Abstract
Background:
Some chemokines like C C motif chemokine ligand (CCL) 2 and 5 and their receptors (CCR) 2 and 5 are mediators of chronic inflammation and cancer development. Moreover, physical exercise can increase the activity of antioxidant enzymes. However, its effect on cancer cells has not been reported at present.Objectives:
Therefore, the present study aimed to ascertain the effect of 12-week aerobic exercise training (AET) on CCL2, CCR2, CCL5, and CCR5 in mice with breast cancer.Methods:
Sixteen Balb/c mice aged 4 - 5 weeks (n = 16; approximate weight: 18 ± 2 g) were divided into two groups: AET group (AETG) and control group (CG) (n = 8 per group). The AETG performed 12-week treadmill running at 18 m/min for 40 min and five times a week. Plasma levels of CCL2 and CCL5 were measured by ELISA, and the CCR2 and CCR5 were evaluated by Western blotting. Two independent sample t-test was applied to compare the differences between AETG and CG.Results:
The analysis displayed after 12 weeks showed a significant reduction in AETG compared to CG in CCL2 (3.94 ± 1.12 vs. 15.40 ± 3.29 pg/mL; P = 0.001), CCR2 (0.56 ± 0.19 vs. 1.00 ± 0.001; P = 0.002), CCL5 (138.59 ± 15.72 vs. 267.57 ± 49.06 ng/mL; P = 0.001) and CCR5 (0.36 ± 0.12 vs. 1.00 ± 0.001; P = 0.001), respectively.Conclusions:
We concluded that one of the main mechanisms of a positive effect of exercise on breast cancer is reducing the inflammation via CCL2 and CCL5 and their related receptors CCR2 and CCR5, respectively. Since these molecules can be triggered off oxidative stress and tumorigenesis, these results can pave the way for further studies in this field.Keywords
Cancer Physical Activity Chronic Inflammation Chemokines Oxidative Stress, Enzymes
1. Background
Breast cancer (BC) is the most common cancer in women, and it is generally the second most common cancer worldwide (WHO) (1, 2). Due to the role of tumor microenvironment components in most malignancies, special attention is paid to inflammatory mediators such as cytokines and their respective receptors and oxidative stress (3-5). This pro-inflammatory such as tumor necrosis factor (TNF)-α, interleukin (IL-1β, IL-6, IL-12 and IL-17) cytokines promote pyrogenesis and acute inflammatory responses such as leukocytosis (neutrophilia), by promoting granulocyte colony-stimulating factor (G-CSF) and chemokines (chemotactic cytokines) such as IL-8 and monocyte chemotactic protein (MCP)-1 (6, 7). Oxidative stress may activate several transcription factors, such as activator protein 1 (AP-1), nuclear factor kappa-light chain-activated B cell enhancer (NF-kB), and the tumor protein p53. The activation of these factors may lead to the expression of more than 500 different genes, such as anti-inflammatory molecules, cell cycle regulatory molecules, chemokines, inflammatory cytokines, and growth factors (8). Cancer cells have arisen from cellular redox imbalance due to oxidative stress (8). Inflammation caused by adipose tissue dysfunction in obesity is associated with increased free radicals and oxidative stress. This may indicate a direct link between obesity and tumor growth that leads to cancer (9).
It has been shown that the effect of chemokines on malignancy can be in various ways, enhancing tumor growth or metastasis (10-12). The biological effects of chemokines include invoking host immune cells to the site of infection, regulating lymphocytes and other leukocytes, transmitting through peripheral lymph tissues, and developing non-lymphatic organs (13). Many BC researchers paid special attention to C C motif chemokine ligand (CCL) 2, MCP-1, and CCL5 (regulated on activation, normal T expressed and secreted: RANTES) (14). CCL2 binds specifically to the chemokine receptor (CCR) 2 (15-17), through CCR2 it activates down (18) stream signaling pathways, including mitogen-activated protein kinases (p42/p44MAPK), phospholipase C-γ, and protein kinase (PKC) through G protein-dependent mechanisms in regulating cell adhesion and motility (19-21). CCL5 production is concerned with developing appropriate immune responses against tumors (22, 23). CCL5, in turn, is connected with cancer progression and metastasis. CCL5/CCR5 interaction can produce tumor growth in several ways, such as recruitment of additional stromal and inflammatory cells, regulation of extracellular matrix, angiogenesis, activation of growth factors, and involvement in immune escape mechanisms (24).
While practicing exercise, mitochondria´s inner membrane is a major source of reactive oxygen species (ROS), and exercise´s volume and intensity condition the production of free radicals that may lead to various levels of oxidative damage (25). Cytokine-induced inflammation has been related to increased ROS production and the development of BC (26). Comparing two legs of healthy individuals with one-way training has shown that exercise can gradually increase the antioxidant enzyme activity of the target tissue in exercise compared to untrained tissue. These local changes indicate the effect of exercise on the antioxidant capacity of healthy individuals, but there are currently no reports of cancer cells (27).
There are also a small number of studies on oxidative stress and exercise in cancer patients. In these studies, conflicting results of increasing or decreasing oxidative stress conditions have been reported, and studies have not yet reached an agreement (28). A previous report mentioned that extracellular in-vivo levels of CCL5 and CCL2 were 3 to 5 times higher in women´s cancerous tissue with BC than in normal breast tissue (18). According to Troseid et al., aerobic exercise training (AET) could lower plasma levels of CCL2 in patients with metabolic syndrome (29). Therefore, considering the scarce studies carried out on the effect of AET on CCL2 and CCL5 and their receptors (i.e., CCR2 and CCR5), further research is needed to shed light on this aspect.
2. Objectives
The present study was conducted to answer whether AET affects CCL2, CCL5, and their receptors.
3. Methods
3.1. Laboratory Animals
The present research was experimental with a post-test design with an AET group (AETG) and a control group (CG). Four to five weeks Balb/c mice (n = 16), weighing approximately 18 ± 2 g, were maintained under standard light conditions (12 hours of daylight and 12 hours of darkness), a constant temperature of 23 ± 1°C and a relative humidity of 50 ± 3% in cages. The animals received rodent pellet diet and had permanent access to water. Food intake was measured on a daily basis.
3.2. Cell Culture
4T1 BC cell line was purchased from Iranian Genetic Resources (Tehran, IRAN) and cultured according to the American type culture collection (ATCC) protocol. The cells were transferred to 25 cm2 flasks and passaged in the Roswell Park Memorial Institute (RPMI) 1640 medium following their growth. For the passage of cells, the culture medium was first evacuated; then, the cells were washed each time with 2 mL of the PBS solution and then incubated with trypsin (1 mL) for 15 min to detach the cells, and culture medium was first evacuated; then, the cells were washed each time with 2 mL of the PBS solution and then incubated with trypsin (1 mL) for 15 min to detach the cells from the flask. Then, 10 mL of the culture medium containing the 10% FBS serum was added and centrifuged at 1000 rpm for 10 min, the cell pellet was flashed, and the cells were discarded in flasks. Consequently, the cells were transferred to 25 cm2 flasks for passage. They were kept in the CO2 incubator with 97% humidity, 5% CO2, and a temperature of 37°C for 1 week; the culture medium was completely changed every two days. The culture medium consisted of RPMI 1640 + FBS 10% + 1% NEAA + 1% Pen-Str. Then, each of the anesthetized Balb/c mice was anesthetized with an appropriate dose of ketamine and Xylazine (10 mg to 1 mg), and 2 × 106 cells in 200 μL of PBS were subcutaneously injected into the upper right thigh of the animal. About 10 - 20 days after cancer cell injection (second 4-week period of AETG) the tumor was appreciable in the injected area (30).
3.3. Aerobic Exercise Training Program
One week after adaptation to the environment, the mice were randomly assigned into two groups of 8 per cage, and animals were familiarized with the treadmill activities for two weeks (6 - 18 m/min for 10 min). AETG was performed for 3-4 weeks’ continuous periods in both cancerous and non-cancerous called CG. The tumor was formed in the second 4-week period. AETG was performed with the intensity of 40 - 50% of maximal oxygen consumption. For all groups, AETG at a speed of 18 m/min was performed for 20 min, five times per week, at 0 degrees (31). It should be noted that to create completely identical conditions, the CG was placed on the immobilized treadmill under the same conditions. During the tumor growth, 2 × 106 cells from the 4T1 cell line were subcutaneously injected into the upper right thigh. The injection was made with the mice anesthetized and utilizing an adequate dose of ketamine and xylazine. After 3 - 4 weeks, the tumor was palpable under their skin. The tumor was weighed after extraction under sterile conditions. It was then frozen in liquid nitrogen. Tissue samples were preserved at -70°C for subsequent processes. The tumor size was measured longitudinally and transversely. After tumor appearance, tumor length and width were measured with a digital caliper weekly. We used the following formula to estimate the tumor volume [V = π/6 (w × L 2)]. Maximum tumor volume was reported to be 1851.67 mm3. Moreover, the tumor of the mice was not damaged during the experiment, and the intensity of the exercise was controlled and moderate. Thus, the mice did not die during the intervention. Additionally, no shocker was used to avoid stressing the animals, and we used the tail-touching method. Free access to water and food was also provided. Finally, all mice were anesthetized using ketamine (90 mg/kg) and xylazine (10 mg/kg) to be sacrificed at the end of the training interval. Then, their tissue and serum were harvested.
3.4. Sampling and Analysis (ELISA & Western Blot)
After the last session, animals were anesthetized using ketamine + tyrosine injection, and their blood samples were collected using heparin from the large underlying vein. Plasma was separated after centrifugation at 10,000 g (for 10 min) and stored at -80°C before being analyzed. Plasma CCL2 and CCL5 levels were examined utilizing the enzyme-linked immunosorbent assay (ELISA) kits (Minneapolis MN R&D Systems).
Tumors were excised from the dermis and adnexal tissues. They were then homogenized with protein extraction solution (PRO-PREPTM, Intron Biotechnology, Seoul, Korea) and 10 mg/mL proteinase inhibitor (Roche) before incubating them at 4°C with gentle agitation between 2 and 3 h. The samples were then centrifuged in an Eppendorph 5415 R centrifuge at 4°C at 12,000 rpm for 10 min. The supernatant containing the protein was extracted and used for Western blotting. Protein concentration was estimated by Bradford protein assay.
A total of 20 micrograms of protein per sample were separated and transferred onto a nitrocellulose membrane on a 15% SDS-PAGE gel. Blots were blocked for one h at room temperature using blocking solution (Roche) and next incubated with primary antibody, CCR2 (anti-mouse or human) and CCR5 (anti-mouse or human) (all 1: 1000, Abcam) overnight at 4°C along with a control (β-actin 1: 1000, Abcam) followed by washing with 0.1% TBST solution three times, each time 5 min with vigorous shaking. The paper was next incubated with HRP-conjugated secondary antibody for one hour (1: 3000, Abcam). The paper was coated with the ECL kit and observed using the radiology film. The j software image was used to convert the Western blot images into quantitative data.
3.5. Ethics Issue
All the experiments were approved by the Ethical Committee of the University of Isfahan (approval number: IR.UI.REC.1399.004, Iran). All institutional, national, and international recommendations were applied in the care and use of the animals.
3.6. Statistical Methods
The normality of the data was verified using the Shapiro-Wilk test and the homoscedasticity with Levene’s test. After ensuring the normality of the data and checking the homogeneity, the t-test for independent samples was used to compare the means of both groups. The significance level was considered at P ≤ 0.05. The statistical analysis was conducted using the SPSS statistical software version 22 (22.0; IBM SPSS Inc., Chicago, IL, USA)
4. Results
Means and standard deviations of the CCL2, CCL5, CCR2, and CCR5 levels in the test for AETG and CG are presented in Table 1.
Mean and Standard Deviation of the Research Variables in the AETG and CG
| Variables | AETG | CG | T | P |
|---|---|---|---|---|
| CCL2 (pg/mL) | 3.94 ± 1.12 | 15.40 ± 3.29 | 8.07 | 0.001 |
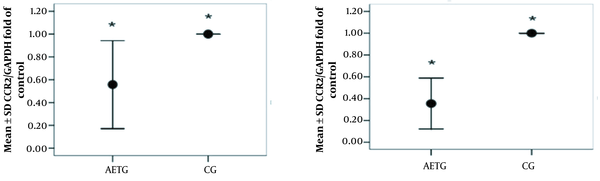
| CCR2 fold of control | 0.56 ± 0.19 | 1.00 ± 0.001 | 5.64 | 0.002 |
| CCL5 (ng/mL) | 138.59 ± 15.72 | 267.57 ± 49.06 | 6.13 | 0.001 |
| CCR5 fold of control | 0.36 ± 0.12 | 1.00 ± 0.001 | 13.55 | 0.001 |
Independent t-test results showed that there was a significant difference in CCL2 level (t = 8.07, P = 0.001) and CCL5 level (t = 6.13, P = 0.001) between the AETG and CG (Figure 1). According to the results of the t-test analysis, a significant difference between the groups was also observed in terms of CCR2 level (t = 5.64, P = 0.002) and CCR5 (t = 13.55, P = 0.001) (Figure 2).
ELISA results of chemokine ligand (CCL) 2 and CCL5 levels in the aerobic exercise training group (AETG) and control group (CG); * P < 0.05.
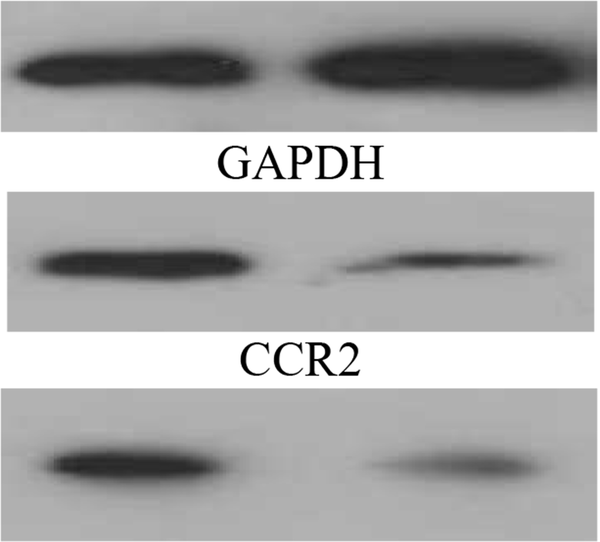
Western blot results of chemokine ligand (CCR) 2 and CCR5 levels in the aerobic exercise training group (AETG) and control group (CG); * P < 0.05.
5. Discussion
This study aimed to evaluate the effect of 12-week AET on CCL2, CCR2, CCL5, and CCR5 levels in the tumor cells of mice suffering from BC. According to the results, after 12 weeks of AETG, significant decreases in CCL2, CCL5, CCR2, and CCR5 levels were observed compared to CG.
Physical activity (PA) can prevent the progression and metastasis of cancer by inhibiting interleukin 6 (IL-6), TNF-α, and CCL2 activity (32). Previous studies suggest that co-expression of CCL2, CCL5, TNF-α, and IL-1β may be important for disease metastasis and that TNF-α and IL-1β may increase disease return (33).
In a study by Mandal et al., there was a significant decrease in CCL2, CCL5, and CCL2 levels and a decrease in the number of metastatic lung nodes using an inhibitor in a Balb/c mouse model of 4T1 cancer (34). Carlin et al. found that PA inhibits the gene expression of the CCL2 and CXCL10 chemokines in the cortex (35). In another study, Chiu et al. showed that high-grade cancer cells expressed higher levels of CCL2 than low-grade cancer and had greater migration. Although activation of CCL2/CCR2 signals does not significantly affect cell growth, it induces cell migration and invasion through activation of protein kinase C and phosphorylation and tyrosine in Paxillin. It has been reported that CCL2 and CCR2 blocking with shCCL2 inhibited cell migration via CCL2/CCR2 (36). In the study of Saji et al., a significant correlation was found between CCL2 and the progression of BC. The expression of CCL2 in tumor cells was found to have a significant correlation with the expression of thymidine phosphorylation and the type of membrane matrix-1 metalloproteinase, which showed that CCL2 might play a key role in macrophages, in the expression of angiogenic factors and matrix metalloproteinase activation in patients with BC (37).
In a study by Goh et al., they found that pre-tumor exercise reduces BC in older mice (38). PA is a moderating factor in lifestyle that reduces the risk of BC and improves the quality of life after diagnosing BC (39, 40). Exercise reduces the expression of Toll-like receptors (TLRs) in monocytes and macrophages by reducing visceral fat mass, increasing the production and release of inflammatory cytokines and reactive oxygen species, and ultimately preventing macrophages from penetrating adipose tissue, which can prevent the progression of chronic inflammation. Inflammation is a key event in the growth and spread of BC and other types of tumors. There is a clear link between cancer and inflammation. Therefore, the prevention of inflammation is a potential goal in preventing tumor progression and treating cancer (41).
In recent years, new areas of PA have emerged in developed countries that look at sports as a therapeutic approach (41). In connection with cancer, research results show that exercise has positive psychological and immunological effects in the treatment of patients with cancer. Evidence suggests that PA reduces the risk of various malignant tumors such as colon, breast, prostate, uterine, and lung cancers (42). Exercise has a special role in preventing chronic diseases, especially BC, by strengthening the immune system and reducing body fat percentage (41-43). Epidemiological evidence suggests that increasing PA is related to a reduced risk of BC. Thus, rates of BC in women who exercise regularly are estimated to be 70% lower than in sedentary women (44). Randomized clinical trials on the positive effects of PA on cardiopulmonary and biological function show that exercise reduces sex hormones, body fat mass, insulin, insulin-like growth factor-1 (IGF-1), adipocytokines, and mammographic density, as well as improves immune function (45, 46). PA can decrease insulin resistance in the liver and muscle and increase glucose metabolism through various mechanisms, such as increasing post-insulin receptor signaling. This decrease in insulin resistance may decrease circulating insulin levels, decreasing the available IGF-1 through insulin-induced changes in IGFBP levels (47). Obesity leads to increased inflammation, and exercise can improve some of the negative health outcomes associated with obesity (35, 41). Hyperlipidemia can increase tumor cells by inducing chronic inflammation and mitogenic action of cholesterol in the tumor. Exercise may reduce chronic inflammation, lower serum lipids, enhance the immune system's ability to detect and kill tumor cells (41), and by normalizing the tumor vascular network, reduce hypoxia to counteract the effect of the tumor, and as a result, may inhibit BC progression (48).
Finally, it is necessary to mention the strengths and limitations of the present study. As for the strengths, unlike previous studies that analyzed the antioxidant capacity of aerobic exercise on healthy individuals, the current work examines the antioxidant effect of aerobic exercise training on cancer cells. Furthermore, it is a randomized controlled study conducted under laboratory conditions with mice to ensure the genetic homogeneity of the subjects. Regarding the limitations, since the existing studies on the effect of exercise in cancer patients focused on its preventive ability rather than its anti-inflammatory capacity, the scope of the results of the present study cannot be compared with previous randomized controlled trials. Similarly, since there was only one intervention group, it was not possible to verify the effect of different exercise regimens. Therefore, future studies might contrast the impact of different physical activity programs to establish the appropriate exercise dose to maximize its anti-inflammatory effects.
5.1. Conclusions
One of the main positive effects of exercise on BC is the reduction of CCL2 and CCL5 chemokines and their receptors. Also, the role of oxidative stress in cancer, particularly BC, has been investigated to identify better the factors involved in the onset and spread of cancer and redefine treatment targets. This significant result, which can pave the way for further studies in this field, has been obtained for the first time in the present study.
References
-
1.
Akbari A, Razzaghi Z, Homaee F, Khayamzadeh M, Movahedi M, Akbari ME. Parity and breastfeeding are preventive measures against breast cancer in Iranian women. Breast Cancer. 2011;18(1):51-5. [PubMed ID: 20217489]. https://doi.org/10.1007/s12282-010-0203-z.
-
2.
Burkman RT. Berek & Novak’s Gynecology. Jama. 2012;308(5):516. https://doi.org/10.1001/jama.308.5.516.
-
3.
Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66(1):1-9. [PubMed ID: 17913510]. https://doi.org/10.1016/j.critrevonc.2007.07.004.
-
4.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539-45. [PubMed ID: 11229684]. https://doi.org/10.1016/s0140-6736(00)04046-0.
-
5.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860-7. [PubMed ID: 12490959]. [PubMed Central ID: PMCPmc2803035]. https://doi.org/10.1038/nature01322.
-
6.
Suzuki K. Cytokine Response to Exercise and Its Modulation. Antioxidants. 2018;7(1):17. https://doi.org/10.3390/antiox7010017.
-
7.
Ruhee RT, Suzuki K. The Integrative Role of Sulforaphane in Preventing Inflammation, Oxidative Stress and Fatigue: A Review of a Potential Protective Phytochemical. Antioxidants (Basel). 2020;9(6). [PubMed ID: 32545803]. [PubMed Central ID: PMC7346151]. https://doi.org/10.3390/antiox9060521.
-
8.
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603-16. [PubMed ID: 20840865]. [PubMed Central ID: PMCPmc2990475]. https://doi.org/10.1016/j.freeradbiomed.2010.09.006.
-
9.
Crujeiras AB, Díaz-Lagares A, Carreira MC, Amil M, Casanueva FF. Oxidative stress associated to dysfunctional adipose tissue: a potential link between obesity, type 2 diabetes mellitus and breast cancer. Free Radic Res. 2013;47(4):243-56. [PubMed ID: 23409968]. https://doi.org/10.3109/10715762.2013.772604.
-
10.
Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev. 2006;25(3):357-71. [PubMed ID: 17016763]. https://doi.org/10.1007/s10555-006-9003-5.
-
11.
Conti I, Rollins BJ. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol. 2004;14(3):149-54. [PubMed ID: 15246049]. https://doi.org/10.1016/j.semcancer.2003.10.009.
-
12.
Zlotnik A. Involvement of chemokine receptors in organ-specific metastasis. Contrib Microbiol. 2006;13:191-9. [PubMed ID: 16627966]. https://doi.org/10.1159/000092973.
-
13.
Palomino DC, Marti LC. Chemokines and immunity. Einstein (Sao Paulo). 2015;13(3):469-73. [PubMed ID: 26466066]. [PubMed Central ID: PMCPmc4943798]. https://doi.org/10.1590/s1679-45082015rb3438.
-
14.
Ben-Baruch A. The Tumor-Promoting Flow of Cells Into, Within and Out of the Tumor Site: Regulation by the Inflammatory Axis of TNFα and Chemokines. Cancer Microenviron. 2012;5(2):151-64. [PubMed ID: 22190050]. [PubMed Central ID: PMCPmc3399063]. https://doi.org/10.1007/s12307-011-0094-3.
-
15.
Ernst CA, Zhang YJ, Hancock PR, Rutledge BJ, Corless CL, Rollins BJ. Biochemical and biologic characterization of murine monocyte chemoattractant protein-1. Identification of two functional domains. J Immunol. 1994;152(7):3541-9. [PubMed ID: 8144933].
-
16.
Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192(6):899-905. [PubMed ID: 10993920]. [PubMed Central ID: PMCPmc2193286]. https://doi.org/10.1084/jem.192.6.899.
-
17.
Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193(6):713-26. [PubMed ID: 11257138]. [PubMed Central ID: PMCPmc2193420]. https://doi.org/10.1084/jem.193.6.713.
-
18.
Svensson S, Abrahamsson A, Rodriguez GV, Olsson AK, Jensen L, Cao Y, et al. CCL2 and CCL5 Are Novel Therapeutic Targets for Estrogen-Dependent Breast Cancer. Clin Cancer Res. 2015;21(16):3794-805. [PubMed ID: 25901081]. https://doi.org/10.1158/1078-0432.ccr-15-0204.
-
19.
Jiménez-Sainz MC, Fast B, Mayor FJ, Aragay AM. Signaling pathways for monocyte chemoattractant protein 1-mediated extracellular signal-regulated kinase activation. Mol Pharmacol. 2003;64(3):773-82. [PubMed ID: 12920215]. https://doi.org/10.1124/mol.64.3.773.
-
20.
Johnson Z, Power CA, Weiss C, Rintelen F, Ji H, Ruckle T, et al. Chemokine inhibition--why, when, where, which and how? Biochem Soc Trans. 2004;32(Pt 2):366-77. [PubMed ID: 15046611]. https://doi.org/10.1042/bst0320366.
-
21.
Mellado M, Rodríguez-Frade JM, Aragay A, del Real G, Martín AM, Vila-Coro AJ, et al. The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J Immunol. 1998;161(2):805-13. [PubMed ID: 9670957].
-
22.
Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309-22. [PubMed ID: 22439926]. https://doi.org/10.1016/j.ccr.2012.02.022.
-
23.
Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267(2):271-85. [PubMed ID: 18439751]. https://doi.org/10.1016/j.canlet.2008.03.018.
-
24.
Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13(8):525-41. [PubMed ID: 23880905]. https://doi.org/10.1038/nrc3565.
-
25.
Nobari H, Nejad HA, Kargarfard M, Mohseni S, Suzuki K, Carmelo Adsuar J, et al. The Effect of Acute Intense Exercise on Activity of Antioxidant Enzymes in Smokers and Non-Smokers. Biomolecules. 2021;11(2). [PubMed ID: 33513978]. https://doi.org/10.3390/biom11020171.
-
26.
Roque AT, Gambeloni RZ, Felitti S, Ribeiro ML, Santos JC. Inflammation-induced oxidative stress in breast cancer patients. Med Oncol. 2015;32(12):263. [PubMed ID: 26541769]. https://doi.org/10.1007/s12032-015-0709-5.
-
27.
Parise G, Phillips SM, Kaczor JJ, Tarnopolsky MA. Antioxidant enzyme activity is up-regulated after unilateral resistance exercise training in older adults. Free Radic Biol Med. 2005;39(2):289-95. [PubMed ID: 15964520]. https://doi.org/10.1016/j.freeradbiomed.2005.03.024.
-
28.
Repka CP, Hayward R. Oxidative Stress and Fitness Changes in Cancer Patients after Exercise Training. Med Sci Sports Exerc. 2016;48(4):607-14. [PubMed ID: 26587845]. [PubMed Central ID: PMCPmc4979000]. https://doi.org/10.1249/mss.0000000000000821.
-
29.
Trøseid M, Lappegård KT, Claudi T, Damås JK, Mørkrid L, Brendberg R, et al. Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur Heart J. 2004;25(4):349-55. [PubMed ID: 14984925]. https://doi.org/10.1016/j.ehj.2003.12.006.
-
30.
Shalamzari SA, Agha-Alinejad H, Alizadeh S, Shahbazi S, Khatib ZK, Kazemi A, et al. The effect of exercise training on the level of tissue IL-6 and vascular endothelial growth factor in breast cancer bearing mice. Iran J Basic Med Sci. 2014;17(4):231-58. [PubMed ID: 24904714]. [PubMed Central ID: PMCPmc4046231].
-
31.
Al-Jarrah M, Pothakos K, Novikova L, Smirnova IV, Kurz MJ, Stehno-Bittel L, et al. Endurance exercise promotes cardiorespiratory rehabilitation without neurorestoration in the chronic mouse model of parkinsonism with severe neurodegeneration. Neuroscience. 2007;149(1):28-37. [PubMed ID: 17869432]. [PubMed Central ID: PMCPmc2099399]. https://doi.org/10.1016/j.neuroscience.2007.07.038.
-
32.
Hou N, Ndom P, Jombwe J, Ogundiran T, Ademola A, Morhason-Bello I, et al. An epidemiologic investigation of physical activity and breast cancer risk in Africa. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2748-56. [PubMed ID: 25242052]. https://doi.org/10.1158/1055-9965.epi-14-0675.
-
33.
Soria G, Ofri-Shahak M, Haas I, Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T, et al. Inflammatory mediators in breast cancer: coordinated expression of TNFα & IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition. BMC Cancer. 2011;11:130. [PubMed ID: 21486440]. [PubMed Central ID: PMCPmc3095565]. https://doi.org/10.1186/1471-2407-11-130.
-
34.
Mandal PK, Biswas S, Mandal G, Purohit S, Gupta A, Majumdar Giri A, et al. CCL2 conditionally determines CCL22-dependent Th2-accumulation during TGF-β-induced breast cancer progression. Immunobiology. 2018;223(2):151-61. [PubMed ID: 29107385]. https://doi.org/10.1016/j.imbio.2017.10.031.
-
35.
Carlin JL, Grissom N, Ying Z, Gomez-Pinilla F, Reyes TM. Voluntary exercise blocks Western diet-induced gene expression of the chemokines CXCL10 and CCL2 in the prefrontal cortex. Brain Behav Immun. 2016;58:82-90. [PubMed ID: 27492632]. [PubMed Central ID: PMCPmc5352157]. https://doi.org/10.1016/j.bbi.2016.07.161.
-
36.
Chiu HY, Sun KH, Chen SY, Wang HH, Lee MY, Tsou YC, et al. Autocrine CCL2 promotes cell migration and invasion via PKC activation and tyrosine phosphorylation of paxillin in bladder cancer cells. Cytokine. 2012;59(2):423-32. [PubMed ID: 22617682]. https://doi.org/10.1016/j.cyto.2012.04.017.
-
37.
Saji H, Koike M, Yamori T, Saji S, Seiki M, Matsushima K, et al. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer. 2001;92(5):1085-91. [PubMed ID: 11571719]. https://doi.org/10.1002/1097-0142(20010901)92:5<1085::aid-cncr1424>3.0.co;2-k.
-
38.
Goh J, Endicott E, Ladiges WC. Pre-tumor exercise decreases breast cancer in old mice in a distance-dependent manner. Am J Cancer Res. 2014;4(4):378-84. [PubMed ID: 25057440]. [PubMed Central ID: PMCPmc4106655].
-
39.
Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(11):815-40. [PubMed ID: 22570317]. [PubMed Central ID: PMCPmc3465697]. https://doi.org/10.1093/jnci/djs207.
-
40.
Löf M, Bergström K, Weiderpass E. Physical activity and biomarkers in breast cancer survivors: a systematic review. Maturitas. 2012;73(2):134-42. [PubMed ID: 22840658]. https://doi.org/10.1016/j.maturitas.2012.07.002.
-
41.
Suzuki K. Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules. 2019;9(6). [PubMed ID: 31181700]. [PubMed Central ID: PMCPmc6628010]. https://doi.org/10.3390/biom9060223.
-
42.
Na HK, Oliynyk S. Effects of physical activity on cancer prevention. Ann N Y Acad Sci. 2011;1229:176-83. [PubMed ID: 21793853]. https://doi.org/10.1111/j.1749-6632.2011.06105.x.
-
43.
Amani-Shalamzari S, Aghaalinejad H, Alizadeh SH, Kazmi AR, Saei MA, Minayi N, et al. [The effect of endurance training on the level of tissue IL-6 and VEGF in mice with breast cancer]. J Shahrekord Univ Med Sci. 2014;16(2):10-21. Persian.
-
44.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-86. [PubMed ID: 25220842]. https://doi.org/10.1002/ijc.29210.
-
45.
Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. Bmj. 2012;344. e70. [PubMed ID: 22294757]. [PubMed Central ID: PMC3269661]. https://doi.org/10.1136/bmj.e70.
-
46.
Fernández Ortega JA, de Paz Fernández JA. Cáncer de mama y ejercicio físico: Revisión. Hacia la Promoción de la Salud. 2012;17(1):135-53. Spanish.
-
47.
Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2003;12(8):721-7. [PubMed ID: 12917202].
-
48.
Buss LA, Dachs GU. The Role of Exercise and Hyperlipidaemia in Breast Cancer Progression. Exerc Immunol Rev. 2018;24:10-25. [PubMed ID: 29461968].
