Abstract
Background:
To present our findings obtained from the treatment of wide-necked intracranial aneurysms with flow diverter stents.Patients and Methods:
Intracranial aneurysms were determined in 19 patients (14 females, 5 males; mean age, 56.6 years) between May 2011 and June 2014, and the patients were treated using flow diverter stents. A total of 28 flow diverter stents, including 9 Silk and 19 Pipeline stents were used. Occlusion rates evaluated with computed tomography at the end of month 6 and digital subtraction angiography after one year, intraoperative success, morbidity and mortality rates were determined.Results:
A total of 20 endovascular interventions, including two interventions in two different aneurysms in one patient were performed in 19 patients. Flow diverter stents were successfully implemented for wide-necked aneurysms at a success rate of 100%. Coil treatment had previously been performed in four patients. Aneurysm occlusion rates were 77.2% at month 6 and 90.9% at the end of month 12.Conclusion:
The use of flow diverter stents is a reliable and effective method in the treatment of wide-necked intracranial aneurysms with high aneurysm occlusion, and low morbidity and mortality rates.Keywords
1. Background
Flow diverter stents are safe and reliable therapeutic modalities in the treatment of wide-necked intracranial aneurysms (1). In the treatment of wide-necked large and giant aneurysms inappropriate for embolization with coils, the use of flow diverter stents are becoming more widespread with increased rates of aneurysm occlusion, and decreased complications and mortality levels (2-4).
2. Objectives
In the present study, we aimed at demonstrating treatment success, effectiveness and occlusion rates of flow diverter stents used to treat wide-necked intracranial aneurysms.
3. Patients and Methods
3.1. Patients
After obtaining an approval from the local ethical committee, data obtained from patients who were diagnosed with wide-necked intracranial aneurysms between May 2011 and June 2014 and treated with flow diverter stents were retrospectively evaluated in the department of interventional radiology in the Medical faculty of Baskent University hospital. A total of 23 aneurysms in 19 patients were treated with flow diverter stents. The decision of using flow diverter stents in all patients was taken by an experienced interventional radiology and neurosurgery team based on the characteristics of aneurysms, such as dimension, neck width, dome length and localization.
3.2. Preoperative Evaluation
Contrast computed tomography (CT) and magnetic resonance imaging (MRI) were performed in all patients before the procedure to assess the nature of aneurysms. Multiplan reconstructions based on maximum intensity projection images of reconstructions were used to identify suitable aneurysms and optimum stents for intervention.
3.3. Materials
Consisting of two commercial types, 28 flow diverter stents were used in the study for 23 intracranial aneurysms detected in 19 patients. While 19 were Pipeline type of stents (ev3 Neurovascular, Chestnut Medical Technologies, Inc, Menlo Park, CA, USA), nine were Silk type (Balt, Montmorency, France). More than one stent was placed in some patients during a single session.
3.4. Procedure
All patients were informed regarding the treatment regime before performing the procedure. Complications were explained, and written consent forms were obtained from all patients. Procedures were performed in the angiography department by a team composed of interventional radiology physicians, neurosurgeons, and anesthetists. A digital subtraction angiography (DSA) Siemens Artis Zee-branded device (Siemens Medical Solutions, Forchheim, Germany) was used in implementing interventions.
Following patients were taken to the angiography table, and necessary conditions were obtained for sterilization in the setting. Prilocaine was administered as a local anesthetic agent guided by ultrasonography (USG). A 5F-6F vascular introducer (Terumo, Tokyo, Japan) was placed into the femoral artery of each patient using Seldinger’s method guided by USG and fluoroscopy. Diagnostic angiography was carried out with the use of 4F Davis catheters (Cordis Corp, Johnson and Johnson, Miami Lakes, FL, USA) and 0.035-inch hydrophilic guide wires (Terumo Corp, Tokyo, Japan). Selective catheterization was performed in both common carotid arteries and vertebral arteries, and aneurysms were evaluated based on different projection images. After diagnostic angiography, all patients were sedated under general anesthesia. By positioning each patient to open the neck of the aneurysm and artery with rotational angiography, long vascular sheath (Arrow Destination, Terumo, Tokyo, Japan) was first placed, and then a guide catheter (6F Envoy; Corp, Johnson and Johnson, 6F Penumbra Neuron Penumbra Inc, Harbor Bay Pkwy Alameda, CA, USA / Chaperon; Micromention Terumo, Japan / 6F Fargomax; Balt Extrusion, France) was located to reach an appropriate state in the cavernous segment of the internal carotid artery (ICA) or in the V3 segment of the vertebral artery.
Micro catheter and guide wire were advanced into distal arteries by passing the neck of the aneurysm with the help of manipulations via micro catheter and glide wire. The transporter catheter was advanced through coaxial method along a neurovascular micro guide to the distal of the aneurysmal neck. Then, flow diverter stents were aligned with the distal end of the micro catheter by pushing forward through the transporter catheter. The stent was removed out of its sheath by withdrawing the micro catheter slightly. The “push and pull” method was utilized to fix and open the stent in a better position, and the stent was placed along the aneurysmal neck. Angiographic control images were performed in order to investigate the exact stent openness and the involvement of the aneurysmal neck. On these images, aneurysmal filling and emptying times, and stagnation of intra aneurysmal flow were investigated. On conditions where the necks of the aneurysms were not completely filled, or no anticipated stagnation was achieved in the filling pattern of the aneurysms, additional stents were placed. While Vasco+21 and Vasco+25 micro catheters (Balt Extrusion, Montmorency, France) were used to treat intracranial aneurysms with Silk stents, micro catheters branded by Marksman (ev3 Neurovascular, Irvine, CA, USA) or Rebar (ev3 Neurovascular, Irvine, CA, USA) were chosen to treat intracranial aneurysms with Pipeline stents. In both therapeutic interventions, micro guide wires branded by Transend 205 EX floppy (Boston Scientific, Natek, NA, USA), Terumo Radifocus (0.016”, Tokyo, Japan), Xpedion (0.014”, ev3 Neurovascular, Irvine, CA, USA) or Synchro 14 (Boston Scientific, Natek, NA, USA) were used. In patients for whom coils were used along with flow diverter stents, 0.010-inch micro coils (Axium, ev3 Neurovascular, Irvine, CA, USA; Compass, Cosmos or Hypersoft, Terumo, Tokyo, Japan) and 0.014-inch micro catheters (Echleon, ev3 Neurovascular, Irvine, CA, USA) were placed into the lumens of the aneurysms with the guidance of 0.012-inch micro guide wires (Terumo Radifocus, Tokyo, Japan) before placing flow diverter stents.
3.5. Medical Therapy
Only elective patients with no ruptured aneurysms were treated with a combination of 300 mg/day acetyl salicylic acid (Bayer, Germany) and 300 mg clopidogrel bisulfate as the loading dose and 75 mg/day thereafter (Sanofi Winthrop Industrie, France), three days prior to the procedure. Prophylactic antibiotic treatment was given to all patients prior to endovascular interventions.
Each patient was initially sedated with a combination of 0.1 mg of fentanyl and 2 mg of midazolam, and then anesthesia was intra-interventionally maintained with propofol between the doses of 150 mg and 400 mg. In addition, to achieve optimal activated clotting time (250 - 300 sec), 5000 - 10,000 units of heparin (50 - 100 units/kg) were administered intravenously.
A combined treatment with acetyl salicylic acid (300 mg/day) and clopidogrel bisulfate (75 mg/day) was initially started for three months after the procedure, and then the treatment including only acetyl salicylic acid (300 mg/day) was planned as a lifetime regime.
3.6. Follow-Up
To examine whether the stents were correctly localized, CT and MRI angiographies were performed in each patient within a week following the procedure. For all patients, control images were formed via CT angiographies at month 6, and the evaluations of DSA were conducted at the end of the first year. Additional control investigations were carried out in patients for whom CT and MRI angiographies were considered to be necessary.
4. Results
4.1. Patients
A total of 23 aneurysms in 19 patients were treated with flow diverter stents. Nineteen Pipeline stents (ev3 Neurovascular, Chestnut Medical Technologies, Inc, Menlo Park, CA, USA) were used for 15 aneurysms detected in 11 patients, while nine Silk stents (Balt, Montmorency, France) were used for eight aneurysms in eight patients. Our patients were composed of 14 women (73.7%) and five men (26.3%). Patients’ ages ranged from 38 to 74 (mean 56.6 years). A total of 20 interventional procedures were performed in 19 patients. In one patient, the treatment was performed on different aneurysms detected in both ICAs at different times. Patients’ clinical symptoms are presented in Table 1.
Patients’ Clinical Presentation
| Presentation | No. | % |
|---|---|---|
| Asymptomatic | 6 | 31.5 |
| SAH | 5 | 26.3 |
| Headache | 4 | 21 |
| Speech Disorders | 2 | 10.5 |
| Blurred Vision | 1 | 5.2 |
| Dizziness | 1 | 5.2 |
4.2. Technical Success and Characteristics of Aneurysms
Stents were successfully placed in a total of 20 procedures in 19 patients as an adequate coverage of aneurysmal necks without intraprocedural complications. The characteristics of aneurysms are shown in Table 2.
Distribution of Aneurysms According to Location, Morphology and Size
| Variables | No. of Aneurysms | % |
|---|---|---|
| Location | ||
| ICA-cavernous | 11 | 47.8 |
| ICA-supraclinoid | 9 | 39.1 |
| ACA | 1 | 4.3 |
| PCA | 1 | 4.3 |
| Basilar Artery | 1 | 4.3 |
| Morphology | ||
| Saccular | 21 | 91.3 |
| Fusiform | 2 | 8.6 |
| Size, mm | ||
| Small (< 10) | 12 | 52.1 |
| Large (10 - 25) | 10 | 43.4 |
| Giant (> 25) | 1 | 4.3 |
4.3. Patients Exposed to Previous Endovascular Treatment
Of 19 patients treated with flow diverter stents, four had been exposed to previous endovascular treatment with coils. In three patients developing subarachnoid hemorrhage due to intracranial aneurysmal ruptures, urgent coil embolization was performed. However, because the necks of ruptured aneurysms were too wide and because of coil dangle, we decided to treat all patients’ aneurysmal necks with flow diverter stents. In one of these patients, the anterior chorodial artery originated from the aneurysm neck. After placing a flow diverter stent, the patency was obtained in this artery.
In a patient whose aneurysm in the supraclinoid segment of right ICA was treated with coil, a flow diverter stent was placed in a way to cover the whole neck due to a saccular type of recurred aneurysm 10 mm in size in the aneurysm neck.
As a result of selective angiography performed in another patient due to acute infarction in the left middle cerebral artery, a saccular type of partial thrombosized aneurysm nearly 15 mm in size was observed in the supraclinoid segment of left ICA. Additionally, the left middle cerebral artery was totally occluded from the origin of the inferior truncus. So, selective thrombolytic treatment was performed, and the inferior truncus was seen to be 80% recanalized on angiographic control images. Afterwards, a flow diverter stent was placed into the aneurysm.
4.4. Follow-Up
Of 19 patients exposed to the treatment of flow diverter stents, the general status of a patient with subarachnoid hemorrhage due to an aneurysmal rupture in the supraclinoid segment of the ICA became increasingly impaired based on abundant hemorrhage. The patient was also surgically treated but died on the seventh day after surgery due to sepsis.
According to Raymond-Roy occlusion criteria, occlusion levels in 22 aneurysms detected in the other 18 patients were assessed (5). According to the assessments, aneurysm occlusion rates were found as 77.2% (17/22) and 90.9% (20/22) at the end of 6-month and 1-year follow-ups, respectively.
In controls at month 6, while total occlusions were observed in 17 aneurysms, partial filling was detected in three aneurysms. No occlusion, however, was determined in two aneurysms. In control investigations at the end of the first year, three aneurysms of partial filling were totally occluded. In one of two aneurysms with no occlusion, partial filling was observed, while filling was seen as slow-flow in another.
In 6-month controls, the patient with no occlusion, for whom a flow diverter stent had been placed into the aneurysm in the supraclinoid segment of the right ICA, was retreated with a second flow diverter stent. In the control of the same patient at the end of the first year, partial filling was observed in the neck of the aneurysm on DSA images.
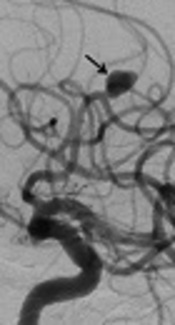
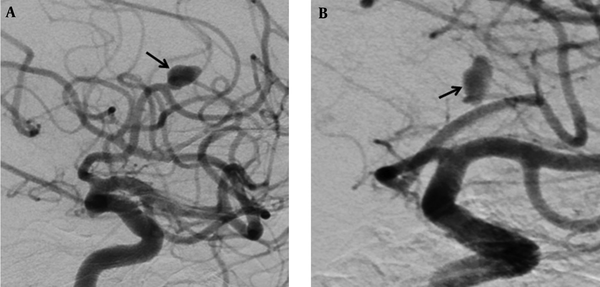
In another patient with no occlusion in the control investigations at month 6, for whom a flow diverter stent had previously been placed into the aneurysm in the A2 segment of the anterior cerebral artery, no interventional procedure was performed. On 1-year angiographic images, slow-flow filling was observed in the aneurysm (Figure 1A and B). Treatment of clopidogrel bisulfate (Sanofi Winthrop Industrie, Fransa) was discontinued. Control CT angiography was planned one month after drug discontinuation.
Digital subtraction angiographic images of a 69-year-old man with wide-necked aneurysm in the anterior cerebral artery. A, Preoperative digital subtraction angiography (DSA) shows a wide-necked aneurysm (black arrow), B, Including one-year DSA follow-up for repletion of the aneurysm with slow flow (black arrow).
In the patient with partial thrombosized saccular aneurysm in the cavernous segment of the left ICA, although in-stent minimal hyperplasia was observed on images performed at month 3, the image of minimal hyperplasia disappeared in 1-year angiography.
4.5. Complications
After the endovascular treatment, the morbidity and mortality rates were found as 5.5% and 5.6%, respectively. The patient developing subarachnoid hemorrhage due to aneurysmal rupture in the supraclinoid segment of ICA died of sepsis on the 7th day after surgery in the intensive care unit.
In the patient with a 20-mm thrombosized saccular aneurysm in the cavernous segment of the right ICA, speech disorder and loss of sense on the left side of the body developed one day after treatment. On diffusion weighted MRI, regions of diffusion restrictions consistent with large acute infarction were detected in the right cerebral hemisphere.
In another patient whose right common femoral artery was entered, pseudoaneurysm developed after treatment. With the guidance of USG, thrombin (500 U/mL) (Tisseel VH, Baxter, Glendale, California, USA) was injected into the pseudoaneurysm, and occlusion was achieved. The pseudoaneurysm was not included into the rate of morbidity because it did not affect the rest of the patient’s life.
5. Discussion
Endovascular coil embolization of intracranial aneurysms has become so influential due to incredible developments in micro catheter and micro wire methods and technologies. However, the migration of coils to common arteries in the treatment of wide-necked and fusiform aneurysms has led to some technical difficulties (6). Additionally, the reconstruction of defective vessel walls is quite difficult without a supportive frame such as stents. Such a condition also causes the development of recurrences in wide-necked aneurysms treated with coils. In their study, Murayama et al. reported recurrence rates of 35.3% for large aneurysms and 59.1% for giant aneurysms. In addition, the endovascular treatment of this type of aneurysms with coils is too expensive and leads to financial burdens (7, 8).
Such different methods as balloon remodeling, stent-first, balloon-in-stent, stent-jack, jailed catheter and telescopic stenting are used in order to stabilize coils in aneurysmal sacs. In the study performed by Lylyk et al., the rates of mortality and morbidity in patients treated with stents in company with coils were accounted for as 6.3% and 10.9%, respectively. Interventional procedures were required to be repeated in 19% of these patients due to insufficient occlusions (9).
Flow diverter stents lead to slow occlusion in time by decreasing the flow in the aneurysm. Complementing occlusion with flow diverter stents takes longer time compared with surgical and coil treatments (10, 11). In a study by Malatesta et al., the rates of aneurysmal occlusions were reported as 60% at month 3, 73% at month 6, and 89% at month 12 (12). In our study, while partial filling was observed in aneurysmal necks of three patients on control angiographies performed at month 6, the aneurysms were entirely occluded on 1-year control angiographies. Also, in the study performed by Brinjikji et al., in 1451 patients with 1654 aneurysms, aneurysm occlusion rates were detected as 76% (1). In our study, occlusion rates of aneurysms were also found as 77.2 % at the sixth month and 90.9% at the end of the first year.
In reports, recurrence rates are pointed out to be lower in the treatment of wide-necked aneurysms with flow diverter stents, and recurred aneurysms are also reported as minimal due to lower likelihood of occlusions in time (13). In one of our patients with recurred aneurysm whose aneurysm was treated with coil, a flow diverter stent was placed in a way to cover the whole neck. On control angiography at month 6, the patient was reoperated due to continuation of filling on the aneurysmal neck at the same rate, and another flow diverter stent was placed. On angiography performed at month 6 after the second intervention, a partial residual filling was observed on the aneurysmal neck.
A potential adverse effect of endovascular treatment of aneurysms with stents is stent-induced thrombosis, and the main reason of the problem is hyperactivated thrombocytes (14). Ischemic strokes may develop as a result of thrombus and distal thromboembolic events forming along the stent wall. Encountering ischemic strokes is higher in large and giant aneurysms (1). Also in our study, a multiple number of embolic infarctions developed in the right cerebral hemisphere in one patient one day after intervention. A left hemiplegia developed in the patient.
In-stent stenosis is another complication seen in the treatment of aneurysms with stents. Among the reasons leading to in-stent stenosis, possible inflammation and neointimal proliferation after stent placement are considered to be culprits (14). In our study, minimal in-stent hyperplasia was observed in one patient’s angiographic investigations performed at month 3. The patient reported that he had not regularly complied with anti-thrombocyte treatment; so the medical treatment was planned again. On one-year angiographic images, no in-stent or intimal hyperplasia was determined.
The risk of developing perforating infarctions is higher in posterior circulation compared to anterior circulation. The reason is insufficiency of collateral circulation and sensitivity of brain stem formations to perfusions (1). So, no endovascular treatments should be performed in posterior circulation, unless necessary. In our study, a flow diverter stent was placed in a patient’s aneurysm in partially thrombosized basilar artery due to the occurrence of frequently repeated infarctions. No complications developed in intra- and postoperative periods. On one-year control angiographic images, it was observed that aneurysmal filling completely disappeared, and the flow diverter stent placed was preserving the patency.
The incidence of subarachnoid hemorrhages based on delayed aneurysmal ruptures in patients treated with flow diverter stents is approximately 4%, although exactly unknown (1). However, worries about the risk of delayed aneurysmal ruptures are so serious that one of the leading companies in the field has announced a warning notice on not using Silk flow diverter stents without coils due to risk of death (15). Despite the existence of studies reporting that using coils decreases such complication rates, the extent to which Silk flow diverter stents lead to death without using coils still remains unknown. In our study, there are no patients developing subarachnoid hemorrhages led by delayed aneurysmal ruptures.
Intraparenchymal hemorrhages are also other complications developing as a result of using flow diverter stents with no relationships to aneurysmal ruptures. However unknown the mechanism leading to these hemorrhages remains, some factors such as hemorrhagic transformation and use of anti-platelet are alleged as guilty culprits (16-18). No intraparenchymal hemorrhages were observed in our study although the rates of the hemorrhages were reported to range between 0% and 10% in several studies (16-18).
One of the most significant advantages of flow diverter stents is to obtain the openness of arteries originated from the wall of the aneurysm or from the common artery wall to be covered with a stent. As opposed to closed stents (without gaps), porous structure of flow diverter stents (with gaps) allows blood to pass. In the study by Szikora et al., flow diverter stents were used to close a total of 28 origins of arteries, 17 consisting of ophthalmic arteries, five of posterior communicant arteries and four of anterior choroidal arteries (13). Szikora et al. had to place second, third or fourth flow diverter stents in the same patient. In one of these patients, no flow was observed in the ophthalmic artery shortly after the treatment, and the occlusion of retinal artery was observed in the patient. In control investigations at month 6, occlusions of ophthalmic arteries were detected in two patients exposed to third or fourth placements of flow diverter stents (13). In our study, however, anterior choroidal artery was originated from the aneurysm neck in one patient. In angiographic investigations performed at month 6 after placing a flow diverter stent, it was seen that the aneurysm was entirely occluded, and the anterior choroidal artery was preserving the patency.
In the treatment with flow diverter stents, more than one stent may overlappingly be used or placed in a telescopical way. The ability of stents to be placed telescopically allows them to be reused in the treatment process with flow diverter stents in the existence of residual or recurrent aneurysms (19, 20). In one of our patients previously treated with a flow diverter stent, in whom a difference was not seen in terms of aneurysmal filling in 6-month angiographic investigations, a second flow diverter stent was telescopically placed. On 6-month angiographic images after the intervention, minimal residual filling was observed at the aneurysmal neck. As well as this patient, two additional stents in one patient and three additional stents in another were telescopically placed in our study.
In conclusion, flow diverter stents are safe and reliable treatment modalities, especially in the treatment of wide-necked intracranial aneurysms due to high aneurysmal occlusion rates, and lower rates of morbidity and mortality. Flow diverter stents are also known as life-saving procedures in aneurysms where arteries are originated from the aneurysmal wall to be covered by stenting or in patients that cannot be treated with surgical interventions. We consider that more comprehensive clinical and angiographic studies including long-term periods are needed to evaluate the effectiveness and safety of flow diverter stents.
References
-
1.
Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013;44(2):442-7. [PubMed ID: 23321438]. https://doi.org/10.1161/STROKEAHA.112.678151.
-
2.
Ansari SA, Lassig JP, Nicol E, Thompson BG, Gemmete JJ, Gandhi D. Thrombosis of a fusiform intracranial aneurysm induced by overlapping neuroform stents: case report. Neurosurgery. 2007;60(5):E950-1. discussion E950-1. [PubMed ID: 17460508]. https://doi.org/10.1227/01.NEU.0000255427.08926.DC.
-
3.
Murthy SB, Shah S, Shastri A, Venkatasubba Rao CP, Bershad EM, Suarez JI. The SILK flow diverter in the treatment of intracranial aneurysms. J Clin Neurosci. 2014;21(2):203-6. [PubMed ID: 24128768]. https://doi.org/10.1016/j.jocn.2013.07.006.
-
4.
Wong GK, Kwan MC, Ng RY, Yu SC, Poon WS. Flow diverters for treatment of intracranial aneurysms: current status and ongoing clinical trials. J Clin Neurosci. 2011;18(6):737-40. [PubMed ID: 21514166]. https://doi.org/10.1016/j.jocn.2010.10.011.
-
5.
Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32(9):1998-2004. [PubMed ID: 11546888]. https://doi.org/10.1161/hs0901.095600.
-
6.
David CA, Vishteh AG, Spetzler RF, Lemole M, Lawton MT, Partovi S. Late angiographic follow-up review of surgically treated aneurysms. J Neurosurg. 1999;91(3):396-401. [PubMed ID: 10470813]. https://doi.org/10.3171/jns.1999.91.3.0396.
-
7.
Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. 2003;98(5):959-66. [PubMed ID: 12744354]. https://doi.org/10.3171/jns.2003.98.5.0959.
-
8.
Sluzewski M, van Rooij WJ, Slob MJ, Bescos JO, Slump CH, Wijnalda D. Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology. 2004;231(3):653-8. [PubMed ID: 15118115]. https://doi.org/10.1148/radiol.2313030460.
-
9.
Lylyk P, Cohen JE, Ceratto R, Ferrario A, Miranda C. Endovascular reconstruction of intracranial arteries by stent placement and combined techniques. J Neurosurg. 2002;97(6):1306-13. [PubMed ID: 12507128]. https://doi.org/10.3171/jns.2002.97.6.1306.
-
10.
Fiorella D, Woo HH, Albuquerque FC, Nelson PK. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62(5):1115-20. discussion 1120-1. [PubMed ID: 18580809]. https://doi.org/10.1227/01.neu.0000325873.44881.6e.
-
11.
Fiorella D, Kelly ME, Albuquerque FC, Nelson PK. Curative reconstruction of a giant midbasilar trunk aneurysm with the pipeline embolization device. Neurosurgery. 2009;64(2):212-7. discussion 217. [PubMed ID: 19057425]. https://doi.org/10.1227/01.NEU.0000337576.98984.E4.
-
12.
Malatesta E, Nuzzi NP, Divenuto I, Fossaceca R, Lombardi M, Cerini P, et al. Endovascular treatment of intracranial aneurysms with flow-diverter stents: preliminary single-centre experience. Radiol Med. 2013;118(6):971-83. [PubMed ID: 23801392]. https://doi.org/10.1007/s11547-013-0944-9.
-
13.
Szikora I, Berentei Z, Kulcsar Z, Marosfoi M, Vajda ZS, Lee W, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the pipeline embolization device. AJNR Am J Neuroradiol. 2010;31(6):1139-47. [PubMed ID: 20150304]. https://doi.org/10.3174/ajnr.A2023.
-
14.
Lozen A, Manjila S, Rhiew R, Fessler R. Y-stent-assisted coil embolization for the management of unruptured cerebral aneurysms: report of six cases. Acta Neurochir (Wien). 2009;151(12):1663-72. [PubMed ID: 19618104]. https://doi.org/10.1007/s00701-009-0436-9.
-
15.
Lubicz B, Collignon L, Raphaeli G, Pruvo JP, Bruneau M, De Witte O, et al. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: a prospective study in 29 patients with 34 aneurysms. Stroke. 2010;41(10):2247-53. [PubMed ID: 20798369]. https://doi.org/10.1161/STROKEAHA.110.589911.
-
16.
Byrne JV, Beltechi R, Yarnold JA, Birks J, Kamran M. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PLoS One. 2010;5(9). [PubMed ID: 20824070]. https://doi.org/10.1371/journal.pone.0012492.
-
17.
Berge J, Biondi A, Machi P, Brunel H, Pierot L, Gabrillargues J, et al. Flow-diverter silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. AJNR Am J Neuroradiol. 2012;33(6):1150-5. [PubMed ID: 22300924]. https://doi.org/10.3174/ajnr.A2907.
-
18.
Cruz JP, Chow M, O'Kelly C, Marotta B, Spears J, Montanera W, et al. Delayed ipsilateral parenchymal hemorrhage following flow diversion for the treatment of anterior circulation aneurysms. AJNR Am J Neuroradiol. 2012;33(4):603-8. [PubMed ID: 22403783]. https://doi.org/10.3174/ajnr.A3065.
-
19.
Fiorella D, Albuquerque FC, Masaryk TJ, Rasmussen PA, McDougall CG. Balloon-in-stent technique for the constructive endovascular treatment of "ultra-wide necked" circumferential aneurysms. Neurosurgery. 2005;57(6):1218-27. discussion 1218-27. [PubMed ID: 16331170].
-
20.
de Paula Lucas C, Piotin M, Spelle L, Moret J. Stent-jack technique in stent-assisted coiling of wide-neck aneurysms. Neurosurgery. 2008;62(5 Suppl 2):ONS414-6. discussion ONS416-7. [PubMed ID: 18596523]. https://doi.org/10.1227/01.neu.0000326028.47090.5f.