Abstract
Context:
Gut microbiota composition plays a pivotal role in health and emerging evidence supports the ability of exercise training programs to alter the gut microbiota’s composition and function, which could counteract dysbiosis and the effects of chronic diseases. This study aims to investigate the effectiveness of different exercise methods on the composition of intestinal microbiota.Methods:
We searched for published peer-reviewed articles in PubMed and Google Scholar databases up until January 2023. We searched using the terms "exercise", "education", "microbiome" and "microbiota".Results:
By electronic search until January 2023 in the databases with keywords made in Mesh by mentioning Title/Abstract, several 1814 articles were collected. By repeating and performing filtering at each stage, 15 clinical trials were finally left in the study. The results showed varying degrees of efficacy and high inter-individual variations. In conclusion, the baseline microbiome profile was shown to have a decisive role in microbiome responsiveness to training intervention and training dose and duration seem to be a determining factor in all exercise modalities.Conclusions:
In general, can be said that exercise can balance gut microbiota (GM). More importantly, exercise is proposed to present a stressor to the gut that stimulates beneficial adaptations and improves long-term gut barrier flexibility over time through regular physical activity. It seems that the GM changes caused by aerobic exercise are reversible after returning to a sedentary lifestyle. Therefore, it is recommended that exercise initially causes a disrupts in GM that, with continued exercise and thus adaptation, revenues to the pre-exercise state.Keywords
Gut Microbiota Exercise Aerobic Exercise Resistance Training Combined Training
1. Context
Gut microbiota (GM) composition can influence human metabolism and has been shown to influence metabolic health and decrease the risk of metabolic diseases (1). Exercise is increasingly reported to positively influence GM by improving the diversity and abundance of health-promoting species, and altering its functional capacity through increasing metabolites (2).
However, the physiologic adaptations to different types of exercise such as resistance and aerobic training are distinctly different and they elicit several pathways in our body (3). Because of the differences in metabolic pathways and the fuel source utilized in these exercise modalities, their impact on the human GM might be different (4). For example, the contribution of anaerobic energy in resistance exercises is predominant (5) and it has been shown that high-intensity anaerobic exercises produce lactate which enters the gut lumen through circulation and provides a selective advantage for lactate-utilizing species (6). Moreover, aerobic exercise specifically enhances cardiorespiratory fitness which is associated with greater diversity in GM (3).
Gut microbiota can be effective in regulating energy metabolism, hydration, inflammatory response, and oxidative stress (7). Exercise can affect the GM due to its metabolic and immune effects. Exercise-induced changes in GM may depend on the type of exercise (8). The effect of physical activity on GM has been investigated in many studies, most of which are animal models that have investigated the effect of a specific exercise with a food combination (7, 9, 10). This study is a pulmonary systemic investigation that examines the effect of various exercise interventions on GM in human populations.
2. Methodology
2.1. Type of Study
The present study is a secondary study of review type. How to conduct the study is shown in Figure 1.
Steps for including articles in the review
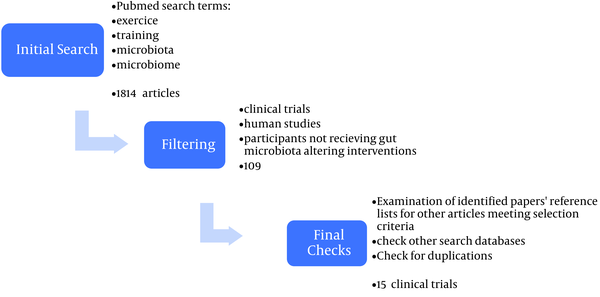
2.2. Search Methodology
A PubMed search using the terms “exercise”, “training”, “microbiome” and “microbiota” was conducted for articles published until January 2023. The secondary search was extended to the Google Scholar database. To ensure that all relevant literature was included, the reference lists of identified articles were also searched, and relevant papers were identified.
2.3. Search Strategy
In this study, we classified exercises into three classes (aerobic, resistance (strength) training, or combined aerobic and resistance training) and their effects on GM characteristics (alpha diversity, beta diversity, changes in microbial composition and abundance, and metabolites produced by specific species). Then, the keywords used in this search were combined with the medical subject profiles (Mesh) and with the abstract and title (Table 1).
Searched Keywords and Terms
| Search Strategy | ||
|---|---|---|
| Keyword | Aerobic, Resistance (strength) Training, Combined Aerobic , Exercise and Resistance Training | microbiome, microbiota, alpha diversity, beta diversity, changes in microbial composition and abundance, and metabolites produced by specific species |
| Search terms | (Aerobic OR Resistance Training OR Combined Aerobic OR Exercise OR Resistance Training) AND (microbiota OR microbiome OR alpha diversity OR beta diversity OR changes in microbial composition OR metabolites produced by specific species) | |
2.4. Inclusion Criteria
In this study, only longitudinal studies were examined.
2.5. Exclusion Criteria
Studies, where intervention participants received specific medications, supplements, or diets (other than exercise intervention) were excluded (11).
2.6. Quality Assessment
To evaluate the quality of the articles, the Jadad scale checklist was used due to its ability to score quantitatively (12).
3. Results
By electronic search until January 2023 in the databases with keywords made in Mesh by mentioning Title/Abstract, several 1814 articles were collected. By repeating and performing filtering at each stage, 15 clinical trials were finally left in the study (Figure 1). A summary of the characteristics and findings of the included studies are presented in separate tables in each section (Table 2).
| Authors | Country | Sample Size | Participants | Intervention | Outcome | Follow-up Period | Conclusion |
|---|---|---|---|---|---|---|---|
| Barton et al. (13) | Ireland | 86 | Athletes/sedentary individuals | AE | GM | 12 weeks | A significant difference in fecal microbiota between athletes and sedentary individuals cannot indicate the effect of exercise. |
| Morita et al. (2) | Japan | 32 | Elderly women | AE | GM | 12 weeks | Aerobic exercise increases intestinal bacteroids in healthy elderly women. |
| Quiroga et al. (14) | Spain | 39 | Childhood obesity | CT | Proteobacteria phylum/ Gammaproteobacteria class | 12 weeks | Exercise training can be considered as an efficient non-pharmacological treatment, reducing inflammatory signaling pathways caused by obesity in children through microbiota modulation. |
| Cronin et al. (15) | Ireland | 90 | Sedentary adults | Exercise | GM | 8 weeks | Little changes in gut microbial composition and function were observed following increased physical activity. |
| Liu et al. (10) | China | 39 | Diabetic men | Exercise | GM | 12 weeks | Exercise increases GT in diabetics |
| Dupuit et al. (16) | France | 17 | Women with overweight | HIIT | GT | 12 weeks | HIIT has a positive effect on gut GT in postmenopausal women. |
| Durk et al. (17) | San Francisco | 37 | Healthy participants | Exercise | GT | 7 days | There is a relationship between the number and proportion of GT and cardiorespiratory fitness. |
| Motiani et al. (8) | Turku | 26 | Sedentary subjects | MIC/SIT | GT | 2 weeks | Exercise improves the intestinal microbial profile. |
| Resende et al. (3) | Brazil | 24 | Healthy men | AE | GT | 10 weeks | Moderate intensity AE improves VO2 and boosts men's GT. |
| Taniguchi et al. (18) | Japanese | 33 | Elderly men | STE | GT | 5 weeks | STE has little effect on GT in the elderly. |
| Kern et al. (4) | Denmark | 88 | Overweight or obesity | BIKE/MOD/VIG | GT | 6 months | Exercise produces little change in GT. |
| Donati Zeppa et al. (19) | Italy | 17 | Seventeen healthy male | HIIT | GT | 9 weeks | HIIT moves the number of GT in the gut towards healthier GT. |
| Rettedal et al. (20) | New Zealand | 29 | Overweight men | HIIT | GT | 3 weeks | Short-term HIIT has no effect on GT composition |
| Zhong et al. (21) | China | 14 | Older women | Exercise | GT | 8 weeks | Exercise can increase the frequency of GT |
| Erlandson et al. (22) | USA | 22 | Older, sedentary adults | RT | GT | 24 weeks | Performing exercise interventions is associated with changes in GT |
Findings of the included studies are presented in three sections: (1) Aerobic Exercise; (2) Resistance Training; and (3) Combined Training.
3.1. Aerobic Exercise Training
Most of the studies included in our review had aerobic exercise training (AE) as one of their primary educational interventions. Aerobic exercise training has effects in several ways such as improving endurance and cardiopulmonary fitness and improving body composition through increasing lean body mass and decreasing body fat percentage (23). Cardiorespiratory fitness (VO2 max and maximum power) showed a positive correlation with the characteristics of GM such as increased bacterial diversity and butyrate-producing bacteria (10). VO2 max has been shown to predict more than 20% of the variance of an individual's relative gut bacteria and is positively correlated with GM diversity (3). However, the results of longitudinal studies in this area are varied. For example, a moderate-intensity AE intervention has shown changes in GM composition, without improvements in cardiorespiratory fitness parameters (8). While a similar intervention by Resende et al. (3) improved VO2peak and affected GM composition in non-obese men, it did not alter alpha or beta variability.
Overall, it seems that exercise-induced changes in GM might not be a result of improved VO2 max and cardiorespiratory fitness and other physiological changes that occur during AE. Instead, improved cardiorespiratory fitness and improved diversity of GM composition might sometimes co-occur as a mutual outcome of exercise and no causality relationship might exist between VO2 max and GM composition. Probably the effect of this type of AE depends on the GM composition and domestic characteristics of the analyzed subject; in particular on the BMI and age of the subject studied (3).
3.2. Richness and Diversity
The majority of AE interventions induced no changes in α-diversity. In the studies reviewed, only Kern et al. observed a 5% increase in the Shannon index in the vigorous-intensity cycling group after 3 months (24). Most AE interventions that we reviewed had employed a frequency of 3 training sessions per week, but Kern et al. intervention was performed 5 times per week (24). However, Morita et al. (2) which implemented daily training sessions for 3 months did not observe the same results. It is also noteworthy that Morita’s study participants were healthy elderly over 66 years old, while Kern’s study population aged between 20 - 45 years old, which might be a determining factor for the different results. In Kern et al. study, three intervention groups with different intensities were compared: Moderate, leisure, and vigorous; and only vigorous AE resulted in changes in alpha diversity. Interventions with moderate intensity AE did not induce changes in alpha diversity (25). Moreover, when comparing a 2-week high-intensity and moderate-intensity AE training, no differences in microbiome richness and diversity were reported (8). Therefore, it can be inferred that a low to moderate-intensity AE or a short-term vigorous AE might not be enough to alter alpha diversity. Regarding AE effects on β-diversity, the data have been heterogeneous even among the participants of one intervention group with the same intervention protocol (3). These varied results can be due to confounding factors such as age, diet, fitness level, BMI, and overall, factors that determine the baseline GM profile.
3.3. Differences in Baseline GM Profile
Large interindividual differences in human GM have been observed even in healthy populations. Moreover, it is well recognized that there is great individual variability in microbiome change patterns in response to exercise training (26), even between individuals of the same group (27). For example, some studies (10) have shown that exercise non-responders or low responders have an attenuated microbiome response to exercise training. In Munukka et al.’s study (25) only half of the subjects’ gut microbiome responded to PA significantly. Moreover, in lean participants, GM modulations were significantly associated with body composition changes, whereas in obese participants these changes were associated with changes in VO2 max. In Allen et al.’s study, five butyrate-producing bacterial genera and consequently, fecal concentrations of SCFAs (acetate and butyrate) were increased in lean individuals (28). In contrast, obese subjects showed a decrease in the abundance of Faecalibacterium spp. and an increase in Bacteroides species. In another study (20), 3 weeks of AE intervention showed that the Subdoligranulum genus was increased in lean subjects, while it decreased in obese subjects. Allen et al. showed a marked improvement in VO2 max in both lean and obese subjects. The basal GM profile plays a decisive role in the regulation of physiological adaptations and exercise-induced changes in GM composition, the effect of exercise on GM strongly depends on the basic profile of intestinal microbiota as well as the type and intensity of exercise (25).
3.4. GM Changes in Response to AE Training
Due to variations in AE protocols (intensity, duration, and frequency) and because of the interpersonal variations between subjects, the influence of AE training on GM has been very diverse. For instance, Taniguchi et al. (18), showed that a five-week AE program in the elderly had modest effects on GM composition. In this study no changes in the GM’s richness and diversity were reported; however, the relative abundance of Clostridium difficile and Oscillospira genus were decreased and increased, respectively, even if the changes in Oscillospira abundance was no longer significant after adjusting for dietary changes that had occurred. Furthermore, in the study by Motiani et al. (8) high and moderate intensity AE has been shown to decrease the ratio of Firmicutes/Bacteroidetes, mainly due to the increase in the relative abundance of Bacteroidetes phyla. Moreover, the Clostridium genus and Blautia genus were decreased in both training modes (8).
3.5. GM Alterations After AE Training Are Reversible
Allen et al. have shown that GM changes after an AE intervention are reversed once the sedentary lifestyle is resumed (28). Notably, some studies have shown that even without going back to a sedentary lifestyle and while the intervention is still ongoing, the initial GM alterations tend to reverse and diminish (24). Inconsistent, in a study by Bycura et al., GM composition changed after the first few weeks of the exercise program, but after the completion of the intervention, individuals’ GM composition was no longer significantly different from the baseline point (29).
3.6. Resistance Training
In resistance training (RT), the contribution of anaerobic energy is predominant and it has been shown that high-intensity anaerobic exercises produce lactate which enters the gut lumen through circulation and provides a selective advantage for lactate-utilizing bacterial species (1). Moreover, it has been shown that changes in GM due to exercise are associated with modifications in body composition, especially lean mass (28). Animal study have also shown that RT can affect GM and alter its composition (30) however, human intervention studies on RT and GM are scarce, with various protocols and doses of intervention that will be discussed in the following text.
3.7. Combined Training
Greater plasticity of the gut microbiome in young makes them more responsive to the effect of exercise on microbial composition, above all at the phylum level (31). In obese children (aged 7 - 12 years old), Quiroga et al. (14) have shown that combined exercise training reduces the abundance of obesity-associated bacteria, and changes the microbiota toward a non-obese profile, according to Allen et al. (28) and Donati Zeppa et al. (19) results that showed a shift towards healthier microbiome profile after AE training.
Training interventions are shown to induce changes in habitual diet, toward healthier dietary patterns (32) and it could be quite challenging to maintain the habitual diet during a training intervention. For example, Donati Zeppa et al. (19) have shown that despite instructions to maintain their usual diets, participants showed significant increases in protein, carbohydrate, fiber, and vitamin C intake. These changes were recorded using consistent daily records, which no other study has applied. Since dietary changes drastically influence the GM composition, not all the changes in longitudinal studies are linked to exercise training and the results should be interpreted with this fact in mind. In another study (29), even with subjects reporting no major changes in their dietary pattern, significant increases in body weight of half of the participants were observed, which could indicate an unintended increase in energy intake.
A further influential factor to be considered is the protocol of training. In combined training (CT) interventions, it is suggested that the modality, frequency, and duration of the selected aerobic training, influence the effects of resistance training. E.g., running, but not cycling, seems to decrease hypertrophy and strength alterations through RT (33). Even the sequence of training modes (AE or RT) in a mixed exercise intervention affects its efficacy (34).
4. Conclusions
In general, can be said that exercise can balance GM. More importantly, exercise is proposed to present a stressor to the gut that stimulates beneficial adaptations and improves long-term gut barrier flexibility over time through regular physical activity. It seems that the GM changes caused by aerobic exercise are reversible after returning to a sedentary lifestyle. Therefore, it is recommended that exercise initially causes a disruption in GM that, with continued exercise and thus adaptation, revenues to the pre-exercise state. The disorder returns. Therefore, GM differences between athletes and non-athletes may be the result of long-term lifestyle differences, including diet and exercise training.
4.1. Limitation
This study is a systematic review study, and due to the variety of exercise interventions, there was a large heterogeneity of the studied population, which did not allow us to perform a meta-analysis.
4.2. Suggestion
It is suggested to carry out meta-analysis studies separately for each type of sport and population (athletes/sedentary individuals) in order to make a better decision about the results of exercise in GM.
References
-
1.
Fava F, Rizzetto L, Tuohy KM. Gut microbiota and health: connecting actors across the metabolic system. Proc Nutr Soc. 2019;78(2):177-88. [PubMed ID: 30561288]. https://doi.org/10.1017/S0029665118002719.
-
2.
Morita E, Yokoyama H, Imai D, Takeda R, Ota A, Kawai E, et al. Aerobic Exercise Training with Brisk Walking Increases Intestinal Bacteroides in Healthy Elderly Women. Nutrients. 2019;11(4):868. [PubMed ID: 30999699]. [PubMed Central ID: PMC6520866]. https://doi.org/10.3390/nu11040868.
-
3.
Resende AS, Leite GSF, Lancha Junior AH. Changes in the Gut Bacteria Composition of Healthy Men with the Same Nutritional Profile Undergoing 10-Week Aerobic Exercise Training: A Randomized Controlled Trial. Nutrients. 2021;13(8):2839. [PubMed ID: 34444999]. [PubMed Central ID: PMC8398245]. https://doi.org/10.3390/nu13082839.
-
4.
Kern T, Blond MB, Hansen TH, Rosenkilde M, Quist JS, Gram AS, et al. Structured exercise alters the gut microbiota in humans with overweight and obesity-A randomized controlled trial. Int J Obes (Lond). 2020;44(1):125-35. [PubMed ID: 31467422]. https://doi.org/10.1038/s41366-019-0440-y.
-
5.
Vianna JM, Lima JP, Saavedra FJ, Reis VM. Aerobic and Anaerobic Energy During Resistance Exercise at 80% 1RM. J Hum Kinet. 2011;29A:69-74. [PubMed ID: 23487002]. [PubMed Central ID: PMC3588899]. https://doi.org/10.2478/v10078-011-0061-6.
-
6.
Bycura D, Santos AC, Shiffer A, Kyman S, Winfree K, Sutliffe J, et al. Impact of Different Exercise Modalities on the Human Gut Microbiome. Sports (Basel). 2021;9(2):14. [PubMed ID: 33494210]. [PubMed Central ID: PMC7909775]. https://doi.org/10.3390/sports9020014.
-
7.
Mach N, Fuster-Botella D. Endurance exercise and gut microbiota: A review. J Sport Health Sci. 2017;6(2):179-97. [PubMed ID: 30356594]. [PubMed Central ID: PMC6188999]. https://doi.org/10.1016/j.jshs.2016.05.001.
-
8.
Motiani KK, Collado MC, Eskelinen JJ, Virtanen KA, Loyttyniemi E, Salminen S, et al. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med Sci Sports Exerc. 2020;52(1):94-104. [PubMed ID: 31425383]. [PubMed Central ID: PMC7028471]. https://doi.org/10.1249/MSS.0000000000002112.
-
9.
Zhao X, Zhang Z, Hu B, Huang W, Yuan C, Zou L. Response of Gut Microbiota to Metabolite Changes Induced by Endurance Exercise. Front Microbiol. 2018;9:765. [PubMed ID: 29731746]. [PubMed Central ID: PMC5920010]. https://doi.org/10.3389/fmicb.2018.00765.
-
10.
Liu Y, Wang Y, Ni Y, Cheung CKY, Lam KSL, Wang Y, et al. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab. 2020;31(1):77-91-e5. [PubMed ID: 31786155]. https://doi.org/10.1016/j.cmet.2019.11.001.
-
11.
Tzemah Shahar R, Koren O, Matarasso S, Shochat T, Magzal F, Agmon M. Attributes of Physical Activity and Gut Microbiome in Adults: A Systematic Review. Int J Sports Med. 2020;41(12):801-14. [PubMed ID: 32455454]. https://doi.org/10.1055/a-1157-9257.
-
12.
Kim SY, Kim KN, Kim DW, Kang MS. Reporting Quality Analysis of Randomized Controlled Trials in Journal of Neurosurgical Anesthesiology: A Methodological Assessment. J Neurosurg Anesthesiol. 2021;33(2):154-60. [PubMed ID: 31702588]. https://doi.org/10.1097/ANA.0000000000000662.
-
13.
Barton W, Penney NC, Cronin O, Garcia-Perez I, Molloy MG, Holmes E, et al. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67(4):625-33. [PubMed ID: 28360096]. https://doi.org/10.1136/gutjnl-2016-313627.
-
14.
Quiroga R, Nistal E, Estebanez B, Porras D, Juarez-Fernandez M, Martinez-Florez S, et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp Mol Med. 2020;52(7):1048-61. [PubMed ID: 32624568]. [PubMed Central ID: PMC8080668]. https://doi.org/10.1038/s12276-020-0459-0.
-
15.
Cronin O, Barton W, Skuse P, Penney NC, Garcia-Perez I, Murphy EF, et al. A Prospective Metagenomic and Metabolomic Analysis of the Impact of Exercise and/or Whey Protein Supplementation on the Gut Microbiome of Sedentary Adults. mSystems. 2018;3(3):e00044-18. [PubMed ID: 29719871]. [PubMed Central ID: PMC5915698]. https://doi.org/10.1128/mSystems.00044-18.
-
16.
Dupuit M, Rance M, Morel C, Bouillon P, Boscaro A, Martin V, et al. Effect of Concurrent Training on Body Composition and Gut Microbiota in Postmenopausal Women with Overweight or Obesity. Med Sci Sports Exerc. 2022;54(3):517-29. [PubMed ID: 34628447]. https://doi.org/10.1249/MSS.0000000000002809.
-
17.
Durk RP, Castillo E, Marquez-Magana L, Grosicki GJ, Bolter ND, Lee CM, et al. Gut Microbiota Composition Is Related to Cardiorespiratory Fitness in Healthy Young Adults. Int J Sport Nutr Exerc Metab. 2019;29(3):249-53. [PubMed ID: 29989465]. [PubMed Central ID: PMC6487229]. https://doi.org/10.1123/ijsnem.2018-0024.
-
18.
Taniguchi H, Tanisawa K, Sun X, Kubo T, Hoshino Y, Hosokawa M, et al. Effects of short-term endurance exercise on gut microbiota in elderly men. Physiol Rep. 2018;6(23):e13935. [PubMed ID: 30536648]. [PubMed Central ID: PMC6286434]. https://doi.org/10.14814/phy2.13935.
-
19.
Donati Zeppa S, Amatori S, Sisti D, Gervasi M, Agostini D, Piccoli G, et al. Nine weeks of high-intensity indoor cycling training induced changes in the microbiota composition in non-athlete healthy male college students. J Int Soc Sports Nutr. 2021;18(1):74. [PubMed ID: 34922581]. [PubMed Central ID: PMC8684107]. https://doi.org/10.1186/s12970-021-00471-z.
-
20.
Rettedal EA, Cree JME, Adams SE, MacRae C, Skidmore PML, Cameron-Smith D, et al. Short-term high-intensity interval training exercise does not affect gut bacterial community diversity or composition of lean and overweight men. Exp Physiol. 2020;105(8):1268-79. [PubMed ID: 32478429]. https://doi.org/10.1113/EP088744.
-
21.
Zhong F, Wen X, Yang M, Lai HY, Momma H, Cheng L, et al. Effect of an 8-week Exercise Training on Gut Microbiota in Physically Inactive Older Women. Int J Sports Med. 2021;42(7):610-23. [PubMed ID: 33321523]. https://doi.org/10.1055/a-1301-7011.
-
22.
Erlandson KM, Liu J, Johnson R, Dillon S, Jankowski CM, Kroehl M, et al. An exercise intervention alters stool microbiota and metabolites among older, sedentary adults. Ther Adv Infect Dis. 2021;8. [PubMed ID: 34262758]. [PubMed Central ID: PMC8246564]. https://doi.org/10.1177/20499361211027067.
-
23.
Pan B, Ge L, Xun YQ, Chen YJ, Gao CY, Han X, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act. 2018;15(1):72. [PubMed ID: 30045740]. [PubMed Central ID: PMC6060544]. https://doi.org/10.1186/s12966-018-0703-3.
-
24.
Kern T, Blond MB, Hansen TH, Rosenkilde M, Quist JS, Gram AS, et al. Structured exercise alters the gut microbiota in humans with overweight and obesity-A randomized controlled trial. Int J Obes (Lond). 2020;44(1):125-35. [PubMed ID: 31467422]. https://doi.org/10.1038/s41366-019-0440-y.
-
25.
Munukka E, Ahtiainen JP, Puigbo P, Jalkanen S, Pahkala K, Keskitalo A, et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is not Reflected in Systemic Metabolism in Over-weight Women. Front Microbiol. 2018;9:2323. [PubMed ID: 30337914]. [PubMed Central ID: PMC6178902]. https://doi.org/10.3389/fmicb.2018.02323.
-
26.
Montero D, Lundby C. Refuting the myth of non-response to exercise training: 'non-responders' do respond to higher dose of training. J Physiol. 2017;595(11):3377-87. [PubMed ID: 28133739]. [PubMed Central ID: PMC5451738]. https://doi.org/10.1113/JP273480.
-
27.
Moitinho-Silva L, Wegener M, May S, Schrinner F, Akhtar A, Boysen TJ, et al. Short-term physical exercise impacts on the human holobiont obtained by a randomised intervention study. BMC Microbiol. 2021;21(1):162. [PubMed ID: 34078289]. [PubMed Central ID: PMC8170780]. https://doi.org/10.1186/s12866-021-02214-1.
-
28.
Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, et al. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc. 2018;50(4):747-57. [PubMed ID: 29166320]. https://doi.org/10.1249/MSS.0000000000001495.
-
29.
Bycura D, Santos AC, Shiffer A, Kyman S, Winfree K, Sutliffe J, et al. Impact of Different Exercise Modalities on the Human Gut Microbiome. Sports (Basel). 2021;9(2):14. [PubMed ID: 33494210]. [PubMed Central ID: PMC7909775]. https://doi.org/10.3390/sports9020014.
-
30.
Cook MD, Allen JM, Pence BD, Wallig MA, Gaskins HR, White BA, et al. Exercise and gut immune function: evidence of alterations in colon immune cell homeostasis and microbiome characteristics with exercise training. Immunol Cell Biol. 2016;94(2):158-63. [PubMed ID: 26626721]. https://doi.org/10.1038/icb.2015.108.
-
31.
Cataldi S, Poli L, Sahin FN, Patti A, Santacroce L, Bianco A, et al. The Effects of Physical Activity on the Gut Microbiota and the Gut-Brain Axis in Preclinical and Human Models: A Narrative Review. Nutrients. 2022;14(16):3293. [PubMed ID: 36014798]. [PubMed Central ID: PMC9413457]. https://doi.org/10.3390/nu14163293.
-
32.
Donati Zeppa S, Sisti D, Amatori S, Gervasi M, Agostini D, Piccoli G, et al. High-intensity Interval Training Promotes the Shift to a Health-Supporting Dietary Pattern in Young Adults. Nutrients. 2020;12(3):843. [PubMed ID: 32245173]. [PubMed Central ID: PMC7146399]. https://doi.org/10.3390/nu12030843.
-
33.
Wilson JM, Marin PJ, Rhea MR, Wilson SM, Loenneke JP, Anderson JC. Concurrent training: a meta-analysis examining interference of aerobic and resistance exercises. J Strength Cond Res. 2012;26(8):2293-307. [PubMed ID: 22002517]. https://doi.org/10.1519/JSC.0b013e31823a3e2d.
-
34.
Methenitis S. A Brief Review on Concurrent Training: From Laboratory to the Field. Sports (Basel). 2018;6(4):127. [PubMed ID: 30355976]. [PubMed Central ID: PMC6315763]. https://doi.org/10.3390/sports6040127.