Abstract
Background:
Superselective transarterial embolization (TAE) is the most commonly used treatment for lower gastrointestinal (LGI) bleeding when endoscopic management is impossible or fails. Its effectiveness and safety are increased using advanced techniques, instruments, and embolic materials.Objectives:
To evaluate the outcome and safety of TAE for LGI bleeding and to analyze various influencing factors, including embolic material, embolization site, and anticoagulant or antiplatelet medication.Patients and Methods:
Fifty-two patients who underwent superselective TAE for LGI bleeding between 2003 and 2011 were included, and their clinical and imaging information were retrospectively reviewed. Outcome and safety measures, including technical and clinical success, early and delayed rebleeding, and complications, were evaluated. Logistic regression analysis was used to determine whether the clinical success rate was associated with specific variables.Results:
Technical and clinical success was achieved in 52 (100%) and 43 (83%) patients, respectively. The prior embolization site was the point of rebleeding in five of the nine patients with early rebleeding. Delayed rebleeding was documented in four patients, including two patients with angiodysplasia. Logistic regression analysis showed that embolization site, embolic material, and anticoagulant or antiplatelet medication were not statistically significant factors affecting the clinical success rate of TAE for LGI bleeding. A major complication, ischemic colitis, occurred in one patient.Conclusion:
Superselective TAE for LGI bleeding has a high success and low complication rate. There are no statistical correlations between the clinical success rate and several variables, including embolic material, embolization site, and anticoagulant or antiplatelet medication.Keywords
Angiography Therapeutic Embolization Gastrointestinal Bleeding
1. Background
Lower gastrointestinal (LGI) bleeding is defined as hemorrhage distal to the ligament of Treitz, including the small bowel, colon, rectum, and anus. The annual incidence of hospitalization for LGI bleeding is approximately 21 per 100,000 persons (1). About 10 - 15% of patients with life-threatening hemorrhage require invasive interventions for hemostasis, and approximately 4% of patients die from LGI bleeding (2, 3). The incidence of LGI bleeding also increases with the usage of aspirin, nonsteroidal anti-inflammatory drugs, and anticoagulants (4-6).
Nowadays, endoscopy is considered the primary method for the investigation and treatment of LGI bleeding. However, it requires preparation time and frequently fails to diagnose and treat LGI bleeding because of blood clots and stools (1, 7, 8). Furthermore, small bowel bleeding cannot be treated via colonoscopy. Alternative treatments include vasopressin infusion, surgery, and transarterial embolization (TAE). Vasopressin infusion has high rates of complication and rebleeding (9). Also, surgery is associated with high mortality and morbidity rates, especially in emergency cases (more than 50% of patients) (10).
TAE is effective for upper gastrointestinal (UGI) bleeding and is considered the first-line therapy for massive bleeding refractory to endoscopic management (11). In the past, TAE was less applicable to LGI bleeding because of the tenuous blood supply in the distal bowel, for which the risk of bowel infarction exceeded 10% (12). Recent advances in the microcatheter system, embolic materials, and intervention radiologists’ skills, however, have improved the effectiveness and safety of superselective TAE in the treatment of LGI bleeding. Although many studies have dealt with the outcome and safety of superselective TAE for LGI bleeding, they included small cohorts and did not consider other influencing factors, including embolic agents or anticoagulant or antiplatelet medication.
2. Objectives
The aim of this study was to evaluate the outcome and safety of TAE in LGI bleeding and to analyze the various factors that potentially affect outcome and safety, including embolic material, embolization site, and anticoagulant or antiplatelet medication.
3. Patients and Methods
3.1. The Study Population
The institutional review board of Korea university medical center approved this study and waived the informed consent requirements. Consecutive patients who underwent superselective TAE for LGI bleeding and were followed up at Korea university medical center between January 2003 and December 2011 were enrolled in the study. Indications for TAE for LGI bleeding were modified from the quality improvement guidelines for transcatheter embolization for acute gastrointestinal nonvariceal hemorrhage (13), including: (a) a consensus between radiologists and clinicians, (b) acute LGI bleeding with an endoscopically untreatable or unrevealed source of bleeding or the impossibility of proceeding with endoscopy, and (c) showing direct or indirect signs of bleeding on angiography. An absolute contraindication for TAE was a history of hypersensitivity to iodine-contrast media. A total of 52 consecutive patients were included in the study. Medical records were retrospectively reviewed for information on clinical signs and symptoms, laboratory findings, endoscopy, and imaging studies (computed tomography and angiography). Fifty-one patients were referred for embolization due to the failure to detect bleeding foci or hemostasis on colonoscopy or sigmoidoscopy. One patient showed active bleeding through a drainage tube after gastrojejunostomy, and embolization was performed without the preceding endoscopy.
The cohort consisted of 35 men and 17 women whose median age was 61 years (range 19 - 86 years). The most common cause of LGI bleeding was a colonic diverticulum (n = 15). However, the cause was not determined in 16 patients. Half of the patients (n = 26) had two or more comorbidities, and 17 patients were taking anticoagulant (heparin or warfarin) or antiplatelet (aspirin or clopidogrel) medication. The demographic characteristics of the patients and the etiologies of LGI bleeding are presented in Tables 1 and 2.
Patients' Information
| Number | Percentage | |
|---|---|---|
| Demographics, n = 52 | ||
| Mean age ± standard deviation | 60.50 ± 15.73 | Range: 19 - 86 |
| Gender (Male/Female) | 35/17 | 69%/31% |
| Comorbidities | ||
| Hypertension | 23 | 44% |
| Diabetes mellitus | 14 | 27% |
| Malignancy | 11 | 21% |
| Liver cirrhosis | 7 | 13% |
| Renal impairment | 5 | 10% |
| Number of comorbidities | ||
| No comorbidity | 7 | 13% |
| Two or more comorbidities | 26 | 50% |
| Medication | ||
| Anticoagulant or antiplatelet | 17 | 33% |
Etiology of LGI Bleeding
| Etiologies | Number | Percentage |
|---|---|---|
| Colonic diverticulum | 15 | 29% |
| Tumor | 8 | 15% |
| Post operation bleeding | 3 | 6% |
| Behçet ulcer | 3 | 6% |
| Angiodysplasia | 3 | 6% |
| Tumor and angiodysplasia | 1 | 2% |
| Appendicitis with necrosis | 1 | 2% |
| Crohn’s disease | 1 | 2% |
| Post-endoscopic polypectomy bleeding | 1 | 2% |
| Unknown | 16 | 31% |
3.2. Embolization Procedures
The right or left common femoral artery was punctured for arterial access. Diagnostic superior mesenteric and inferior mesenteric angiography was performed using the 4-French Yashiro (Terumo, Tokyo, Japan) or the 5-French Cobra catheter (Cook, Bloomington, IN). If the angiogram showed direct (extravasation of the contrast medium) or indirect signs of bleeding (pseudoaneurysm, hyperemia, intramural contrast pooling, or arterial wall abnormality), a 2.0- to 3.0-French coaxial microcatheter (Progreat, Terumo, Tokyo, Japan; Microferret, Cook, Blomington, IN; MiraFlex, Cook, Bloomington, IN; Renegade, Boston Scientific Corporation, Natick, MA; Jamiro, Kaneka Corporation, Hyogo, Japan) was advanced to the bleeding site. TAE was performed at the angiographic bleeding site at the level of the vasa recta or marginal artery. Several embolic materials were selected according to the preference of the operators, including an absorbable gelatin sponge (Gelfoam, Pharmacia and Upjohn, New York, NY) that was cut into 1 × 1 mm segments and injected manually with contrast at the bleeding site or microcoils (a 3 × 2 mm or 4 × 2 mm Tornado embolization microcoil) (Cook, Bloomington, IN) deployed in the selected artery (Figure 1). In some cases, additional gelatin sponge embolization was performed for consolidation; polyvinyl alcohol particles (PVA; Contour, Boston Scientific Corporation, Natick, MA) between 150 and 500 nm were infused manually with contrast; glue (N-butyl-2-cyanoacrylate [histoacry]; B. Braun Melsungen AG, Melsungen, Germany) mixed with ethiodized oil (glue-to-ethiodized oil ratio 1:2 - 8) was injected manually through a microcatheter (Figure 2); ethiodized oil (Lipiodol Ultrafluide, Laboratoire Guerbet, Aulnay-Sous-Bois, France) was also injected manually though a microcatheter. Post-embolization angiography was performed to confirm the absence of bleeding at the embolization area.
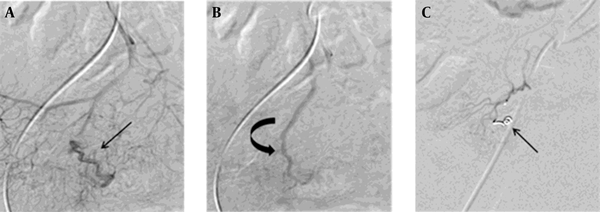
Successful microcoil embolization in a 72-year-old woman with angiodysplasia. A and B, Selective superior mesenteric angiogram showing a tortuous arterial supply (straight arrow) and an early draining vein (curved arrow) without evident nidus in the distal ileum. These findings suggest angiodysplasia. C, After microcoil embolization of the tortuous artery (arrow), no evidence of contrast filling was observed in the angiodysplasia.
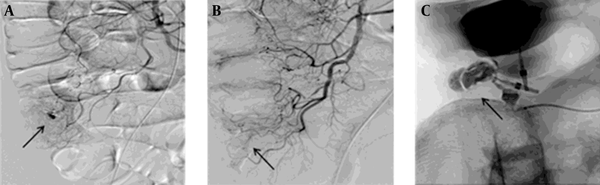
Successful glue embolization in a 54-year-old man with cecal diverticulum. A, Selective superior mesenteric arteriography representing contrast extravasation (arrow) in the distal ileocolic artery. B, Embolization of the distal vasa recta was performed using radiopaque glue (histoacryl, arrow). C, A postembolization angiogram showing hemostasis and packing of the radiopaque glue (arrow).
All the patients were monitored for complications or rebleeding after the procedure. All patients were followed up clinically, on either an inpatient or outpatient basis, to determine whether they had clinical symptoms and signs of rebleeding. Twenty-seven patients underwent follow-up sigmoidoscopy or colonoscopy for complications or recurrence within one week. The average follow-up time was 439 days (range 1 - 2,798 days).
3.3. Data Analysis
Technical success was defined as the cessation of bleeding on postembolization angiography. Clinical success was defined as the technical success of the clinical cessation of bleeding in the patient, without early rebleeding and further surgical, endoscopic, or repeated angiographic procedures within 30 days of TAE. Early rebleeding was defined as clear laboratory or clinical signs of LGI bleeding within 30 days of TAE. Delayed rebleeding was defined as LGI bleeding more than 30 days after TAE. These were determined according to the quality improvement guidelines for transcatheter embolization for acute gastrointestinal nonvariceal hemorrhage (13). Procedure-related complications were classified either as major or minor according to the Society of Interventional Radiology Standards of Practice Committee classification of complications (14).
A Kaplan-Meier analysis was performed to identify the time interval between TAE and early rebleeding. Logistic regression analysis was applied to the data set to identify the statistically significant relationships, if any, between clinical success rate and multiple variables, including sex, age, number of underlying diseases (≥ 2 or none), anticoagulant or antiplatelet medication, embolization site, and embolic material (among gelatin sponge, microcoil and microcoil + gelatin sponge groups). Statistical analysis was performed using SPSS ver. 20 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.).
4. Results
Technical success was achieved in 52 patients (100%). All the patients showed no signs of bleeding on post-embolization angiography. Clinical success was achieved in 43 patients (83%). Among the nine patients with clinical failure, four underwent repeated TAE at the sites of active bleeding, which had been previously embolized. The procedure protocol for the repeated TAE was the same as that for the first TAE, except for the different embolic agents that were used in three patients (Table 3). Among the remaining five patients, three patients underwent colonoscopy. For one patient, electrocoagulation was performed at the site of active bleeding, which was in the same anatomic region as the embolization site. Two patients showed no sites of active bleeding or suspicious sites on colonoscopy, so conservative management was indicated. These seven patients who underwent endoscopy or embolization after early rebleeding did not show further rebleeding.
An Overview of Early Rebleeding Patients
| Patient Number | Sex | Age | Anticoagulant or Antiplatelet Medication | Etiology | Bleeding Focus | Embolization Site | Embolic Agents | Early Rebleeding Time Interval, d | Rebleeding Management | Rebleeding Site | Progress |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 76 | Aspirin | Unknown | Jejunal branch | Vasa recta + Marginal artery | Microcoil + gelatin sponge | 0 | Conservative | Unknown | Expired |
| 2 | M | 62 | - | Behçet ulcer | Ileocolic branch | Vasa recta | Microcoil | 0 | Endoscopy | No focus | Spontaneous hemostasis |
| 3 | F | 66 | Aspirin | Diverticulum | Right colic branch | Vasa recta | Microcoil + gelatin sponge | 1 | Endoscopy | No focus | Spontaneous hemostasis |
| 4 | F | 62 | - | Colonic metastasis from hepatocellular carcinoma | Left colic branch | Marginal artery | Ethiodized oil + microcoil + gelatin sponge | 1 | Embolization with ethiodized oil | The same site | Clinical success |
| 5 | M | 55 | Aspirin | Diverticulum | Ileocolic branch | Vasa recta | Microcoil + gelatin sponge | 0 | Embolization with gelatin sponge | The same site | Clinical success |
| 6 | M | 47 | - | Post operation | Right colic branch | Vasa recta | Microcoil + gelatin sponge | 6 | Embolization with gelatin sponge | The same site | Clinical success |
| 7 | M | 35 | - | Diverticulum | Right colic branch | Vasa recta | Microcoil | 10 | Conservative | Unknown | Expired |
| 8 | M | 48 | Aspirin | Unknown | Jejunal branch | Marginal artery | Gelatin sponge | 7 | Embolization with gelatin sponge | The same site | Clinical success |
| 9 | M | 19 | - | Behçet ulcer | Ileocolic branch | Marginal artery | Gelatin sponge | 1 | Endoscopic coagulation | The same anatomic region | Clinical success |
The other two patients with clinical failure expired within 30 days. One patient with underlying chronic kidney disease and hypertension underwent TAE at the jejunal branch of the superior mesenteric artery. Temporary hemostasis was achieved immediately after TAE; however, continuous hematochezia recurred soon thereafter. His condition worsened because of hypovolemia and metabolic acidosis, and he expired one day after TAE. The other patient, who had liver cirrhosis, diabetes, and hypertension, underwent TAE at the cecal branch of the right colic artery. Successful hemostasis was achieved for nine days. However, during these nine days, his condition worsened due to septic shock and disseminated intravascular coagulation. On the 10th day after TAE, hematochezia recurred and esophageal variceal bleeding developed. Despite intensive medical management, he expired 21 days after TAE.
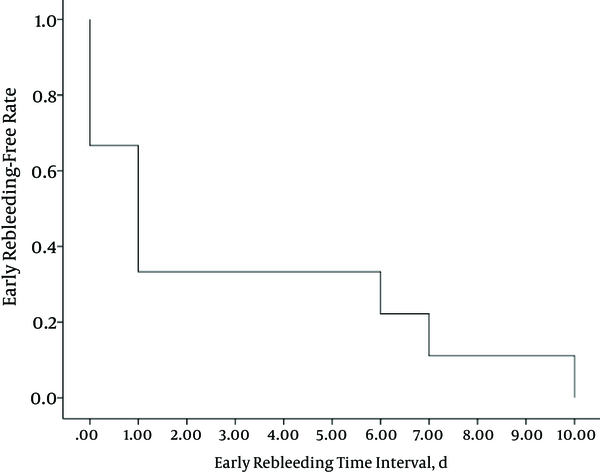
The average and median time intervals between TAE and early rebleeding were approximately three days and one day, respectively. All the time intervals were within 10 days, and eight out of nine patients (89%) showed early rebleeding within seven days (Figure 3). An overview of the nine patients with clinical failure is presented in Table 3.
Delayed rebleeding occurred in four patients after TAE. The median interval between TAE and delayed rebleeding was 311 days (range 195 - 426 days). Two patients had angiodysplasia, and two had unknown etiologies of LGI bleeding. For the patient with angiodysplasia in the terminal ileum, angiography revealed that the site of active bleeding was the prior embolization site. After the repeated TAE, no rebleeding occurred. In the other patient with angiodysplasia, a different bleeding site was disclosed on endoscopy and successful endoscopic electrocoagulation was performed. One patient with an unknown etiology of LGI bleeding underwent endoscopic clipping and electrocoagulation at the site of active bleeding, which was in the same anatomic region as the prior embolization. The remaining one patient with an unknown etiology of LGI bleeding was treated conservatively without angiography or endoscopy.
The Kaplan-Meier curve for the time interval between TAE and early rebleeding in nine patients
In 31 patients (60%), TAE was performed at the vasa recta. In 20 patients (38%), TAE was performed at the marginal artery. In one patient, TAE was performed at both the marginal artery and the vasa recta (Table 4).
Embolization Procedure
| Characteristics | Total Number | Successful Number | Clinical Success Rate |
|---|---|---|---|
| Embolization sites, n = 52 | |||
| Marginal artery | 20 | 17 | 85% |
| Vasa recta | 31 | 26 | 84% |
| Marginal + vasa recta | 1 | 1 | 100% |
| Embolic agents, n = 52 | |||
| Gelatin sponge | 25 | 23 | 92% |
| Microcoil + gelatin sponge | 16 | 12 | 75% |
| Microcoil | 4 | 2 | 50% |
| Glue | 4 | 4 | 100% |
| PVA particle + gelatin sponge | 1 | 1 | 100% |
| PVA particle | 1 | 1 | 100% |
| Ethiodized oil + microcoil + gelatin sponge | 1 | 0 | 0% |
The most commonly used embolic agent was a gelatin sponge (n = 25, 48%), followed by a microcoil with a gelatin sponge (n = 16, 31%) and a microcoil alone (n = 4, 8%; Table 4).
Seventeen patients (33%) had taken anticoagulant or antiplatelet medication because of various underlying diseases. The time interval from cessation of the anticoagulant or antiplatelet medication to embolization was within seven days (median 1.5 days; range 0 - 7 days). Clinical success was achieved in 14 (82%) of these patients. Meanwhile, clinical success was achieved in 30 (86%) of the patients who had not taken anticoagulant or antiplatelet medication (n = 35).
In the logistic regression analysis, the older the patients, the better their chances of clinical success (odds ratio 1.07; 95% confidence interval at 1.01 - 1.14; p = 0.03). Gelatin sponge embolization had a higher success rate than that achieved by microcoil embolization, and borderline significance was observed (odds ratio 7.27; 95% confidence interval, 1.06 - 61.53; P = 0.07). Sex, underlying disease, anticoagulant or antiplatelet medication, and embolization site were not statistically significant factors (Table 5).
Logistic Regression Analysis for the Association Between the Clinical Success Rate and Multiple Variables
Minor complications, including fever, chills, headache, and mild abdominal pain, occurred in 12 patients (23%) and were relieved by conservative management. One patient (2%) developed bowel ischemia. The patient was a 70-year-old man who was hospitalized for sepsis and had underlying liver cirrhosis. Superior mesenteric arteriography revealed hypervascularity at the cecum and terminal ileum. Gelatin sponge embolization was performed at the level of the vasa recta, and bleeding stopped. A few days after TAE, endoscopy revealed bowel ischemia. On the eighth day after TAE, he expired from the worsened septic condition.
5. Discussion
Since TAE for intra-abdominal bleeding was introduced in 1965 (15), it has been considered a good alternative therapeutic method when gastrointestinal bleeding is refractory or impossible to treat with endoscopy. Advances in microcatheter systems have made superselective TAE possible and decreased complications, including bowel ischemia and recurrent bleeding via collateral flow.
Current studies report high technical success rates for superselective TAE for lower GI bleeding, ranging from 85 to 100% (2, 16 - 22). In this study, technical success was achieved in 52 patients (100%), which is comparable to rates in other studies. Generally, technical difficulty, vasospasm, and prior surgery are the causes of embolization failure (16). However, no such obstacles affected the outcomes of embolization in the present study.
Clinical success was achieved in 43 patients (83%). This result was also comparable to the clinical success rates in other studies, ranging from approximately 63 to 92% (2, 16-22). In the nine patients classified as clinical failures, the time interval between TAE and early rebleeding was usually short, and the average was approximately three days. Other studies also showed relatively short time intervals, within one day or an average three days (17, 23). The prior embolization site was the site of rebleeding in five patients. Other studies also show the rebleeding site after TAE to be the site of the prior TAE, which can be explained by collateral blood flow or the insufficient reduction of pulse pressure (24, 25).
Some studies point out that the etiology of LGI bleeding is the most important factor in delayed rebleeding (2, 19, 20). Angiodysplasia is the most common cause of delayed rebleeding (18, 26, 27). Other frequently attributed lesions are extensive diverticulosis and tumors that can progress or metastasize (2, 19, 20). In our study, delayed rebleeding occurred in four patients (8%) two were cases of angiodysplasia at the distal ileum, and the remaining two were LGI bleeding of unknown etiology. Angiodysplasia could have been the cause of the delayed rebleeding in two patients, although no investigation into the causes of rebleeding was conducted.
Many authors prefer the use of microcoil in TAE for LGI bleeding embolization (2, 16, 17, 20, 28). However, the precise deployment of microcoil TAE requires radiologists with a high skill level and experience. Difficulties in the precise deployment and incomplete control of the distal blood flow can be attributed to clinical failure. In some cases, supplementary embolization using PVA particles or a gelatin sponge may be necessary to consolidate the coil-embolized artery after microcoil deployment (2, 29-32). In our series, a gelatin sponge was used as the supplementary embolic material in 16 patients.
A gelatin sponge, the most commonly used embolic agent in this study, is widely used because it is safe, inexpensive, and easy to use (2). Although no statistically significant differences in success rates were observed between the microcoil and gelatin sponge groups, the gelatin sponge group had a higher success rate. In the gelatin sponge group, marginal artery embolization was performed in 15 out of 25 cases. However, marginal artery embolization was performed in two out of 20 cases in the microcoil group. Therefore, we can speculate that a gelatin sponge could effectively prevent rebleeding from backflow via collateral circulation, even in marginal artery embolization. Also, supplemental particle embolization after microcoil deployment may be helpful in controlling LGI bleeding.
A recent study by Hur et al. shows the safety and effectiveness of glue as a primary embolic agent for LGI bleeding (22). Although glue embolization is useful for patients with coagulopathy, it requires the operator to have a high level of skill and experience and sufficient training to avoid complications. In this study, glue embolization was performed in only four patients. If the complexity of handling glue is overcome, however, glue could be just as good an embolic agent as a gelatin sponge or microcoil.
The use of anticoagulant or antiplatelet medication has been shown to be a significant risk factor for clinical failure in UGI bleeding (33), but this study found no statistically significant association between anticoagulant or antiplatelet medication and clinical success rate. The patients who had taken anticoagulant or antiplatelet medication only had a slightly lower clinical success rate than patients who had not taken these medications. Considering the effective time of anticoagulant or antiplatelet in the bloodstream and the time interval between cessation of the medication and TAE in our patients, anticoagulant or antiplatelet medication might have had no effect on the embolization result in terms of LGI bleeding. Nonetheless, a prospective study would be warranted for further evaluation.
This study has two limitations. First, it was designed retrospectively. A prospective randomized study comparing variables would help to obtain more exact information on clinical outcomes, effectiveness, and safety. Second, the cause of LGI bleeding was not thoroughly investigated in many cases because of clinicians’ inclinations or patients’ wishes.
In conclusion, superselective TAE for LGI bleeding is safe and effective with a high success rate and a low complication rate. No statistically significant differences in success rates were observed between embolic material, embolization site, and anticoagulant or antiplatelet medication, although further study is required.
References
-
1.
Lhewa DY, Strate LL. Pros and cons of colonoscopy in management of acute lower gastrointestinal bleeding. World J Gastroenterol. 2012;18(11):1185-90. [PubMed ID: 22468081]. https://doi.org/10.3748/wjg.v18.i11.1185.
-
2.
Kickuth R, Rattunde H, Gschossmann J, Inderbitzin D, Ludwig K, Triller J. Acute lower gastrointestinal hemorrhage: minimally invasive management with microcatheter embolization. J Vasc Interv Radiol. 2008;19(9):1289-96 e2. [PubMed ID: 18725091]. https://doi.org/10.1016/j.jvir.2008.06.003.
-
3.
Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol. 2008;6(9):1004-10. quiz 955-. [PubMed ID: 18558513]. https://doi.org/10.1016/j.cgh.2008.03.021.
-
4.
Lanas A, Garcia-Rodriguez LA, Polo-Tomas M, Ponce M, Quintero E, Perez-Aisa MA, et al. The changing face of hospitalisation due to gastrointestinal bleeding and perforation. Aliment Pharmacol Ther. 2011;33(5):585-91. [PubMed ID: 21205256]. https://doi.org/10.1111/j.1365-2036.2010.04563.x.
-
5.
Baron JA, Senn S, Voelker M, Lanas A, Laurora I, Thielemann W, et al. Gastrointestinal adverse effects of short-term aspirin use: a meta-analysis of published randomized controlled trials. Drugs R D. 2013;13(1):9-16. [PubMed ID: 23532576]. https://doi.org/10.1007/s40268-013-0011-y.
-
6.
Casado Arroyo R, Polo-Tomas M, Roncales MP, Scheiman J, Lanas A. Lower GI bleeding is more common than upper among patients on dual antiplatelet therapy: long-term follow-up of a cohort of patients commonly using PPI co-therapy. Heart. 2012;98(9):718-23. [PubMed ID: 22523056]. https://doi.org/10.1136/heartjnl-2012-301632.
-
7.
Green BT, Rockey DC, Portwood G, Tarnasky PR, Guarisco S, Branch MS, et al. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: a randomized controlled trial. Am J Gastroenterol. 2005;100(11):2395-402. [PubMed ID: 16279891]. https://doi.org/10.1111/j.1572-0241.2005.00306.x.
-
8.
Jensen DM, Machicado GA, Jutabha R, Kovacs TO. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342(2):78-82. [PubMed ID: 10631275]. https://doi.org/10.1056/NEJM200001133420202.
-
9.
Darcy M. Treatment of lower gastrointestinal bleeding: vasopressin infusion versus embolization. J Vasc Interv Radiol. 2003;14(5):535-43. [PubMed ID: 12761306].
-
10.
Schuetz A, Jauch KW. Lower gastrointestinal bleeding: therapeutic strategies, surgical techniques and results. Langenbecks Arch Surg. 2001;386(1):17-25. [PubMed ID: 11405084].
-
11.
Shin JH. Recent update of embolization of upper gastrointestinal tract bleeding. Korean J Radiol. 2012;13 Suppl 1:S31-9. [PubMed ID: 22563285]. https://doi.org/10.3348/kjr.2012.13.S1.S31.
-
12.
Rosenkrantz H, Bookstein JJ, Rosen RJ, Goff W2, Healy JF. Postembolic colonic infarction. Radiology. 1982;142(1):47-51. [PubMed ID: 6975953]. https://doi.org/10.1148/radiology.142.1.6975953.
-
13.
Valek V, Husty J. Quality improvement guidelines for transcatheter embolization for acute gastrointestinal nonvariceal hemorrhage. Cardiovasc Intervent Radiol. 2013;36(3):608-12. [PubMed ID: 23150119]. https://doi.org/10.1007/s00270-012-0462-5.
-
14.
Brown DB, Nikolic B, Covey AM, Nutting CW, Saad WE, Salem R, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2012;23(3):287-94. [PubMed ID: 22284821]. https://doi.org/10.1016/j.jvir.2011.11.029.
-
15.
Baum S, Nusbaum M, Blakemore WS, Finkelstein AK. The preoperative radiographic demonstration of intra-abdominal bleeding from undetermined sites by percutaneous selective celiac and superior mesenteric arteriography. Surgery. 1965;58(5):797-805. [PubMed ID: 5294694].
-
16.
Teng HC, Liang HL, Lin YH, Huang JS, Chen CY, Lee SC, et al. The efficacy and long-term outcome of microcoil embolotherapy for acute lower gastrointestinal bleeding. Korean J Radiol. 2013;14(2):259-68. [PubMed ID: 23483780]. https://doi.org/10.3348/kjr.2013.14.2.259.
-
17.
Kwak HS, Han YM, Lee ST. The clinical outcomes of transcatheter microcoil embolization in patients with active lower gastrointestinal bleeding in the small bowel. Korean J Radiol. 2009;10(4):391-7. [PubMed ID: 19568468]. https://doi.org/10.3348/kjr.2009.10.4.391.
-
18.
Tan KK, Wong D, Sim R. Superselective embolization for lower gastrointestinal hemorrhage: an institutional review over 7 years. World J Surg. 2008;32(12):2707-15. [PubMed ID: 18843444]. https://doi.org/10.1007/s00268-008-9759-6.
-
19.
d'Othee BJ, Surapaneni P, Rabkin D, Nasser I, Clouse M. Microcoil embolization for acute lower gastrointestinal bleeding. Cardiovasc Intervent Radiol. 2006;29(1):49-58. [PubMed ID: 16328695]. https://doi.org/10.1007/s00270-004-0301-4.
-
20.
Funaki B, Kostelic JK, Lorenz J, Ha TV, Yip DL, Rosenblum JD, et al. Superselective microcoil embolization of colonic hemorrhage. AJR Am J Roentgenol. 2001;177(4):829-36. [PubMed ID: 11566683]. https://doi.org/10.2214/ajr.177.4.1770829.
-
21.
Bandi R, Shetty PC, Sharma RP, Burke TH, Burke MW, Kastan D. Superselective arterial embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2001;12(12):1399-405. [PubMed ID: 11742013].
-
22.
Hur S, Jae HJ, Lee M, Kim HC, Chung JW. Safety and efficacy of transcatheter arterial embolization for lower gastrointestinal bleeding: a single-center experience with 112 patients. J Vasc Interv Radiol. 2014;25(1):10-9. [PubMed ID: 24286939]. https://doi.org/10.1016/j.jvir.2013.09.012.
-
23.
Yap FY, Omene BO, Patel MN, Yohannan T, Minocha J, Knuttinen MG, et al. Transcatheter embolotherapy for gastrointestinal bleeding: a single center review of safety, efficacy, and clinical outcomes. Dig Dis Sci. 2013;58(7):1976-84. [PubMed ID: 23361570]. https://doi.org/10.1007/s10620-012-2547-z.
-
24.
Weldon DT, Burke SJ, Sun S, Mimura H, Golzarian J. Interventional management of lower gastrointestinal bleeding. Eur Radiol. 2008;18(5):857-67. [PubMed ID: 18185932]. https://doi.org/10.1007/s00330-007-0844-2.
-
25.
Waugh J, Madan A, Sacharias N, Thomson K. Embolization for major lower gastrointestinal haemorrhage: five-year experience. Australas Radiol. 2004;48(3):311-7. [PubMed ID: 15344979]. https://doi.org/10.1111/j.0004-8461.2004.01313.x.
-
26.
Foutch PG. Colonic angiodysplasia. Gastroenterologist. 1997;5(2):148-56. [PubMed ID: 9193932].
-
27.
Foutch PG. Angiodysplasia of the gastrointestinal tract. Am J Gastroenterol. 1993;88(6):807-18. [PubMed ID: 8389094].
-
28.
Hongsakul K, Pakdeejit S, Tanutit P. Outcome and predictive factors of successful transarterial embolization for the treatment of acute gastrointestinal hemorrhage. Acta Radiol. 2014;55(2):186-94. [PubMed ID: 23904090]. https://doi.org/10.1177/0284185113494985.
-
29.
Bulakbasi N, Kurtaran K, Ustunsoz B, Somuncu I. Massive lower gastrointestinal hemorrhage from the surgical anastomosis in patients with multiorgan trauma: treatment by subselective embolization with polyvinyl alcohol particles. Cardiovasc Intervent Radiol. 1999;22(6):461-7. [PubMed ID: 10556404].
-
30.
Evangelista PT, Hallisey MJ. Transcatheter embolization for acute lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2000;11(5):601-6. [PubMed ID: 10834491].
-
31.
Kuo WT, Lee DE, Saad WE, Patel N, Sahler LG, Waldman DL. Superselective microcoil embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2003;14(12):1503-9. [PubMed ID: 14654483].
-
32.
Peck DJ, McLoughlin RF, Hughson MN, Rankin RN. Percutaneous embolotherapy of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 1998;9(5):747-51. [PubMed ID: 9756061].
-
33.
Lundgren JA, Matsushima K, Lynch FC, Frankel H, Cooney RN. Angiographic embolization of nonvariceal upper gastrointestinal bleeding: predictors of clinical failure. J Trauma. 2011;70(5):1208-12. [PubMed ID: 21610434]. https://doi.org/10.1097/TA.0b013e318213faf1.