Abstract
Background:
Differentiation of patients with central precocious puberty (CPP) from healthy individuals and patients with CPP-like conditions [isolated premature thelarche (IPT) and isolated premature adrenarche (IPA)] is important for selecting an appropriate treatment. The gonadotropin-releasing hormone stimulation test is used as the gold standard for differentiating CPP from other conditions. Despite its high specificity, this test has disadvantages, such as low sensitivity, time-consuming sampling, and need for multiple blood samples.Objectives:
This study aimed to investigate the use of pelvic ultrasonography (US) and its parameters in distinguishing CPP patients from those with similar conditions and healthy individuals.Patients and Methods:
In this case-control study, a total of 183 patients, who were referred to the endocrinology department of Ali Asghar Hospital in Tehran, Iran for the evaluation of CPP, were recruited cconsecutively from 2015 to 2019. All the participants were Iranians and classified based on the clinical and laboratory findings. Pelvic US parameters were evaluated in all groups. One-way analysis of variance (ANOVA) was used to compare the mean values of continuous variables between the groups. Moreover, a post-hoc test was performed for pairwise comparisons between the groups if the result of ANOVA test was statistically significant. Finally, the receiver-operating characteristic (ROC) curve analysis was performed to determine the best cutoff points for US parameters.Results:
Of 183 children, 62 were allocated to the control group (33.87%), 93 to the CPP group (50.81%), 16 to the IPT group (8.74%), and 12 to the IPA group (6.55%). The results showed no significant difference between the groups regarding chronological age and body mass index, while the bone age (107.76 ± 19.81 months) (P < 0.001) and height (129.53 ± 8.97 cm) were significantly higher in the CPP group compared to the other groups (P = 0.003). All US parameters were significantly different between the CPP and control groups. There were also significant differences between CPP patients and those with IPT and IPA in terms of all parameters, except for the cervical anteroposterior diameter and ovarian volume. The best parameters for differentiating CPP from other conditions were the uterine volume (cutoff value, 1.40; 75.27% sensitivity; 75.56% specificity), uterine transverse diameter (cutoff value, 13.5 mm; 72.04% sensitivity; 71.11% specificity), and fundus/cervix (F/C) ratio (cutoff value, 0.98; 78.49% sensitivity; 70% specificity).Conclusion:
The pelvic US parameters can improve the diagnosis of CPP and play an auxiliary role in distinguishing the treatment needed. Based on the findings, the best diagnostic parameter and its cutoff value can vary depending on ethnicity and type of study.Keywords
Precocious Puberty Central Pelvic Examination Ultrasonography
1. Background
Precocious puberty in girls is defined as the development of secondary sexual characteristics, growth spurts, and psychosocial changes before the age of eight years in Caucasian people and before the age of 7 - 8 years in African Americans (1-3). It can be classified into two main groups, namely, gonadotropin-dependent and gonadotropin-independent precocious puberty (4). Conditions, such as gonadal disorders, adrenal gland disorders, pseudo-precocious puberty, peripheral precocious puberty, and exogenous hormone administration, are classified in the gonadotropin-independent group (4). On the other hand, gonadotropin-dependent precocious puberty, known as central precocious puberty (CPP), is associated with the hypothalamic-pituitary-gonadal (HPG) axis activity and accounts for 58 - 90% of all precocious puberty cases (4).
CPP is mostly caused by idiopathic factors, and in rare cases, brain abnormalities, such as tumors, traumas, infections, and malformations, are the main causes of HPG axis activity (5). Although CPP occurs at younger age, natural maturation stages, such as thelarche, pubarche, and menarche, occur at the same time as normal people (6, 7). The main concerns related to CPP are premature bone maturation and reduced height (6, 8). Therefore, timely onset of CPP treatment with gonadotropin-releasing hormone (GnRH) agonists is of great importance (9).
Additionally, differentiation of CPP from isolated premature thelarche (IPT) and isolated premature adrenarche (IPA) is clinically important, because these conditions do not accelerate bone maturation and do not require treatment with GnRH agonists, despite the development of sexual characteristics similar to CPP (10). Besides, it is important to differentiate CPP from pseudo-precocious puberty and exogenous obesity, with increasing prevalence rates in recent years. In case of obesity, bone age may be above normal; however, there is no other evidence on puberty, and therefore, treatment with GnRH agonists is not required (11).
The clinical diagnosis of CPP is based on physical examinations, bone age, and growth rate (12). The GnRH stimulation test is used as the gold standard to differentiate CPP patients from other suspicious or borderline cases. Despite its high specificity, this test has disadvantages, such as low sensitivity, time-consuming sampling, and need for multiple blood sampling (13). Pelvic ultrasonography (US) is almost always requested if there is a clinical suspicion of any type of precocious puberty (14). Pelvic US is a non-invasive, accessible, radiation-free, and inexpensive modality and a useful diagnostic tool for assessing the female pelvis (14). It provides detailed information about the uterine and ovarian size, fundus-to-cervix (F/C) ratio, endometrial thickness, and ovarian follicle size. It can also help diagnose cysts and pelvic masses (14).
Numerous studies have attempted to increase the volume of ovaries and uterus and increase the size and number of follicles in years leading up to puberty (15-17). Also, major attempts have been made to use US for distinguishing healthy girls from those with precocious puberty (15-17). Nevertheless, the diagnostic value of pelvic US parameters remains unclear. Some studies have reported overlapping values for pelvic US parameters between CPP patients and other groups (15), while some others have shown that pelvic US parameters are useful for differentiating CPP patients from healthy individuals. It should be noted that different studies used different cutoff points for US parameters (16, 17).
2. Objectives
This study aimed to evaluate the use of pelvic US and its parameters in differentiating CPP patients from healthy individuals, with or without suspicious characteristics. It also attempted to compare the pelvic US parameters between CPP, IPT, IPA, and healthy individuals to determine safe cutoff limits for each parameter.
3. Patients and Methods
3.1. Study Population
This case-control study was performed on female Iranian patients, aged ≤ 8 years, who were referred to the endocrinology department of our hospital for the evaluation of precocious puberty during 2015 - 2019. The participants were assigned to four groups: control, IPT, IPA, and CPP. The exclusion criteria were as follows: (1) Central nervous system abnormalities; (2) related endocrinological abnormalities, such as adrenal, gonadal, and thyroid abnormalities; (3) iatrogenic or exogenous hormone administration; and (4) lack of proper follow-up. The local ethics committee confirmed this study, and written informed parental consent was obtained according to the revised Declaration of Helsinki. All the participants were clinically followed-up for at least two years.
3.1.1. Control Group
Sixty-two girls, aged ≤ 8 years, were included in the control group after excluding three of them based on the exclusion criteria (lack of proper follow-up). The inclusion criteria for the control group were as follows: (1) Chronological age ≤ 8 years; (2) absence of secondary sexual characteristics; (3) being within two standard deviations (SDs) of bone age with Normal growth velocity; and (4) children with hormonal assessments and pelvic US examination in our hospital.
3.1.2. CPP Group
Ninety-three girls, aged ≤ 8 years, were included in the CPP group after excluding four of them based on the exclusion criteria (two due to hypophyseal microadenoma, one due to hypothyroidism, and one due to estrogen cream treatment for labial adhesion in infancy). The inclusion criteria for the CPP group were as follows: (1) Children ≤ 8 years of chronological age; (2) children with secondary sexual characteristics; (3) children with > 2 SDs of bone chronological age and accelerated height velocity; (4) children with a confirmed diagnosis of precocious puberty based on the GnRH stimulation test; and (5) children undergoing pelvic US in our hospital.
3.1.3. IPT Group
Sixteen girls, aged ≤ 8 years, were included in the IPT group after excluding one of them (due to the normal breast adipose tissue rather than real thelarche). The inclusion criteria for the IPT group were as follows: (1) Chronological age ≤ 8 years; (2) breast development in the absence of any other signs of puberty; (3) being within 2 SDs of bone age with a normal height velocity; and (4) undergoing hormonal assessments and pelvic US in our hospital.
3.1.4. IPA Group
Twelve girls, aged ≤ 8 years, were included in the IPA group after eliminating two of them due to the diagnosis of congenital adrenal hyperplasia. The inclusion criteria for the IPT group were as follows: (1) Chronological age ≤ 8 years; (2) pubic and/or axillary hair growth in the absence of any other signs of puberty; (3) being within 2 SDs of bone age with a normal height velocity; and (4) undergoing hormonal assessments and pelvic US in our hospital.
3.2. Study Design
The secondary sexual characteristics of all the participants were precisely examined according to the Tanner staging (2). The patients’ height and weight, as well as height velocity (height gained within a year), were measured within at least a six-month interval and interpreted as either accelerated or non-accelerated (18). Bone age was defined by Greulich scoring and evaluated using non-dominant hand radiographs (19). The hormonal assessments included the baseline levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and estradiol (E2) in all participants. The levels of GnRH-stimulated LH and FSH were measured using radioimmunoassay for only CPP and CPP-like patients. The stimulated LH and FSH levels were measured after 30 and 60 minutes of GnRH injection for only the CPP, IPT, and IPA groups. The diagnostic level of stimulated LH for CPP was set at ≥ 5 IU/L for patients with pubertal characteristics (20).
The pelvic US examinations were carried out in the radiology department of our hospital by a single radiologist. The Philips EPIQ 7 Ultrasound Machine (China), equipped with a 7.5-MHz linear array transducer and a 5-MHz convex array transducer, was used for evaluating the patients. All the patients were examined with a full bladder in a completely calm condition. The US parameters were as follows: (1) Anteroposterior (AP) diameter (mm) of the uterine fundus; (2) AP diameter (mm) of the uterine body; (3) AP diameter (mm) of the uterine cervix; (4) F/C ratio (uterine fundus AP diameter divided by the cervix AP diameter); (5) uterine length (mm); (6) uterine transverse diameter (mm); (7) uterine volume calculated by the ellipse formula [V (mL) = uterine length (mm) × transverse diameter (mm) × AP diameter (mm) × 0.5236]; (8) endometrial thickness (mm); (9) left ovarian volume (mL); (10) right ovarian volume (mL); and (11) average maximum diameter of the largest follicles in both ovaries (mm). The volume of each ovary was also calculated using the same ellipse formula for the uterus.
3.3. Statistical Analysis
Categorical variables were described as frequency and percentage. Continuous data were examined using Anderson-Darling test of normality and Gaussian distribution and reported as mean ± SD. One-way analysis of variance (ANOVA) was used to compare the mean values of continuous variables between the groups, including the CPP, IPT, IPA, and healthy control groups. Moreover, Tukey’s post-hoc test was used for pairwise comparisons between the groups if the initial value of ANOVA test was statistically significant.
Additionally, the receiver operating characteristic (ROC) curve analyses were performed to determine the best cutoff points for US parameters based on the Youden’s index and to identify their sensitivity, specificity, positive likelihood ratio (LR +), and negative likelihood ratio (LR -) to distinguish patients with CPP. Moreover, the equality of area under the ROC curve (AUC) for the US parameters against the ROC curve for uterine volume, as the most accurate measurement, was assessed using the “rocgold” command in Stata software. In this study, Stata version 12 (Stata Corp., College Station, TX, USA) was used for statistical analysis, and a P-value less than 0.05 was considered statistically significant.
4. Results
Of 183 children, 62 were assigned to the control group (33.87%), 93 to the CPP group (50.81%), 16 to the IPT group (8.74%), and 12 to the IPA group (6.55%).
4.1. Comparison of the Demographic Characteristics and Laboratory Hormone Test Findings Between the Groups
ANOVA test and post-hoc analysis were performed for comparison of demographic characteristics. Although there was no significant difference in terms of chronological age and body mass index (BMI) between the groups, significant differences were observed regarding bone age (P < 0.001) and height (P = 0.003); in other words, bone age (107.76 ± 19.81 months) and height (129.53 ± 8.97 cm) were significantly higher in the CPP group compared to the other groups (Table 1).
Comparison of Demographic Characteristics and Laboratory Hormone Test Findings Between the Groups a, b
| Variables | Control (n = 62) | CPP (n = 93) | IPT (n = 16) | IPA (n = 12) | P-value |
|---|---|---|---|---|---|
| Chronological age (m); mean ± SD (min/max) | 86.69 ± 9.79 (70/96) | 88.96 ± 3.40 (82/93) | 88.50 ± 8.05 (79/96) | 85.00 ± 11.350 (74/96) | 0.605 |
| Bone age (m) | 85.51 ± 14.40 A, c | 107.76 ± 19.81 B | 91.43 ± 20.92 A | 94.16 ± 9.04 A | < 0.001 |
| Height (cm) | 124.15 ± 8.70 A, c | 129.53 ± 8.97 B | 126.53 ± 11.58 A | 123.75 ± 8.59 A | 0.003 |
| BMI | 18.66 ± 3.80 | 19.44 ± 3.74 | 20.25 ± 4.09 | 18.69 ± 3.94 | 0.385 |
| Baseline LH (mIU/mL) | 0.26 ± 0.34 A | 1.35 ± 2.13 B | 0.42 ± 0.43 AB | 0.22 ± 0.07 AB | < 0.001 |
| Baseline FSH (mIU/mL) | 2.36 ± 1.58 A | 3.43 ± 2.60 B | 2.85 ± 2.43 AB | 2.30 ± 1.27 AB | 0.024 |
| Estradiol (pg/mL) | 8.65 ± 7.58 A | 22.33 ± 7.58 B | 10.63 ± 6.74 AB | 5.23 ± 1.74 A | < 0.001 |
| Vitamin D (ng/mL) | 27.68 ± 16.72 | 27.86 ± 20.15 | 34.12 ± 16.60 | 24.65 ± 11.56 | 0.664 |
According to one-way ANOVA test, the results of laboratory hormone tests showed significant differences between the groups in terms of the baseline levels of LH (P < 0.001), FSH (P = 0.024), and E2 (P < 0.001). However, based on pairwise post-hoc analysis, only the difference between the CPP and control groups was significant. Also, the IPA and IPT groups were not significantly different from the other groups, according to pairwise comparisons (Table 1). Also, vitamin D level was compared between the groups, and no significant difference was observed (Table 1).
4.2. Comparison of Pelvic US Parameters
The results of one-way ANOVA and post-hoc analysis revealed that the uterine fundus AP diameter, uterine body AP diameter, F/C ratio, uterine length, uterine volume, endometrial thickness, and transverse uterine diameter significantly increased in CPP patients compared to the other groups (P < 0.001) (Figure 1). Three parameters, including the uterine cervix AP diameter, left ovarian volume, and right ovarian volume, were only significantly higher in CPP patients compared to the control and IPA groups, whereas no significant difference was found between CPP and IPT patients regarding the three abovementioned parameters, despite higher values in CPP patients compared to IPT patients (Table 2).
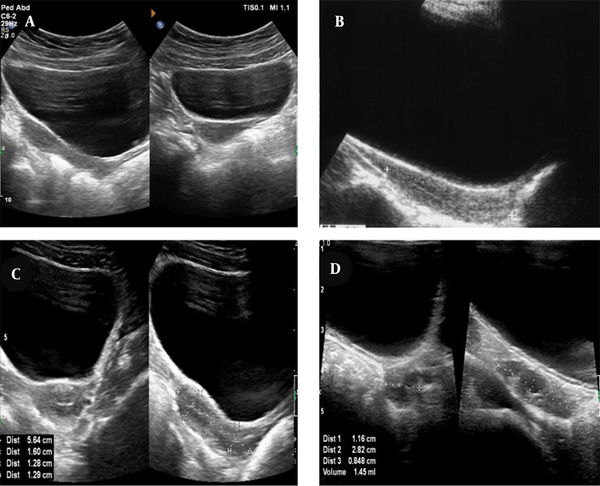
Typical ultrasonography (US) of the uterus and ovaries of a central precocious puberty (CPP) patient and a healthy individual. A, The pear-shaped appearance of a mature uterus in CPP patients; B, The tubular appearance of an immature uterus in a healthy individual; C, The length and anteroposterior diameter of the fundus, body, and cervix in a mature uterus, as well as a transverse view of the uterus; D, The volume of the ovaries measured in a CPP patient.
| Variables | Control (n = 62) | CPP (n = 93) | IPT (n = 16) | IPA (n = 12) | P-value |
|---|---|---|---|---|---|
| Uterine fundus AP diameter (mm) | 5.33 ± 1.92 A | 8.60 ± 3.95 B | 5.00 ± 1.44 A | 4.55 ± 1.25 A | < 0.001 |
| Uterine body AP diameter (mm) | 5.90 ± 2.01 A | 8.26 ± 3.49 B | 5.82 ± 1.39 A | 4.85 ± 1.40 A | < 0.001 |
| Uterine cervix AP diameter (mm) | 6.00 ± 1.51 A | 7.34 ± 2.51 B | 6.21 ± 0.836 AB | 5.54 ± 1.01 A | < 0.001 |
| F/C ratio | 0.90 ± 0.28 A | 1.16 ± 0.323 B | 0.79 ± 0.17 A | 0.84 ± 0.32 A | < 0.001 |
| Uterine length (mm) | 28.96 ± 4.81 A | 38.04 ± 8.33 B | 31.03 ± 5.27 A | 32.16 ± 3.51 A | < 0.001 |
| Uterine volume (mL) | 1.13 ± 0.95 A | 3.13 ± 2.64 B | 1.16 ± 0.38 A | 0.96 ± 0.51 A | < 0.001 |
| Endometrial thickness (mm) | 0.37 ± 0.30 A | 1.09 ± 0.69 B | 0.45 ± 0.16 A | 0.35 ± 0.13 A | < 0.001 |
| Left ovarian volume (mL) | 1.08 ± 0.64 A | 1.94 ± 1.47 B | 1.45 ± 0.92 AB | 1.08 ± 0.77 A | < 0.001 |
| Right ovarian volume (mL) | 1.10 ± 0.73 A | 2.01 ± 1.14 B | 1.51 ± 0.83 AB | 1.14 ± 0.74 A | < 0.001 |
| Transverse uterine diameter (mm) | 11.66 ± 4.12 A | 17.02 ± 6.49 B | 11.90 ± 3.17 A | 10.87 ± 3.51 A | < 0.001 |
| Average maximum diameter of the largest follicle (mm) | 2.60 ± 2.46 A | 5.00 ± 6.08 B | 3.90 ± 2.19 AB | 4.25 ± 1.81 AB | 0.022 |
The average maximum diameter of the largest follicle was only significantly different between CPP patients (5.00 ± 6.08 mm) and the control group (2.60 ± 2.46 mm). However, this parameter showed no significant difference between other groups (Table 2). None of the US parameters were significantly different between the control, IPA, and IPT groups, according to the pairwise post-hoc analysis (Table 2).
4.3. ROC Curve Analysis and Measurement of Cutoff Values for US Parameters
The ROC curve analysis was carried out with each pelvic US parameter as an independent measurement in CPP patients and other groups (Figure 2). Three of the parameters showed acceptable and excellent sensitivity and specificity, respectively for distinguishing CPP patients from other groups. The uterine volume was one of the parameters used to distinguish CPP patients from other groups at a cutoff value of 1.40 mL (sensitivity, 75.27%; specificity, 75.56%; AUC, 0.826; SE, 0.030). The remaining two parameters, which showed acceptable sensitivity and specificity, were transverse uterine diameter (cutoff value, 13.5 mm; sensitivity, 72.04%; specificity, 71.11%; AUC, 0.780; SE, 0.034) and F/C ratio (cutoff value, 0.98; sensitivity, 78.49%; specificity, 70%; AUC, 0.788; SE, 0.034) (Table 3).
The receiver operating characteristic (ROC) curve parameters of variables for c patients with central precocious puberty (CPP) from other groups.
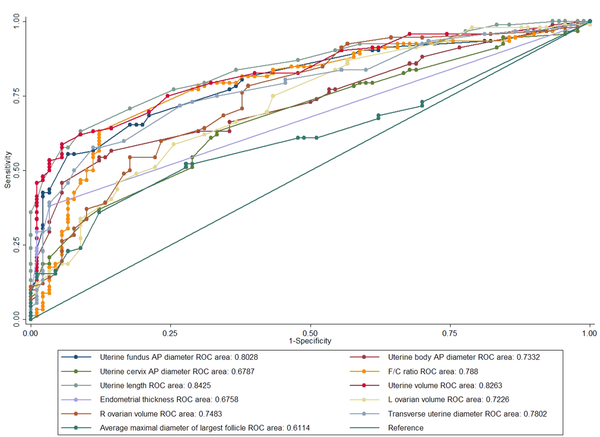
The Receiver Operating Characteristic (ROC) Curve Parameters for the Study Variables to Distinguish Patients with Central Precocious Puberty (CPP) from Other Groups
| Area | AUC | Cutoff point | Sensitivity (%) | Specificity (%) | LR+ | LR- | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Uterine fundus AP diameter | 0.802 | 5.50 | 80.65 | 62.22 | 2.134 | 0.311 | 68.79 | 75.69 |
| Uterine body AP diameter | 0.733 | 6.30 | 66.67 | 64.44 | 1.875 | 0.517 | 66.94 | 65.19 |
| Uterine cervix AP diameter | 0.678 | 6.20 | 63.44 | 66.67 | 1.903 | 0.548 | 66.28 | 63.85 |
| F/C ratio | 0.788 | 0.98 | 78.49 | 70.00 | 2.616 | 0.307 | 72.98 | 75.91 |
| Uterine length | 0.842 | 35.00 | 70.97 | 82.22 | 3.991 | 0.353 | 80.47 | 73.28 |
| Uterine volume | 0.826 | 1.40 | 75.27 | 75.56 | 3.079 | 0.327 | 76.08 | 74.74 |
| Endometrial thickness | 0.675 | 1.00 | 37.63 | 96.67 | 11.290 | 0.645 | 92.11 | 60.02 |
| Left ovarian volume | 0.722 | 1.30 | 66.30 | 63.33 | 1.808 | 0.532 | 65.12 | 64.54 |
| Right ovarian volume | 0.748 | 1.10 | 78.49 | 61.11 | 2.018 | 0.351 | 67.57 | 73.34 |
| Transverse uterine diameter | 0.780 | 13.5 | 72.04 | 71.11 | 2.493 | 0.393 | 72.03 | 71.12 |
| Average maximum diameter of the largest follicle | 0.611 | 4.00 | 61.29 | 52.22 | 1.282 | 0.741 | 56.98 | 56.64 |
Moreover, the ROC curve analysis was carried out to compare the CPP and control groups (Figure 3). The three abovementioned parameters were again found to be the most practical ones. The first parameter was the uterine volume with a cutoff value of 1.40 mL (sensitivity, 75.27%; specificity, 75.19%; AUC, 0.826; SE, 0.032). The other two parameters were the uterine transverse diameter (cutoff value, 13.5 mm; sensitivity, 72.04%; specificity, 72.58%; AUC, 0.778; SE, 0.037) and F/C ratio (cutoff value, 1; sensitivity, 77.42%; specificity, 69.35%; AUC, 0.769; SE, 0.039) (Table 4). Additionally, endometrial thickness, at a cutoff value of 1 mm, showed higher specificity for distinguishing CPP patients from others, despite lower sensitivity (sensitivity, 37.63%; specificity, 96.67%) (Table 3), and also for distinguishing CPP patients from the controls (sensitivity, 37.63%; specificity, 95.16%) (Table 4).
The receiver operating characteristic (ROC) curve parameters of variables for distinguishing patients with central precocious puberty (CPP) from the healthy control group.
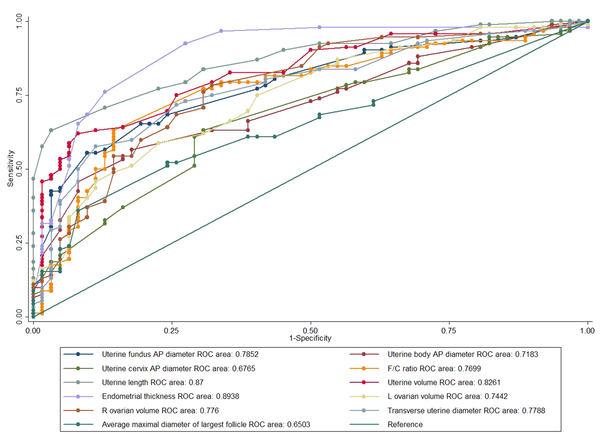
The Receiver Operating Characteristic (ROC) Curve Parameters for the Study Variables to Differentiate Patients with Central Precocious Puberty (CPP) from the Control Group
| Parameters | AUC | Cutoff point | Sensitivity (%) | Specificity (%) | LR+ | LR- | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Uterine fundus AP diameter | 0.785 | 6.50 | 68.82 | 75.81 | 2.844 | 0.411 | 81.02 | 75.81 |
| Uterine body AP diameter | 0.718 | 6.30 | 66.67 | 61.29 | 1.722 | 0.543 | 72.09 | 55.07 |
| Uterine cervix AP diameter | 0.676 | 6.50 | 61.29 | 70.97 | 2.111 | 0.545 | 76 | 55 |
| F/C ratio | 0.769 | 1.00 | 77.42 | 69.35 | 2.526 | 0.325 | 79.12 | 67.19 |
| Uterine length | 0.871 | 33.00 | 79.57 | 72.58 | 2.902 | 0.281 | 81.32 | 70.31 |
| Uterine volume | 0.826 | 1.40 | 75.27 | 75.19 | 2.916 | 0.333 | 81.98 | 66.96 |
| Endometrial thickness | 0.893 | 1.00 | 37.63 | 95.16 | 7.777 | 0.655 | 92.1 | 50.43 |
| Left ovarian volume | 0.744 | 1.2 | 69.57 | 61.29 | 1.797 | 0.496 | 72.94 | 57.32 |
| Right ovarian volume | 0.776 | 1.2 | 76.34 | 69.35 | 2.491 | 0.341 | 78.89 | 66.15 |
| Transverse uterine diameter | 0.778 | 13.5 | 72.04 | 72.58 | 2.627 | 0.385 | 79.76 | 63.38 |
| Average maximum diameter of the largest follicle | 0.650 | 4.00 | 61.29 | 61.29 | 1.583 | 0.631 | 70.37 | 51.35 |
4.4. Evaluation of the Equality of AUC Values for US Parameters Against Uterine Volume As the Most Accurate Parameter
According to the results of the present study and the majority of previous research, uterine volume is a valuable diagnostic parameter for identifying CPP patients. Among all parameters, the uterine volume, with the highest AUC value, was found to be the best parameter with acceptable sensitivity and specificity. Accordingly, the AUC values of other US parameters were compared with the AUC of uterine volume. The AUC values of all parameters, except for the uterine body AP diameter, uterine cervix AP diameter, and average maximum diameter of the largest follicles in both ovaries, were not significantly different from the gold standard curve; in other words, all US parameters, except for the abovementioned parameters, had a comparable diagnostic value to the gold standard in both study designs (CPP group vs. other groups and CPP group vs. control group) (Table 5).
Comparison of the Area Under the Receiver Operating Characteristic (ROC) Curve (AUC) for Each Parameter Against the AUC of Uterine Volume as the Best Differentiating Parameter
| Parameters | AUC | SE | Chi2 a | df | PR > Chi2 b | Bonferroni PR > Chi2 |
|---|---|---|---|---|---|---|
| Uterine volume (standard) | 0.826 | 0.030 | ||||
| Uterine fundus AP diameter | 0.802 | 0.032 | 0.901 | 1 | 0.342 | 1 |
| Uterine body AP diameter | 0.733 | 0.037 | 13.455 | 1 | 0.001 | 0.002 |
| Uterine cervix AP diameter | 0.678 | 0.039 | 21.707 | 1 | 0.001 | 0.001 |
| F/C ratio | 0.788 | 0.034 | 1.180 | 1 | 0.277 | 1 |
| Uterine length | 0.842 | 0.028 | 0.232 | 1 | 0.629 | 1 |
| Endometrial thickness | 0.892 | 0.024 | 4.049 | 1 | 0.044 | 0.441 |
| Left ovarian volume | 0.722 | 0.037 | 7.468 | 1 | 0.006 | 0.062 |
| Right ovarian volume | 0.748 | 0.036 | 4.677 | 1 | 0.030 | 0.305 |
| Transverse uterine diameter | 0.780 | 0.034 | 4.630 | 1 | 0.031 | 0.314 |
| Average maximum diameter of the largest follicle | 0.611 | 0.041 | 18.994 | 1 | 0.001 | 0.001 |
5. Discussion
The gold standard for CPP diagnosis is the GnRH stimulation test, which has different cutoff values according to different studies (20-22). It is a relatively expensive and time-consuming test, which exhibits low sensitivity, despite high specificity (17-24). Meanwhile, pelvic US is a useful, non-invasive, and relatively low-cost method for assessing female pelvic parameters (uterine and ovarian) (14). In the present study, clinical and laboratory data were used for the classification of patients, and all cases were allocated to one of the control, IPA, IPT, or CPP groups. According to the criteria proposed by Kim et al. (20), a stimulated LH level ≥ 5 IU/L is the gold standard for the diagnosis of CPP. Besides, at least two years of follow-up is considered to reduce the possibility of misdiagnosis (especially IPA and IPT forms of precocious puberty) (10, 22).
Differences in pelvic US parameters were analyzed between CPP, IPA, IPT, and control groups, and cutoff values were calculated to differentiate CPP patients from others. To the best of our knowledge, few studies have compared such groups (15, 23, 25). A remarkable aspect of the current study in that the cutoff values were extracted to differentiate patients requiring GnRH treatment from the group without any need for treatment. Also, for the first time, the equality of AUC values for each parameter was evaluated against the most accurate ROC curve (i.e., uterine volume curve), with the highest AUC value and acceptable sensitivity and specificity.
The present study found that most pelvic US parameters were useful for distinguishing CPP from other conditions. Among all parameters, uterine volume was the best diagnostic parameter. Also, three other parameters, namely, the uterine transverse diameter, F/C ratio, and endometrial thickness, played important roles in the optimal diagnosis of CPP (Tables 3 and 4). The cutoff value for the uterine volume, which distinguished CPP patients from other patients and from the control group was 1.40 mL, with sensitivity of 75.27% and 75.27% and specificity of 75.56% and 75.19%, respectively.
The majority of previous studies, similar to the present research, found uterine volume to be the best parameter for CPP diagnosis; however, there were differences in the uterine volume cutoff value. In this regard, Wen et al. and Yu et al. reported sensitivities of 91.66 % and 59.1% and specificities of 77.60% and 71.0% for the cutoff values of 1.09 and 1.07 mL for uterine volume, respectively (23, 26). On the other hand, Haber et al. reported higher values with a cutoff value of 1.8 mL with 100% sensitivity and specificity for the uterine volume (17). Moreover, de Vries et al. reported a cutoff value of 1.96 mL with sensitivity of 88.8% and specificity of 89.4% (27), and Battaglia et al. reported a cutoff value of 4 mL with sensitivity of 87% and specificity of 87.5% (28). According to these results, the most powerful US parameter and the optimal cutoff value may differ according to ethnicity, sample size, and type of study.
In the current study, the second and third most efficient parameters were the uterine transverse diameter and F/C ratio, with cutoff values of 13.5 mm (72.04% sensitivity and 71.11% specificity) and 0.98 mm (78.49% sensitivity and 70.00% specificity) for distinguishing CPP patients from other groups, respectively; also, cutoff values of 13.5 mm (72.04% sensitivity and 72.58% specificity) and 1 mm (77.42% sensitivity and 69.35% specificity) were reported to distinguish CPP patients from only the control group, respectively. These findings are highly similar to the results of a study by Yu et al., in which a cutoff point of 7.6 mm for the transverse uterine diameter showed sensitivity of 71% and specificity of 56.5% (26). Besides, de Vries et al. reported that a cutoff point of 15 mm for the transverse uterine diameter showed sensitivity of 67.9% and specificity of 100% (27). Additionally, Badouraki et al. found that a cutoff point of 1.05 for the F/C ratio showed sensitivity of 82.4% and specificity of 81.8% (25). Also, Binay et al. reported that a cutoff point of 0.98 for the F/C ratio showed sensitivity of 91.9% and specificity of 87.3% (29).
The present study also indicated the high specificity of endometrial thickness and echogenicity in diagnosing CPP, which is consistent with most previous studies (27, 28, 30) and comparable to the findings reported by Wen et al. (23). Previous studies from different countries indicated the good diagnostic value of ovarian volume for identifying CPP patients (16, 25, 27, 31). In the present study, despite a significant difference between CPP patients and the control and IPA groups, there was no significant difference between CPP and IPT patients. Therefore, we do not suggest the ovarian volume as a discriminating parameter to differentiate CPP from other conditions, which is in agreement with some studies (17, 23, 28, 32).
Finally, all US parameters were compared with the ROC curve for the uterine volume, which showed the highest AUC value and exhibited acceptable sensitivity and specificity. According to this analysis, some parameters including uterine fundus AP diameter, F/C ratio, uterine length, uterine volume, endometrial thickness, uterine transverse diameter, and ovarian volume were found to have an equal diagnostic value to uterine volume) (Table 5).
This study had some limitations due to confounding factors, such as sample loss in case files. Also, given the small number of patients younger than six years, age subgroups were not investigated.
In conclusion, timely onset of GnRH agonist therapy for CPP patients is critical to the prevention of premature bone maturation, reduced height, and related physiological stress effects. It is also important to differentiate CPP patients from IPA and IPT patients in the clinical context to avoid unnecessary treatments. In this study, the US parameters were evaluated in females in relation to precise clinical and laboratory data. A comparison of all US parameters in different groups showed that only the uterine volume (with a Youden’s index > 0.5) was a good predictor of central precocious puberty. On the other hand, the uterine transverse diameter and F/C ratio were not useful predictors for differentiating CPP from other conditions, despite good sensitivity and specificity.
Acknowledgements
References
-
1.
Berberoglu M. Precocious puberty and normal variant puberty: definition, etiology, diagnosis and current management. J Clin Res Pediatr Endocrinol. 2009;1(4):164-74. [PubMed ID: 21274291]. [PubMed Central ID: PMC3005651]. https://doi.org/10.4274/jcrpe.v1i4.3.
-
2.
Emmanuel M, Bokor BR. Tanner Stages. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
-
3.
Cheuiche AV, da Silveira LG, de Paula LCP, Lucena IRS, Silveiro SP. Diagnosis and management of precocious sexual maturation: an updated review. Eur J Pediatr. 2021;180(10):3073-87. [PubMed ID: 33745030]. https://doi.org/10.1007/s00431-021-04022-1.
-
4.
Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24(5):668-93. [PubMed ID: 14570750]. https://doi.org/10.1210/er.2002-0019.
-
5.
Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57 Suppl 2:2-14. [PubMed ID: 12065920]. https://doi.org/10.1159/000058094.
-
6.
Adan L, Chemaitilly W, Trivin C, Brauner R. Factors predicting adult height in girls with idiopathic central precocious puberty: implications for treatment. Clin Endocrinol (Oxf). 2002;56(3):297-302. [PubMed ID: 11940040]. https://doi.org/10.1046/j.1365-2265.2002.01488.x.
-
7.
Brito VN, Latronico AC, Cukier P, Teles MG, Silveira LF, Arnhold IJ, et al. Factors determining normal adult height in girls with gonadotropin-dependent precocious puberty treated with depot gonadotropin-releasing hormone analogs. J Clin Endocrinol Metab. 2008;93(7):2662-9. [PubMed ID: 18460564]. https://doi.org/10.1210/jc.2007-2183.
-
8.
Nacinovich R, Buzi F, Oggiano S, Rossi S, Spada S, Broggi F, et al. Body experiences and psychopathology in idiopathic central precocious and early puberty. Minerva Pediatr. 2016;68(1):11-8. [PubMed ID: 26864719].
-
9.
Guaraldi F, Beccuti G, Gori D, Ghizzoni L. MANAGEMENT OF ENDOCRINE DISEASE: Long-term outcomes of the treatment of central precocious puberty. Eur J Endocrinol. 2016;174(3):R79-87. [PubMed ID: 26466612]. https://doi.org/10.1530/EJE-15-0590.
-
10.
Khokhar A, Mojica A. Premature Thelarche. Pediatr Ann. 2018;47(1):e12-5. [PubMed ID: 29323691]. https://doi.org/10.3928/19382359-20171214-01.
-
11.
Ortega MT, McGrath JA, Carlson L, Flores Poccia V, Larson G, Douglas C, et al. Longitudinal Investigation of Pubertal Milestones and Hormones as a Function of Body Fat in Girls. J Clin Endocrinol Metab. 2021;106(6):1668-83. [PubMed ID: 33630047]. [PubMed Central ID: PMC8118584]. https://doi.org/10.1210/clinem/dgab092.
-
12.
Lee PA. Central precocious puberty. An overview of diagnosis, treatment, and outcome. Endocrinol Metab Clin North Am. 1999;28(4):901-18. xi. [PubMed ID: 10609126]. https://doi.org/10.1016/s0889-8529(05)70108-0.
-
13.
Iughetti L, Predieri B, Ferrari M, Gallo C, Livio L, Milioli S, et al. Diagnosis of central precocious puberty: endocrine assessment. J Pediatr Endocrinol Metab. 2000;13 Suppl 1:709-15. [PubMed ID: 10969913]. https://doi.org/10.1515/jpem.2000.13.s1.709.
-
14.
Garel L, Dubois J, Grignon A, Filiatrault D, Van Vliet G. US of the pediatric female pelvis: a clinical perspective. Radiographics. 2001;21(6):1393-407. [PubMed ID: 11706212]. https://doi.org/10.1148/radiographics.21.6.g01nv041393.
-
15.
Buzi F, Pilotta A, Dordoni D, Lombardi A, Zaglio S, Adlard P. Pelvic ultrasonography in normal girls and in girls with pubertal precocity. Acta Paediatr. 1998;87(11):1138-45. [PubMed ID: 9846915]. https://doi.org/10.1080/080352598750031121.
-
16.
Herter LD, Golendziner E, Flores JA, Moretto M, Di Domenico K, Becker E, et al. Ovarian and uterine findings in pelvic sonography: comparison between prepubertal girls, girls with isolated thelarche, and girls with central precocious puberty. J Ultrasound Med. 2002;21(11):1237-46. quiz 1247-8. [PubMed ID: 12418765]. https://doi.org/10.7863/jum.2002.21.11.1237.
-
17.
Haber HP, Wollmann HA, Ranke MB. Pelvic ultrasonography: early differentiation between isolated premature thelarche and central precocious puberty. Eur J Pediatr. 1995;154(3):182-6. [PubMed ID: 7758513]. https://doi.org/10.1007/BF01954267.
-
18.
Kelly A, Winer KK, Kalkwarf H, Oberfield SE, Lappe J, Gilsanz V, et al. Age-based reference ranges for annual height velocity in US children. J Clin Endocrinol Metab. 2014;99(6):2104-12. [PubMed ID: 24601728]. [PubMed Central ID: PMC4037731]. https://doi.org/10.1210/jc.2013-4455.
-
19.
Gaskin CM, Kahn S, Bertozzi J, Bunch PM. Skeletal Development of the Hand and Wrist: A Radiographic Atlas and Digital Bone Age Companion. Oxford, England: Oxford University Press; 2011. https://doi.org/10.1093/med/9780199782055.001.0001.
-
20.
Kim HK, Kee SJ, Seo JY, Yang EM, Chae HJ, Kim CJ. Gonadotropin-releasing hormone stimulation test for precocious puberty. Korean J Lab Med. 2011;31(4):244-9. [PubMed ID: 22016677]. [PubMed Central ID: PMC3190002]. https://doi.org/10.3343/kjlm.2011.31.4.244.
-
21.
Neely EK, Wilson DM, Lee PA, Stene M, Hintz RL. Spontaneous serum gonadotropin concentrations in the evaluation of precocious puberty. J Pediatr. 1995;127(1):47-52. [PubMed ID: 7608810]. https://doi.org/10.1016/s0022-3476(95)70255-5.
-
22.
Palmert MR, Malin HV, Boepple PA. Unsustained or slowly progressive puberty in young girls: initial presentation and long-term follow-up of 20 untreated patients. J Clin Endocrinol Metab. 1999;84(2):415-23. [PubMed ID: 10022394]. https://doi.org/10.1210/jcem.84.2.5430.
-
23.
Wen X, Wen D, Zhang H, Zhang H, Yang Y. Observational study pelvic ultrasound a useful tool in the diagnosis and differentiation of precocious puberty in Chinese girls. Medicine (Baltimore). 2018;97(10). e0092. [PubMed ID: 29517679]. [PubMed Central ID: PMC5882436]. https://doi.org/10.1097/MD.0000000000010092.
-
24.
Aritaki S, Takagi A, Someya H, Jun L. A comparison of patients with premature thelarche and idiopathic true precocious puberty in the initial stage of illness. Acta Paediatr Jpn. 1997;39(1):21-7. [PubMed ID: 9124048]. https://doi.org/10.1111/j.1442-200x.1997.tb03550.x.
-
25.
Badouraki M, Christoforidis A, Economou I, Dimitriadis AS, Katzos G. Evaluation of pelvic ultrasonography in the diagnosis and differentiation of various forms of sexual precocity in girls. Ultrasound Obstet Gynecol. 2008;32(6):819-27. [PubMed ID: 18951545]. https://doi.org/10.1002/uog.6148.
-
26.
Yu J, Shin HY, Lee SH, Kim YS, Kim JH. Usefulness of pelvic ultrasonography for the diagnosis of central precocious puberty in girls. Korean J Pediatr. 2015;58(8):294-300. [PubMed ID: 26388894]. [PubMed Central ID: PMC4573443]. https://doi.org/10.3345/kjp.2015.58.8.294.
-
27.
de Vries L, Horev G, Schwartz M, Phillip M. Ultrasonographic and clinical parameters for early differentiation between precocious puberty and premature thelarche. Eur J Endocrinol. 2006;154(6):891-8. [PubMed ID: 16728550]. https://doi.org/10.1530/eje.1.02151.
-
28.
Battaglia C, Mancini F, Regnani G, Persico N, Iughetti L, De Aloysio D. Pelvic ultrasound and color Doppler findings in different isosexual precocities. Ultrasound Obstet Gynecol. 2003;22(3):277-83. [PubMed ID: 12942501]. https://doi.org/10.1002/uog.154.
-
29.
Binay C, Simsek E, Bal C. The correlation between GnRH stimulation testing and obstetric ultrasonographic parameters in precocious puberty. J Pediatr Endocrinol Metab. 2014;27(11-12):1193-9. [PubMed ID: 25153373]. https://doi.org/10.1515/jpem-2013-0363.
-
30.
Eksioglu AS, Yilmaz S, Cetinkaya S, Cinar G, Yildiz YT, Aycan Z. Value of pelvic sonography in the diagnosis of various forms of precocious puberty in girls. J Clin Ultrasound. 2013;41(2):84-93. [PubMed ID: 23124596]. https://doi.org/10.1002/jcu.22004.
-
31.
Sathasivam A, Rosenberg HK, Shapiro S, Wang H, Rapaport R. Pelvic ultrasonography in the evaluation of central precocious puberty: comparison with leuprolide stimulation test. J Pediatr. 2011;159(3):490-5. [PubMed ID: 21489559]. https://doi.org/10.1016/j.jpeds.2011.02.032.
-
32.
Griffin IJ, Cole TJ, Duncan KA, Hollman AS, Donaldson MD. Pelvic ultrasound findings in different forms of sexual precocity. Acta Paediatr. 1995;84(5):544-9. [PubMed ID: 7633151]. https://doi.org/10.1111/j.1651-2227.1995.tb13691.x.