurs in 0.8% to 30.0% of cases following RA cannulation (9). Therefore, the authors suggest that evaluation of RA diameter and flow parameters would assist clinicians and prove useful in selection of the most appropriate access site and device before the procedure, thereby, reducing possible complications related to the procedure.
Arterial blood pressure measurement and assessment of the integrity of the palmar arch of the hand are recommended before coronary catheterization and removal of a vessel for coronary artery bypass grafting (CABG) (3). Allen’s test is the most frequently used method in evaluation of collateral blood flow to the hand. However, this test remains incapable due to its subjective nature and high false positive and negative results (10). Therefore, we need a more superior test to determine whether the RA is a suitable access point. Doppler ultrasonography (DUS) has been recently introduced into the practice in addition to the use of AT and similar clinical tests (11). Assessment of the functions of the superficial palmar arch using DUS aims at detecting congenital and/or acquired changes in the UA.
2. Objectives
In the present study, we hypothesized that flow parameters of the RA and UA would increase with reciprocal compression and aimed to evaluate the changes occurring in flow parameters and diameter, peak systolic velocity (PSV), and volume flow (VF) values of RA and UA on DUS during reciprocal compression and at rest in healthy individuals having no risk factor for atherosclerosis.
3. Patients and Methods
3.1. Study Group
Among patients who were admitted to the ultrasonography unit of the radiology clinics for non-urgent conditions between April 2016 and June 2016, 105 patients who volunteered for the study and signed an informed consent were included in the study. The study group was composed of volunteers aged 15 to 35 years who did not have a known risk factor for atherosclerosis (i.e. diabetes, hypertension, hyperlipidemia, smoking, and age). Demographic data, height, weight, and gender were recorded. Body mass index (BMI) was calculated by dividing body weight in kilograms by the square of body height in meters. Both RA and UA in the upper extremity of each case were evaluated using DUS. Patients who had prior RA catheterization, arteriovenous fistula, scleroderma, previous upper extremity amputation or trauma to the arm requiring treatment, and extremity abnormality were excluded from the study.
3.2. Ultrasonographic Assessment
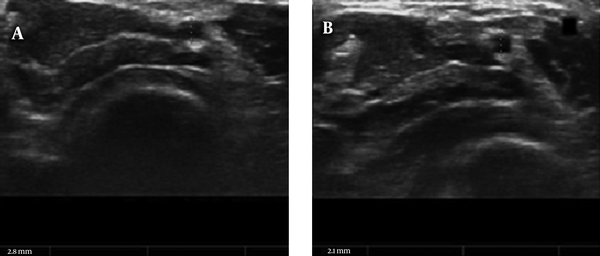
B-mode and DUS examinations of both hands were performed using SSA-390A (Toshiba, Tokyo, Japan) model device using 7.5 to 11 MHz multi-frequency linear-array transducer. Examinations were performed at room temperature, following 10 minutes of rest with the hand in supine position. Initially, the lumen diameters, PSV, and VF were obtained at the wrist level for RA and UA. The measurements were taken in a short axial view by a single operator who had 10 years of experience in DUS. A vertical diameter was obtained for each RA on diastolic phase (from intima to intima). In the presence of intimal thickening, the thickening was also included in the measurement. The measurements were obtained by magnification without disturbing the resolution, and compression was avoided during the measurement. All measurements were performed considering the short axial view of the hypoechogenic vascular area (Figure 1). Flow profiles were obtained in longitudinal planes using color DUS. The VF was calculated by measurement of the mean areas under three consequent spectral waves and the vessel diameter in a short axis.
Radial artery (RA) diameter before compression (A) and during compression (B)
3.3. Ultrasonographic Assessment During Unilateral Reciprocal Compression
The UA was palpated at the level of the wrist joint, at Guyon’s canal. It was then, manually compressed with complete obliteration of the ulnar pulse for a total duration of 60 seconds. The RA Duplex DUS evaluation was performed continuously, and the effect of UA compression was recorded. The VF was calculated at baseline and after UA occlusion by the RA pulsed Doppler envelope. Three sampled beats were analyzed and the mean values were recorded. The PSV of the RA flow envelope before and during UA compression was recorded. Diameter of the RA was recorded before and during UA compression. The RA was palpated just proximal to the wrist joint, pressing the artery on to the anterior surface of the radius. It was then, manually compressed with complete obliteration of the radial pulse for a total duration of 60 seconds. The UA Duplex DUS was performed continuously, and the effect of RA compression was recorded. The VF was calculated at baseline and after RA occlusion by the UA pulsed Doppler envelope. Three sampled beats were analyzed and the mean values were recorded. The PSV of the UA flow envelope before and during RA compression was recorded. The diameter of the UA was recorded before and during RA compression.
3.4. Intra- and Inter-Observer Variability of Ultrasound Assessment
To assess the intra-observer variability, DUS measurements were repeated in 10 participants the following day by the same researcher. The measurements of the RA diameter showed an internal consistency coefficient (ICC) and Kappa values of 0.98 and 0.929, respectively, indicating an almost excellent agreement. In addition, to demonstrate the repeatability of the measurements, the inter-observer variability was assessed by two different researchers performing DUS on the same 25 patients. The inter-observer measurements of the RA diameter were also in almost perfect agreement with the ICC and Kappa values being 0.97 and 0.881, respectively.
3.5. Ethical Considerations
An approval was obtained from the institutional ethics committee. The study was conducted in accordance with the principles of the declaration of Helsinki (approval No: 25.04.2016/29/05). A written informed consent was obtained from each participant.
3.6. Statistical Analysis
Statistical analysis was performed using the statistical package for social sciences (SPSS) for Windows version 22.0 software (SPSS Inc., Chicago, IL, USA). Descriptive data were expressed in mean ± standard deviation, median (interquartile ranges), frequency distribution, and percentage. The Pearson’s chi-square test was used to analyze categorical variables. The variables were tested for conformity to normal distribution using visual (histogram and probability graphs) and analytic methods (Kolmogorov-Smirnov Test). For normally distributed variables, the Student’s t-test was used to compare two independent groups, while Paired Sample t-test was used to compare two dependent groups. The relationship between the variables was analyzed using the Pearson’s correlation coefficients. Independent effects of variable predictors on the RA and UA before compression were evaluated using univariate and multivariate linear regression model. The diagnostic value of RA and UA diameter in predicting a height above 160 cm, body weight above 80 kg, and BMI above 25 kg/m2 was evaluated using the receiver operating characteristic (ROC) curve. A P value of < 0.05 was considered statistically significant.
4. Results
A total of 210 upper extremities (UE) of 105 patients were evaluated. Demographic characteristics of the patients are presented in Table 1.
Baseline Demographic Characteristics of the Study Population
| (n = 105) | Mean ± SD | Minimum | Maximum |
|---|---|---|---|
| Age (years) | 21.49 ± 5.61 | 16 | 35 |
| Male sex [n (%)] | 38 (36.2) | ||
| Height (cm) | 168.54 ± 9.40 | 150 | 190 |
| Weight (kg) | 66.01 ± 14.01 | 45 | 115 |
| BMI (kg/m2) | 23.08 ± 3.35 | 16.2 | 31.9 |
The mean baseline RA diameter was 2.04 ± 0.33 mm and the mean baseline UA diameter was 1.92 ± 0.38 mm. The mean RA diameter was significantly greater than the mean UA diameter (P = 0.005). All participants were right-hand dominant. The diameters of the RA and UA in the dominant hand were greater than the diameters of the corresponding arteries in the other hand (P < 0.05). We also found significant increases in the diameter, PSV, and VF of the RA and UA during reciprocal compression (compression of either the ipsilateral RA or UA while examining the other). The increases in the diameter, PSV, and VF of UA in percent during reciprocal compression were higher, compared to the RA (Table 2).
Changes in the Ultrasonography Measurements of Radial and Ulnar Arteries During Ipsilateral Reciprocal Compressiona
| (N = 105) | Baseline | During Ipsilateral Reciprocal Compression | ∆ | ∆% | P value |
|---|---|---|---|---|---|
| Radial artery | |||||
| Diameter | 2.04 ± 0.32 | 2.08 ± 0.35 | 0.048±0.189 | 2.69 ± 9.73 | 0.011 |
| PSV | 44.64 ± 13.45 | 60.57 ± 21.28 | 15.94±15.81 | 38.95 ± 38.18 | < 0.001 |
| VF | 18.71 ± 12.89 | 28.28 ± 21.14 | 9.57±14.82 | 60.10 ± 85.53 | < 0.001 |
| Ulnar artery | |||||
| Diameter | 1.92 ± 0.38 | 2.04 ± 0.39 | 0.123±0.209 | 7.26 ± 11.02 | < 0.001 |
| PSV | 51.26 ± 15.48 | 102.41 ± 28.36 | 51.15±23.05 | 109.43 ± 64.88 | < 0.001 |
| VF | 22.89 ± 16.15 | 32.58 ± 20.81 | 9.69±13.91 | 56.40 ± 78.50 | < 0.001 |
The RA and UA diameters were greater in men than in women. The right and left upper extremity RA and UA diameters of the men were similar, while the right RA and UA diameters of the women were greater than the left UE. The RA and UA diameters and VF values of the men at rest and during compression were greater than in women. In addition, the increase in the RA diameter during compression was significant in men; whereas, the increase in women was not significant (Table 3).
Mean of Diameter, PSV and VF of Radial and Ulnar Arteries Before and After Compression in Two Gendersa
| Male (N = 38) | Female (N = 67) | P value | |||
|---|---|---|---|---|---|
| Radial artery | Diameter | Before compression | 2.25 ± 0.33 | 1.92 ± 0.26 | < 0.001 |
| After compression | 2.33 ± 0.35 | 1.95 ± 0.27 | < 0.001 | ||
| P value | 0.021 | 0.184 | |||
| PSV | Before compression | 47.81 ± 14.82 | 42.84 ± 12.37 | 0.052 | |
| After compression | 64.87 ± 19.13 | 58.13 ± 22.17 | 0.034 | ||
| P value | < 0.001 | < 0.001 | |||
| VF | Before compression | 23.95 ± 16.84 | 15.74 ± 8.83 | 0.003 | |
| After compression | 38.55 ± 24.71 | 22.46 ± 16.32 | < 0.001 | ||
| P value | < 0.001 | <0.001 | |||
| Ulnar artery | Diameter | Before compression | 2.07 ± 0.33 | 1.84 ± 0.38 | < 0.002 |
| After compression | 2.28 ± 0.37 | 1.91 ± 0.33 | < 0.001 | ||
| P value | < 0.001 | 0.005 | |||
| PSV | Before compression | 53.74 ± 14.10 | 49.83 ± 16.15 | 0.217 | |
| After compression | 108.21 ± 25.71 | 99.07 ± 29.45 | 0.201 | ||
| P value | < 0.001 | < 0.001 | |||
| VF | Before compression | 25.40 ± 14.86 | 21.45 ± 16.79 | 0.047 | |
| After compression | 41.70 ± 21.56 | 27.33 ± 18.58 | 0.001 | ||
| P value | < 0.001 | < 0.001 | |||
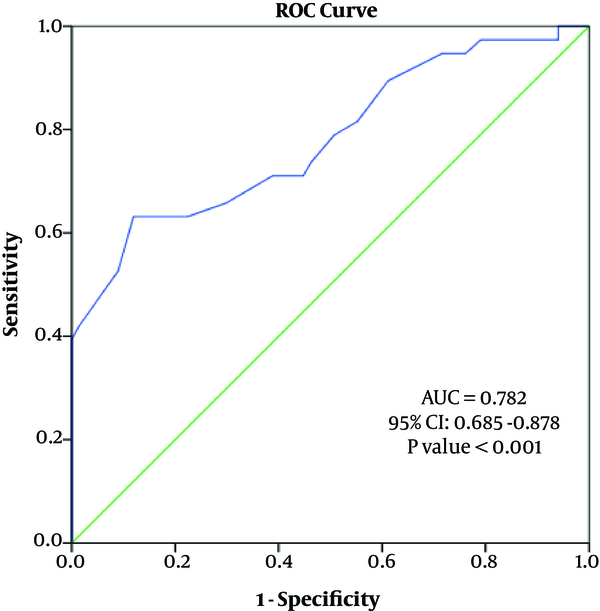
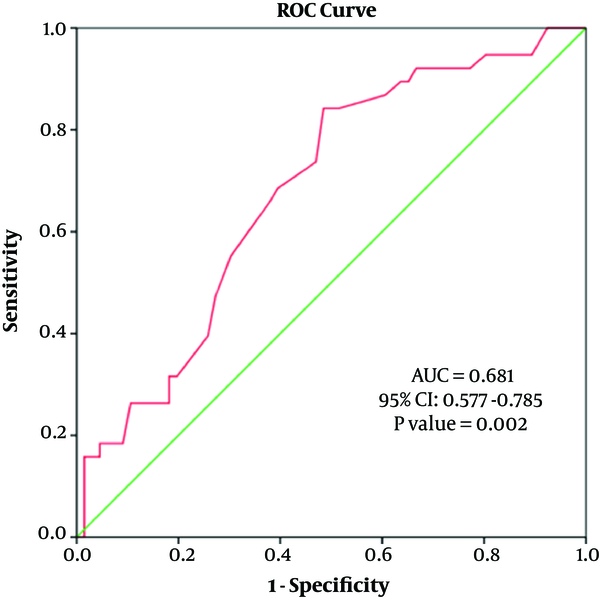
Furthermore, sex was an independent predictor of the RA diameter. Sex and BMI were independent factors predicting the UA diameter (Table 4). The diagnostic value of sex in predicting the RA diameter and UA diameter was evaluated using the ROC curve analysis (area under the curve (AUC) of radial artery diameter = 0.782 (95% confidence interval [CI]: 0.685 - 0.878), P < 0.001; AUC of ulnar artery diameter = 0.681 (95% CI: 0.577 - 0.875) (Figures 2 and 3).
| Radial Artery | Ulnar Artery | |||||
|---|---|---|---|---|---|---|
| Diameter | PSV | VF | Diameter | PSV | VF | |
| Age (years) | ||||||
| r | 0.077 | -0.062 | 0.022 | 0.146 | -0.013 | 0.020 |
| P value | 0.438 | 0.529 | 0.827 | 0.140 | 0.898 | 0.842 |
| Height (cm) | ||||||
| r | 0.299 | 0.111 | 0.213 | 0.301 | 0.101 | 0.268 |
| P value | 0.002 | 0.258 | 0.029 | 0.002 | 0.310 | 0.006 |
| Weight (kg) | ||||||
| r | 0.286 | 0.141 | 0.181 | 0.416 | 0.279 | 0.369 |
| P value | 0.003 | 0.151 | 0.065 | < 0.001 | 0.004 | < 0.001 |
| BMI (kg/m2) | ||||||
| r | 0.172 | 0.093 | 0.081 | 0.359 | 0.307 | 0.312 |
| P value | 0.079 | 0.345 | 0.411 | < 0.001 | 0.002 | 0.001 |
Receiver operating characteristic (ROC) analysis to predict sex by radial artery (RA) diameter
Receiver operating characteristic (ROC) analysis to predict sex by ulnar artery (UA) diameter
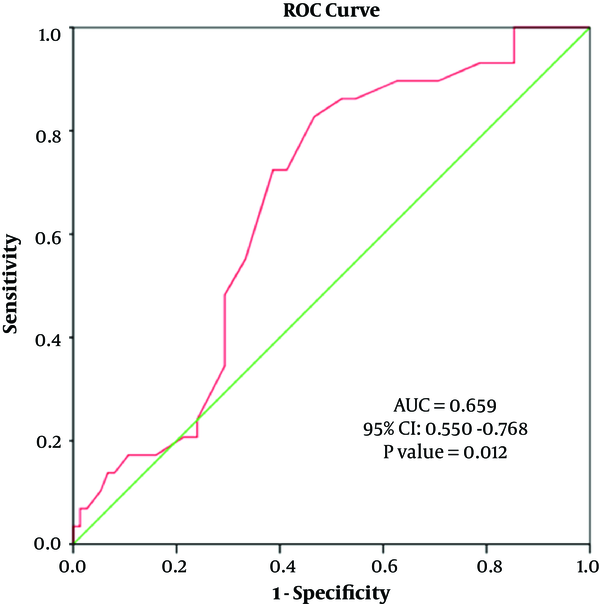
The diagnostic value of BMI in predicting the UA diameter was evaluated using the ROC curve analysis (AUC of ulnar artery diameter = 0.659 (95% CI: 0.550 - 0.768), P = 0.012). A cut-off value of 1.98 mm for UA diameter in participants with a BMI ≥ 25 kg/m2 was associated with a sensitivity of 72.4% and a specificity of 61.3% (Figure 4).
Receiver operating characteristic (ROC) analysis to predict body mass index (BMI) ≥ 25 by ulnar artery (UA) diameter
5. Discussion
The present study found that the diameters, PSV, and VF values of both RA and UA increased during reciprocal compression, compared to baseline values. In addition, the increases in the diameter, PSV and VF of the UA were more prominent compared to the RA-related parameters.
The studies on RA and UA diameters in the literature reported different results. One study using DUS reported no difference between the diameters of the RA and UA in the measurements through the distal wrist (12). In a study, which evaluated 327 patients using DUS, the RA diameter at the wrist level was found to be significantly greater than UA diameter (13). In another study, RA diameter remains unaffected by hand dominance; however, UA diameter was greater in the dominant hand (14). In the present study, RA diameter at the wrist level was greater than UA diameter. In addition diameters of the two arteries were greater in the dominant upper extremity. Therefore, we suggest that UA in the dominant hand can be used as an alternative access site during percutaneous coronary intervention (PCI) in patients with RA occlusion or in those in whom an intervention through the RA fails.
Several studies examined the relationship between sex, race, height, weight, BMI, and RA and UA diameters (6, 14-18). The RA diameter in males was found to be higher than in females. This finding was attributed to the lower height of females (14). Male sex and high BMI were also associated with a greater RA diameter (15). In the good radial artery size predictor (GRASP) study, male sex, wrist circumference, and non-South Asian race were found to be associated with and independent predictors of the greater RA diameter (17). In the present study, RA and UA diameters in males were greater than in females. Thus, PCIs are safer and easier in males than in females. Several studies have shown that the incidence of RAO following PCI is higher in females with low BMI due to presence of a low-caliber RA (15, 18). Although the present study found no effect of BMI on RA diameter, the diameter of UA was greater in subjects with a BMI of ≥ 25 kg/m2.
The use of TRA in cardiac catheterization and interventional procedures has gained wide acceptance in the past decade (19). However, TRA has some limitations such as low caliber of the RA limiting device selection, higher incidence of arterial spasm (10%), and presence of anatomic variations in approximately 5% of the patient population (19). The development of post-procedural occlusion following TRA limits the repetitive use of same artery in the future (9, 20). Moreover, collateral circulation from the UA is insufficient in certain patients. The UA in the opposite hand can be used as an alternative access site in patients developing RAO, particularly in those with insufficient collateral circulation in the UA (20).
A study in the literature reported no ischemic events, despite the occurrence of partial or complete occlusion in more than 25% of patients following TRA (21). Thus, most patients with RAO are asymptomatic (21, 22). Increases in the diameter and flow rates of the other arteries in patients developing RAO or ulnar artery occlusion (UAO) ensure sufficient blood supply to the hand and protect the hand against ischemic events (4). Significant increases in the diameter, velocity and volume flow of the arteries during reciprocal compression of the two arteries in the present study support this assumption. Thus, the use of TUA as an alternative in patients developing RAO, particularly in those with peripheral vascular disease, would avoid transfemoral puncture and difficulties of re-intervention to the already occluded RA.
Blood supply to the forearm completely relies on UA upon removal of the RA for CABG; the studies have observed a steady increase in the flow velocity of UA for 5 to 10 years starting in the early postoperative period (23). If collateral circulation is lack of sufficient reserves to maintain blood supply to the hand at rest after removal of RA, acute hand ischemia develops in the early period, and if collateral circulation is sufficient at rest but lacking sufficient reserves to maintain blood supply to the hand during exercise, then subclinical ischemia and associated exercise intolerance will develop in the same arm in later periods (23). In a series of 3,977 patients reported by Meharwal et al. (24), 5% of the patients developed paresthesia and pulselessness in the early postoperative period after removal of RA and they found weakness in the same hand for four weeks. The decision to perform surgery in this case series was based on AT performed in the preoperative period. In another study, Agrifoglio et al. (10) evaluated hand circulation using DUS during RA compression and the removal of the RA was deemed unsuitable for use in CABG surgery. Therefore, avoiding in 5.3% of the patients, other patients undergoing operation had no hand or wrist ischemia in the postoperative period (25). Abu-Omar used DUS in the presence of abnormal AT to evaluate patients undergoing total arterial revascularization. In preoperative assessment, 38 out of 43 patients with abnormal AT were found to be normal on DUS and no ischemic sequel was observed after removal of the RA (26).
Selection of appropriate arterial conduit for CABG surgery should take into consideration long-life expectancy, particularly in young patients. Hence, we consider that measurement of flow characteristics of RA and UA during reciprocal compression in the preoperative period would be beneficial. The VF calculated by the area under the spectral curve and measurement of diameter would provide sufficient information about circulatory reserves. Therefore, we believe that evaluation of blood supply to the forearm and hand using DUS before PCIs and procedures during which RA is to be used in CABG and assessment of volume flow in addition to flow velocity would provide more reliable and useful data.
Nonetheless, the present study has some limitations. It is a single-center study with a small sample size. Sex and BMI have low specificity and sensitivity in predicting appropriate arterial diameter. Further studies are therefore, required to identify whether changes in the UA volume flow and velocity parameters on DUS in preoperative assessment correlate with postoperative changes in patients undergoing coronary artery bypass graft (CABG) surgery using RA conduit. Such study design would ensure the safety of VF parameter.
In conclusion, our study results showed that an ultrasonographic measurement of diameter, PSV and VF would still be necessary in the preoperative period. The increases in the diameter, PSV, and VF of the RA and UA during reciprocal compression were higher, compared to the increases in the RA. We therefore, suggest that assessment of forearm and wrist arteries in the preoperative period using DUS at rest and during reciprocal compression is a safe and effective method in predicting postoperative hand ischemia, and the use of VF parameter, in addition to PSV would give more accurate data.
References
-
1.
Hahalis G, Tsigkas G, Xanthopoulou I, Deftereos S, Ziakas A, Raisakis K, et al. Transulnar compared with transradial artery approach as a default strategy for coronary procedures: a randomized trial. The Transulnar or Transradial Instead of Coronary Transfemoral Angiographies Study (the AURA of ARTEMIS Study). Circ Cardiovasc Interv. 2013;6(3):252-61. [PubMed ID: 23735472]. https://doi.org/10.1161/CIRCINTERVENTIONS.112.000150.
-
2.
Geng W, Fu X, Gu X, Jiang Y, Fan W, Wang Y, et al. Safety and feasibility of transulnar versus transradial artery approach for coronary catheterization in non-selective patients. Chin Med J (Engl). 2014;127(7):1222-8. [PubMed ID: 24709170].
-
3.
Valgimigli M, Saia F, Guastaroba P, Menozzi A, Magnavacchi P, Santarelli A, et al. Transradial versus transfemoral intervention for acute myocardial infarction: a propensity score-adjusted and -matched analysis from the REAL (REgistro regionale AngiopLastiche dell'Emilia-Romagna) multicenter registry. JACC Cardiovasc Interv. 2012;5(1):23-35. [PubMed ID: 22230147]. https://doi.org/10.1016/j.jcin.2011.08.018.
-
4.
Kwan TW, Ratcliffe JA, Chaudhry M, Huang Y, Wong S, Zhou X, et al. Transulnar catheterization in patients with ipsilateral radial artery occlusion. Catheter Cardiovasc Interv. 2013;82(7):849-55. [PubMed ID: 23008162]. https://doi.org/10.1002/ccd.24662.
-
5.
Hamon M, Pristipino C, Di Mario C, Nolan J, Ludwig J, Tubaro M, et al. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care** and Thrombosis of the European Society of Cardiology. EuroIntervention. 2013;8(11):1242-51. [PubMed ID: 23354100]. https://doi.org/10.4244/EIJV8I11A192.
-
6.
Aykan AC, Hatem E, Kalaycioglu E, Altintas Aykan D, Gokdeniz T, Arslan AO, et al. Prediction of radial artery diameter in candidates for transradial coronary angiography: Is occupation a factor? Turk Kardiyol Dern Ars. 2015;43(5):450-6. [PubMed ID: 26148077]. https://doi.org/10.5543/tkda.2015.75002.
-
7.
Brzezinski M, Luisetti T, London MJ. Radial artery cannulation: a comprehensive review of recent anatomic and physiologic investigations. Anesth Analg. 2009;109(6):1763-81. [PubMed ID: 19923502]. https://doi.org/10.1213/ANE.0b013e3181bbd416.
-
8.
Scheer B, Perel A, Pfeiffer UJ. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit Care. 2002;6(3):199-204. [PubMed ID: 12133178].
-
9.
Rao SV, Bernat I, Bertrand OF. Clinical update: Remaining challenges and opportunities for improvement in percutaneous transradial coronary procedures. Eur Heart J. 2012;33(20):2521-6. [PubMed ID: 22815330]. https://doi.org/10.1093/eurheartj/ehs169.
-
10.
Agrifoglio M, Dainese L, Pasotti S, Galanti A, Cannata A, Roberto M, et al. Preoperative assessment of the radial artery for coronary artery bypass grafting: is the clinical Allen test adequate? Ann Thorac Surg. 2005;79(2):570-2. [PubMed ID: 15680837]. https://doi.org/10.1016/j.athoracsur.2004.07.034.
-
11.
Yadava OP, Dinda AK, Mohanty BK, Mishra R, Ahlawat V, Kundu A. Is radial artery Doppler scanning mandatory for use as coronary bypass conduit? Asian Cardiovasc Thorac Ann. 2015;23(7):822-7. [PubMed ID: 26071450]. https://doi.org/10.1177/0218492315589197.
-
12.
Gokhroo R, Bisht D, Padmanabhan D, Gupta S, Kishor K, Ranwa B. Feasibility of ulnar artery for cardiac catheterization: AJmer ULnar ARtery (AJULAR) catheterization study. Catheter Cardiovasc Interv. 2015;86(1):42-8. [PubMed ID: 25559217]. https://doi.org/10.1002/ccd.25806.
-
13.
Loh YJ, Nakao M, Tan WD, Lim CH, Tan YS, Chua YL. Factors influencing radial artery size. Asian Cardiovasc Thorac Ann. 2007;15(4):324-6. [PubMed ID: 17664207]. https://doi.org/10.1177/021849230701500412.
-
14.
Pancholy SB, Heck LA, Patel T. Forearm arterial anatomy and flow characteristics: a prospective observational study. J Invasive Cardiol. 2015;27(4):218-21. [PubMed ID: 25840406].
-
15.
Velasco A, Ono C, Nugent K, Tarwater P, Kumar A. Ultrasonic evaluation of the radial artery diameter in a local population from Texas. J Invasive Cardiol. 2012;24(7):339-41. [PubMed ID: 22781473].
-
16.
Kotowycz MA, Dzavik V. Radial artery patency after transradial catheterization. Circ Cardiovasc Interv. 2012;5(1):127-33. [PubMed ID: 22338002]. https://doi.org/10.1161/CIRCINTERVENTIONS.111.965871.
-
17.
Kotowycz MA, Johnston KW, Ivanov J, Asif N, Almoghairi AM, Choudhury A, et al. Predictors of radial artery size in patients undergoing cardiac catheterization: insights from the Good Radial Artery Size Prediction (GRASP) study. Can J Cardiol. 2014;30(2):211-6. [PubMed ID: 24461923]. https://doi.org/10.1016/j.cjca.2013.11.021.
-
18.
Kohonen M, Teerenhovi O, Terho T, Laurikka J, Tarkka M. Is the Allen test reliable enough? Eur J Cardiothorac Surg. 2007;32(6):902-5. [PubMed ID: 17889550]. https://doi.org/10.1016/j.ejcts.2007.08.017.
-
19.
Bazemore E, Mann JT. Problems and complications of the transradial approach for coronary interventions: a review. J Invasive Cardiol. 2005;17(3):156-9. [PubMed ID: 15867445].
-
20.
Vassilev D, Smilkova D, Gil R. Ulnar artery as access site for cardiac catheterization: anatomical considerations. J Interv Cardiol. 2008;21(1):56-60. [PubMed ID: 18086137]. https://doi.org/10.1111/j.1540-8183.2007.00314.x.
-
21.
Rashid M, Kwok CS, Pancholy S, Chugh S, Kedev SA, Bernat I, et al. Radial Artery Occlusion After Transradial Interventions: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2016;5(1). [PubMed ID: 26811162]. https://doi.org/10.1161/JAHA.115.002686.
-
22.
Wagener JF, Rao SV. Radial artery occlusion after transradial approach to cardiac catheterization. Curr Atheroscler Rep. 2015;17(3):489. [PubMed ID: 25651786]. https://doi.org/10.1007/s11883-015-0489-6.
-
23.
Gaudino M, Anselmi A, Serricchio M, Flore R, Santoliquido A, Gerardino L, et al. Late haemodynamic and functional consequences of radial artery removal on the forearm circulation. Int J Cardiol. 2008;129(2):255-8. [PubMed ID: 17988752]. https://doi.org/10.1016/j.ijcard.2007.07.096.
-
24.
Meharwal ZS, Trehan N. Functional status of the hand after radial artery harvesting: results in 3,977 cases. Ann Thorac Surg. 2001;72(5):1557-61. [PubMed ID: 11722043].
-
25.
Ronald A, Patel A, Dunning J. Is the Allen's test adequate to safely confirm that a radial artery may be harvested for coronary arterial bypass grafting? Interact Cardiovasc Thorac Surg. 2005;4(4):332-40. [PubMed ID: 17670425]. https://doi.org/10.1510/icvts.2005.110247.
-
26.
Abu-Omar Y, Mussa S, Anastasiadis K, Steel S, Hands L, Taggart DP. Duplex ultrasonography predicts safety of radial artery harvest in the presence of an abnormal Allen test. Ann Thorac Surg. 2004;77(1):116-9. [PubMed ID: 14726046].