Abstract
Background:
Postpartum hemorrhage (PPH), a serious labor-related complication, is the leading cause of maternal mortality, which requires an emergent intervention. Uterine arterial embolization (UAE) is an effective treatment for hemostasis of intractable PPH. Several risk factors have been reported for the failure of UAE.Objectives:
To evaluate the clinical outcomes of UAE for the treatment of primary PPH following cesarean section (CS) and to determine the risk factors associated with the failure of this procedure.Patients and Methods:
This retrospective, single-center study was approved by the institutional review board, and the requirement to obtain informed consent was waived. All patients referred to a tertiary care center, who underwent UAE for primary PPH between January 2018 and December 2020, were included. The patients’ medical records and radiological findings, including the patients’ characteristics, mode of delivery, initial vital signs and laboratory findings after hospitalization, procedure details, and embolization outcomes, were evaluated for data collection. Technical success was defined as appropriate embolization of target vessels on a completion angiogram. Clinical success was defined as adequate cessation of bleeding after the first embolization, without any need for subsequent embolization or surgical intervention. Statistical analysis was performed to determine factors related to the clinical failure of UAE in CS cases.Results:
UAE was performed for 25 patients (mean age, 37.2 years; range, 25 - 45 years). The technical success rate was estimated at 100% (n = 25), and the clinical success rate was 76% (n = 19). There were no patients with permanent adverse sequelae or death. The univariate analyses showed that hemodynamic instability (P = 0.006), lower hemoglobin levels (P = 0.02), and prolonged activated partial thromboplastin time (aPTT) (P = 0.017) were related to clinical failure. The logistic regression analysis adjusted for age showed that the area under the curve (AUC) was 0.86 for hemoglobin (95% CI: 0.7 - 1; cutoff value: 0.667), 0.816 for aPTT (95% CI: 0.625 - 1; cutoff value: 0.411), and 0.868 for hemodynamic instability (95% CI: 0.661 - 1; cutoff value: 0.622).Conclusion:
UAE is a safe and effective treatment for primary PPH following CS. Hemodynamic instability, low hemoglobin levels, and prolonged aPTT can be predictive factors for the poor outcomes of UAE in CS patients. These factors are rapid and straightforward criteria, which can be simply applied, even in emergency situations.Keywords
Postpartum Hemorrhage Uterine Arterial Embolization Transcatheter Embolization Cesarean Section
1. Background
Postpartum hemorrhage (PPH) is defined as blood loss more than 500 mL within 24 hours after vaginal delivery or more than 1000 mL after cesarean section (CS) (1). PPH is classified as primary hemorrhage, which refers to excessive bleeding in the first 24 hours after delivery, and secondary PPH, which may occur between 24 hours and 12 weeks after delivery. PPH is significantly associated with maternal morbidity and mortality, with severe PPH accounting for approximately 25% of maternal deaths worldwide (2).
Initially, PPH is managed conservatively using medicines, such as uterotonic agents and tranexamic acid, vaginal packing, or uterine massage depending on the cause (3). If uterine bleeding persists despite medical treatment, surgical procedures or transcatheter arterial embolization may be required. In recent decades, selective arterial embolization has been shown to be an efficacious and secure alternative to surgery (4). Other advantages of selective arterial embolization include its high efficacy, safety, minimal invasiveness, minimal complications, and preservation of fertility (5, 6). Management decisions are commonly made in collaboration with obstetricians, interventional radiologists, and anesthesiologists.
2. Objectives
Several recent retrospective studies have examined the efficacy of uterine arterial embolization (UAE) for PPH. A previous study on 102 patients with PPH, who underwent transcatheter arterial embolization, suggested that CS was associated with a high failure rate (7). According to this finding, the current study aimed to evaluate the efficacy of UAE and to identify predictive factors associated with its failure in CS women.
3. Patients and Methods
3.1. Patient Characteristics
The protocol of this retrospective study was approved by the institutional review board (CNUH-2021-129), and the requirement to obtain informed consent was waived. Patients who had undergone UAE in the department of radiology between January 2018 and December 2020 were recruited. On the other hand, patients who had undergone UAE for causes other than PPH were excluded.
During 2018 - 2020, a total of 124 UAE procedures were performed on 123 patients. Overall, 80 patients underwent UAE for PPH, while 43 patients underwent UAE for other causes, such as myoma, adenomyosis, or curettage. The causes of curettage included management of uterine arteriovenous malformation, spontaneous or missed abortion, and endometrial mass. Twenty-two patients who underwent embolization for delayed PPH were excluded from the study. Of 58 patients, 33 (56.9%) had a normal vaginal delivery, and 25 (43.1%) had undergone CS. The patients’ medical records and radiological findings were reviewed to collect data regarding the patient characteristics, mode of delivery, initial vital signs and laboratory findings after hospitalization, procedure details, and embolization outcomes.
3.2. Embolization Procedure
All procedures were performed by interventional radiologists using digital subtraction angiography (DSA) with a common femoral arterial approach under local anesthesia with lidocaine. Aortoiliac arteriography and bilateral internal iliac artery angiography were routinely performed to determine the bleeding site. Superselective embolization was performed using embolization agents, such as absorbable gelatin sponge particles and microcoils, according to the operator’s preference and angiographic findings. Specifically, a mixture of gelatin sponge particles (Cali-Gel 350-560/710-1000/1000-1400 μm, Alicon, Hangzhou, China or Eg-Gel 560-710/700-1000 μm, Engain Co., Seongnam, South Korea), contrast medium, and normal saline was used for selective embolization. Detachable microcoils (ConcertoTM, Medtronic, Minneapolis, MN, USA or Interlock, Boston Scientific, Cork, Ireland) were also used as additional embolic agents.
If angiography demonstrated active contrast extravasation, superselective embolization of injured vessel was performed (Figure 1). If the findings of the initial angiogram were negative, bilateral UAE was performed experientially to prevent any possible blood loss that might not have been detectable on the angiogram. The pelvic anastomotic vasculature, including the ovarian, vaginal, and round ligament arteries, as well as the anterior division of internal iliac arteries (internal pudendal artery), was assessed if necessary. The embolization endpoint was absence of extravasation or evident targeted vessel stasis on angiography. Next, post-embolization angiography was performed to verify the absence of active contrast extravasation from the opening of previously spastic vessels, and adequate reduction of blood flow to the uterus was achieved.
Angiograms of a 32-year-old woman with primary postpartum hemorrhage (PPH) after cesarean section (CS). A, Digital subtraction angiogram (DSA) of the left uterine artery. Contrast extravasation is indicated by an arrow; B, DSA after embolization of the left uterine artery. Hemostasis is achieved after superselective coil embolization of the left uterine artery.
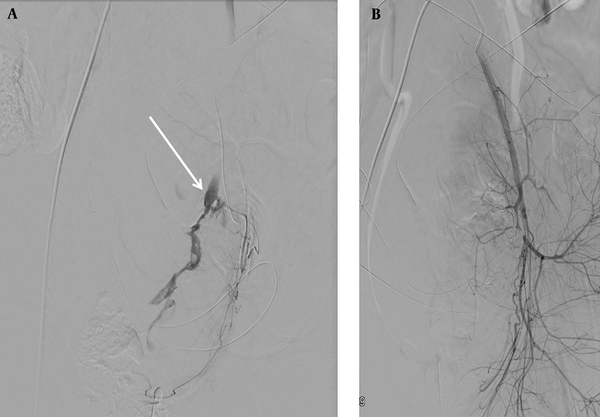
3.3. Treatment Outcomes
Technical success was defined as appropriate embolization of target vessels on a completion angiogram. Clinical success was defined as adequate cessation of bleeding after the first embolization, without any need for a subsequent repeat embolization or an additional surgical intervention (e.g., laparotomy or hysterectomy). Clinical failure was defined as repeated embolization or continuous bleeding following a surgical intervention despite successful embolization (8). The obstetrician decided on whether an additional or emergent surgical intervention was required, depending on the patient’s condition. Hemodynamic instability was defined as systolic blood pressure (SBP) ≤ 90 mmHg or a shock index (heart rate divided by SBP) ≥ 1 (9).
3.4. Statistical Analysis
The outcomes were compared between patients undergoing successful and unsuccessful UAE for primary PPH. Univariate analyses were performed using Fisher’s exact test and Mann-Whitney U test, as appropriate. Various identified risk factors for PPH were analyzed using a logistic regression analysis, and the optimal cutoff values for significant factors were selected based on the receiver operating characteristic (ROC) curves. All statistical analyses were performed in SPSS version 26.0 (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp), and the level of statistical significance (P-value) was set at < 0.05.
4. Results
The patients’ clinical outcomes are summarized in Figure 2. The technical success of initial embolization was 100%, while clinical success was achieved in 19 (76.0%) patients. Of six patients who required further interventions, four required emergency operations, including explorative laparotomy, bleeding control, and hysterectomy, because cessation of bleeding was not achieved. Two patients underwent repeat embolization, as adequate hemostasis was not achieved, and surgery was eventually required. No patients experienced permanent adverse sequelae or death.
Clinical outcomes of uterine arterial embolization (UAE) in patients with postpartum hemorrhage (PPH)
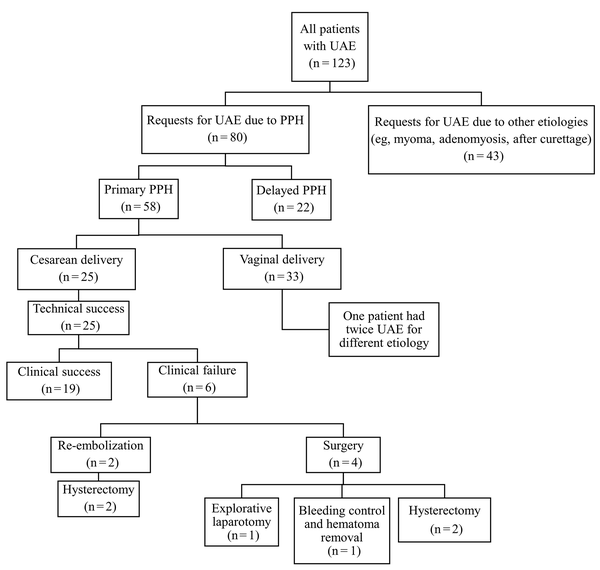
Table 1 presents the patients’ characteristics, including age, interval between delivery and embolization, biological markers, such as hemoglobin level and platelet count before embolization, and number of transfused red blood cells (RBC) expressed as mean, standard deviation (SD), and range. The average age of the patients was 37.24 years (range, 25 - 45 years). The mean interval between delivery and embolization was 395.96 minutes (range, 64 - 910 minutes), and the mean number of transfused RBC units was 4.08 (range, 0 - 20).
The Patients’ Characteristics
| Variables | Mean ± SD (range) |
|---|---|
| Age (y) | 37.24 ± 4.65 (25 - 45) |
| Interval between delivery and embolization (min) | 395.56 ± 218.44 (64 - 910) |
| Embolization duration (min) | 43 ± 20.04 (15 - 99) |
| Hemoglobin level (g/dL) | 10.29 ± 2.78 (4.8 - 18.3) |
| Platelet count (10 × 3/UL) | 139.52 ± 46.12 (66 - 225) |
| Activated partial thromboplastin time (s) | 37.74 ± 12.17 (24.3 - 79.3) |
| Prothrombin time (INR) | 1.28 ± 0.23 (0.96 - 1.91) |
| Fibrinogen level (mg/dL) | 177.24 ± 114.89 (50 - 494.4) |
| Fibrinogen degradation product level (μg/mL) | 223.14 ± 242.96 (7.8 - 640) |
| D-dimer level (mg/L) | 26.51 ± 11.24 (4 - 35) |
| Transfused RBC (unit) | 4.08 ± 4.68 (0 - 20) |
Of 25 patients, 16 (64%) were primiparous, and nine (36%) were multiparous. Meanwhile, eight (32%) patients were hemodynamically unstable, with hypotension (SBP ≤ 90 mmHg) or a shock index ≥ 1. Eight (32%) patients had active contrast extravasation on DSA, and three (12%) patients underwent additional superselective embolization for a vessel other than the uterine artery.
Factors that were significantly associated with the success or failure of embolization are presented in Table 2. Section A represents quantitative variables, such as age, duration of embolization, and diagnostic laboratory findings. Section B shows qualitative variables, including parity, extravasation on angiography, additional embolization, and hemodynamic instability (yes/no).
Factors Associated with the Clinical Effectiveness of Uterine Arterial Embolization (UAE) in Patients with Primary Postpartum Hemorrhage (PPH) After Cesarean Section (CS)
| A | |||
|---|---|---|---|
| Variables | Clinical success (n = 19) | Clinical failure (n = 6) | P-value |
| Age (y) | 36.89 ± 5.02 | 38.33 ± 3.33 | 0.676 |
| Mean interval between delivery and embolization (min) | 406.84 ± 203.44 | 361.5 ± 279.59 | 0.475 |
| Embolization duration (min) | 40.94 ± 16.41 | 49.5 ± 29.86 | 0.964 |
| Hemoglobin level (g/dL) | 11.03 ± 2.61 | 7.95 ± 1.95 | 0.02 |
| Platelet count (10 × 3/UL) | 142.21 ± 47.89 | 131 ± 42.88 | 0.743 |
| aPTT (sec) | 34.51 ± 8.69 | 47.97 ± 16.54 | 0.017 |
| Prothrombin time (INR) | 1.23 ± 0.2 | 1.42 ± 0.29 | 0.102 |
| Fibrinogen level (mg/dL) | 193.89 ± 125.25 | 124.52 ± 49.89 | 0.273 |
| Fibrinogen degradation product level (μg/mL) | 218.37 ± 246.48 | 238.23 ± 253.53 | 0.674 |
| D-dimer level (mg/L) | 25.57 ± 11.56 | 29.5 ± 10.52 | 0.334 |
| Transfused RBC (unit) | 6.26 ± 6.1 | 12.33 ± 6.38 | 0.127 |
| B | |||
| Parity | 0.142 | ||
| Primiparity | 14 | 2 | |
| Multiparity | 5 | 4 | |
| Extravasation on angiography | 0.344 | ||
| Negative | 14 | 3 | |
| Positive | 5 | 3 | |
| Additional embolization of a vessel other than the uterine artery | 0.133 | ||
| Negative | 18 | 4 | |
| Positive | 1 | 2 | |
| Hemodynamic instability | 0.006 | ||
| Negative | 16 | 1 | |
| Positive | 3 | 5 | |
The clinical failure rate was significantly higher in patients with hemodynamic instability, lower hemoglobin levels, and prolonged activated partial thromboplastin time (aPTT). Based on the findings, clinical success was not associated with age (P = 0.676), interval between delivery and embolization (P = 0.475), duration of embolization (P = 0.964), number of transfused RBC units before the procedure (P = 0.127), or laboratory findings, including the platelet count (P = 0.743), prothrombin time (P = 0.102), fibrinogen level (P = 0.273), fibrinogen degradation product (FDP) level (P = 0.674), or D-dimer level (P = 0.334). Also, no significant difference was found between the groups of clinical success and clinical failure in terms of parity, presence of extravasation on angiography, or additional embolization of a vessel other than the uterine artery.
Moreover, a logistic regression analysis was performed to identify significant variables in univariate analyses. Given the small sample size of the study, the analysis was only adjusted for age. Table 3 presents the detailed results of age-adjusted logistic regression analysis. Based on the findings, aPTT was not a significant factor (P = 0.071). However, a lower hemoglobin level was associated with poor outcomes, with an odds ratio (OR) of 0.42 [95% confidence interval (CI): 0.19 - 0.91). Hemodynamic instability was also a significant negative predictor of UAE in CS, with an OR of 33.14 (95% CI: 2.14 - 511.93).
The Results of Age-Adjusted Logistic Regression Analysis Indicating Significant Clinical Predictors of UAE failure in Patients with Primary Postpartum Hemorrhage (PPH) After Cesarean Section (CS)
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
| Hemoglobin | 0.42 | 0.192-0.913 | 0.029 |
| aPTT | 1.104 | 0.992-1.229 | 0.071 |
| Hemodynamic instability | 33.14 | 2.145-511.932 | 0.012 |
Figure 3 indicates the ROC curves for hemoglobin level, aPTT, and hemodynamic instability to predict UAE failure in cases of primary PPH undergoing CS. The area under the curve (AUC) was 0.86 (95% CI: 0.7 - 1) for hemoglobin, 0.816 (95% CI: 0.625 - 1) for aPTT, and 0.868 (95% CI: 0.661 - 1) for hemodynamic instability. The optimal cutoff value for hemoglobin level (presented as probability) was 0.667 at a 95% CI of 0.7 - 1. The corresponding results of aPTT and hemodynamic instability are shown in Table 4.
The receiver operating characteristic (ROC) curves for significant clinical predictors of uterine arterial embolization (UAE) in patients with primary postpartum hemorrhage (PPH) after cesarean section (CS). Abbreviations: AUC, area under the curve; aPTT, activated partial thromboplastin time.
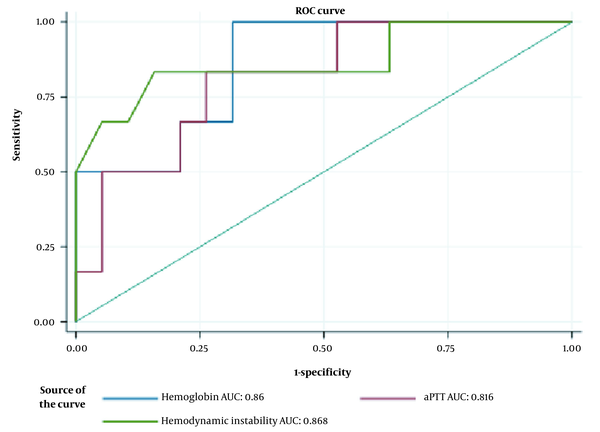
The Receiver Operating Characteristic (ROC) Curve Analysis of Significant Clinical Factors for Predicting UAE failure in Patients with Primary Postpartum Hemorrhage (PPH) After Cesarean Section (CS)
| Variables | AUC | 95% CI | Optimal cutoff value |
|---|---|---|---|
| Hemoglobin | 0.860 | 0.7-1 | 0.667 |
| aPTT | 0.816 | 0.625-1 | 0.411 |
| Hemodynamic instability | 0.868 | 0.661-1 | 0.622 |
5. Discussion
UAE is considered a safe and effective method for the treatment of medically intractable PPH. Currently, the overall technical success rate is estimated at 99% (6). Since the late 1990’s, many retrospective studies have reported excellent clinical outcomes, with recent larger case series reporting clinical success rates of 71 - 92% (4, 7, 10). In the present study, the technical success rate was 100%. Also, the clinical success rate (76%) was comparable to that of other studies.
Many predictive factors have been identified for PPH, including uterine atony, placenta accreta, transfusion volume, and some biological factors (i.e., hemoglobin, prothrombin time [PT], aPTT, and fibrinogen level) (3). Many reports suggest that disseminated intravascular coagulation (DIC) is a significant independent risk factor for UAE failure (10). In this study, the presence of DIC was assessed based on the International Society on Thrombosis and Hemostasis (ISTH) criteria. On the other hand, Lee et al. (4) defined coagulopathy as another concept. Since DIC is an extremely dynamic condition, its definitions are heterogeneous in previous studies, and there are no definite guidelines. Overall, the available findings support the hypothesis that DIC is an important determinant of embolization failure.
Additionally, in the current study, other factors that are among the indicators of DIC, including aPTT, were examined. In this regard, a previous study suggested that a low fibrinogen level and prolonged aPTT in the earliest phase of severe PPH were associated with the need for surgical interventions (11). The current findings suggest that the pre-embolization aPTT level is a significant independent predictor of clinical failure in patients undergoing emergency UAE for primary PPH after CS. Although other known parameters, such as PT, fibrinogen, and platelet count, were not statistically significant, the mean values of these parameters in patients who met the criteria for clinical failure mimicked the DIC profile.
In patients with DIC, the level of PT or aPTT was prolonged in 50-60% of cases (12). However, nearly half of patients with DIC may have normal or shortened PT and aPTT (12, 13); conseuqently, it is difficult to conclude that aPTT reflects the patient’s general condition. Nonetheless, in emergency situations, such as severe PPH, aPTT monitoring is simpler than the measurement of DIC score for predicting the procedure outcomes. Also, in the treatment of patients with PPH with prolonged aPTT, a close discussion between clinicians and interventional radiologists is necessary.
Hemodynamic instability and low hemoglobin levels were predictors of the clinical outcomes of embolization in the current study. DIC, hemoglobin level < 8 g/dL, hemodynamic instability, and extravasation on angiography are frequently associated factors with failed embolization (10). There is an intertwined relationship between shock and DIC, where activation of the kinin system, complement system, and coagulation pathways promotes vascular leakage, and hypotension eventually leads to shock (4, 14); consequently, low pre-embolization levels of hemoglobin can suggest underlying coagulopathy (15).
Despite the insignificance of aPTT (P = 0.071), the result of the logistic regression analysis was close to the significance threshold; therefore, aPTT might have been significant for a larger sample size. Also, the AUC of hemoglobin, aPTT, and hemodynamic instability was 0.86, 0.816, and 0.868, respectively. Based on the results, these factors are helpful for predicting the clinical outcomes of UAE in CS. Therefore, it is important to correct coagulopathies and hemodynamic instability before and after UAE to increase its success rate.
The present study had several major limitations. First, this was a retrospective cohort study, which might have selection or recall bias owing to its design. Second, the small sample size of this study was selected from a single institution. Although previous studies have described other potential predictors of UAE failure, including parity and blood transfusion volume (16, 17), these factors were not significant in the present study, probably because of the small sample size.
Third, the causes of bleeding (e.g., uterine atony, genital tract laceration, and retained placental tissue) and CS (emergency or elective) were not evaluated in this study, as many patients were delivered in other clinics; therefore, information required for the medical referral form was lacking. These factors could not be corrected and might have influenced the results of the study. Finally, in this study, all patients who underwent additional embolization after the first procedure eventually required a surgical intervention. Although this finding raises questions about the efficacy of re-embolization after the failure of primary embolization, it was difficult to determine the underlying cause or associated factors because of the small sample size. Some studies have reported that repeated UAE leads to successful hemostasis (4, 8); consequently, in patients with embolization failure, additional UAE remains a viable treatment option. However, further prospective studies with a larger sample size are needed to confirm the current findings.
In conclusion, UAE is a safe and effective treatment with a high success rate for the management of primary PPH following CS. Despite some limitations, the present results revealed that hemodynamic instability, low hemoglobin levels, and prolonged aPTT could contribute to the identification of patients that may benefit from targeted hemostatic treatment. Overall, the identified factors provide rapid and straightforward criteria that can be simply applied, even in emergency situations.
References
-
1.
Chen C, Lee SM, Kim JW, Shin JH. Recent Update of Embolization of Postpartum Hemorrhage. Korean J Radiol. 2018;19(4):585-96. [PubMed ID: 29962865]. [PubMed Central ID: PMC6005941]. https://doi.org/10.3348/kjr.2018.19.4.585.
-
2.
Souza JP, Gülmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. Lancet. 2013;381(9879):1747-55. [PubMed ID: 23683641]. https://doi.org/10.1016/s0140-6736(13)60686-8.
-
3.
Poujade O, Zappa M, Letendre I, Ceccaldi PF, Vilgrain V, Luton D. Predictive factors for failure of pelvic arterial embolization for postpartum hemorrhage. Int J Gynaecol Obstet. 2012;117(2):119-23. [PubMed ID: 22361480]. https://doi.org/10.1016/j.ijgo.2011.11.025.
-
4.
Lee HY, Shin JH, Kim J, Yoon HK, Ko GY, Won HS, et al. Primary postpartum hemorrhage: outcome of pelvic arterial embolization in 251 patients at a single institution. Radiology. 2012;264(3):903-9. [PubMed ID: 22829685]. https://doi.org/10.1148/radiol.12111383.
-
5.
Gonsalves M, Belli A. The role of interventional radiology in obstetric hemorrhage. Cardiovasc Intervent Radiol. 2010;33(5):887-95. [PubMed ID: 20464555]. https://doi.org/10.1007/s00270-010-9864-4.
-
6.
Zhang XQ, Chen XT, Zhang YT, Mai CX. The Emergent Pelvic Artery Embolization in the Management of Postpartum Hemorrhage: A Systematic Review and Meta-analysis. Obstet Gynecol Surv. 2021;76(4):234-44. [PubMed ID: 33908615]. [PubMed Central ID: PMC8081441]. https://doi.org/10.1097/ogx.0000000000000887.
-
7.
Touboul C, Badiou W, Saada J, Pelage JP, Payen D, Vicaut E, et al. Efficacy of selective arterial embolisation for the treatment of life-threatening post-partum haemorrhage in a large population. PLoS One. 2008;3(11). e3819. [PubMed ID: 19043573]. [PubMed Central ID: PMC2583949]. https://doi.org/10.1371/journal.pone.0003819.
-
8.
Lee HJ, Jeon GS, Kim MD, Kim SH, Lee JT, Choi MJ. Usefulness of pelvic artery embolization in cesarean section compared with vaginal delivery in 176 patients. J Vasc Interv Radiol. 2013;24(1):103-9. [PubMed ID: 23273701]. https://doi.org/10.1016/j.jvir.2012.09.029.
-
9.
Tanahashi Y, Goshima S, Kondo H, Ando T, Noda Y, Kawada H, et al. Transcatheter Arterial Embolization for Primary Postpartum Hemorrhage: Predictive Factors of Need for Embolic Material Conversion of Gelatin Sponge Particles to N-Butyl Cyanoacrylate. Cardiovasc Intervent Radiol. 2017;40(2):236-44. [PubMed ID: 27812783]. https://doi.org/10.1007/s00270-016-1496-x.
-
10.
Kim YJ, Yoon CJ, Seong NJ, Kang SG, An SW, Kim YS, et al. Failed pelvic arterial embolization for postpartum hemorrhage: clinical outcomes and predictive factors. J Vasc Interv Radiol. 2013;24(5):703-9. [PubMed ID: 23622042]. https://doi.org/10.1016/j.jvir.2013.02.013.
-
11.
Gillissen A, van den Akker T, Caram-Deelder C, Henriquez D, Bloemenkamp KWM, de Maat MPM, et al. Coagulation parameters during the course of severe postpartum hemorrhage: a nationwide retrospective cohort study. Blood Adv. 2018;2(19):2433-42. [PubMed ID: 30266818]. [PubMed Central ID: PMC6177656]. https://doi.org/10.1182/bloodadvances.2018022632.
-
12.
Bick RL. Disseminated intravascular coagulation current concepts of etiology, pathophysiology, diagnosis, and treatment. Hematol Oncol Clin North Am. 2003;17(1):149-76. [PubMed ID: 12627667]. https://doi.org/10.1016/s0889-8588(02)00102-8.
-
13.
Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145(1):24-33. [PubMed ID: 19222477]. https://doi.org/10.1111/j.1365-2141.2009.07600.x.
-
14.
Bell TN. Disseminated intravascular coagulation and shock. Multisystem crisis in the critically ill. Crit Care Nurs Clin North Am. 1990;2(2):255-68. [PubMed ID: 2192729].
-
15.
Zhang E, Liu L, Owen R. Pelvic Artery Embolization in the Management of Obstetrical Hemorrhage: Predictive Factors for Clinical Outcomes. Cardiovasc Intervent Radiol. 2015;38(6):1477-86. [PubMed ID: 25876518]. https://doi.org/10.1007/s00270-015-1092-5.
-
16.
Sentilhes L, Gromez A, Clavier E, Resch B, Verspyck E, Marpeau L. Predictors of failed pelvic arterial embolization for severe postpartum hemorrhage. Obstet Gynecol. 2009;113(5):992-9. [PubMed ID: 19384113]. https://doi.org/10.1097/AOG.0b013e3181a114f7.
-
17.
Habitamu D, Goshu YA, Zeleke LB. The magnitude and associated factors of postpartum hemorrhage among mothers who delivered at Debre Tabor general hospital 2018. BMC Res Notes. 2019;12(1):618. [PubMed ID: 31547856]. [PubMed Central ID: PMC6757371]. https://doi.org/10.1186/s13104-019-4646-9.