Abstract
Keywords
Minute Pulmonary Meningothelial - Like Nodule (MPMN) Diffuse Pulmonary Meningotheliomatosis High Resolution Computed Tomography (HRCT) Lung Neoplasms
1. Introduction
Minute pulmonary meningothelial - like nodules (MPMNs) are often incidentally diagnosed in resected lungs or autopsy specimens. They are also known as diffuse pulmonary meningotheliomatosis (1). The etiology of the disease is unknown. Patients are usually asymptomatic. The incidence of MPMNs is variable from 0.07% to 13.8% at autopsy or surgical resection (2-5). These lesions have been thought to be benign in nature. Some studies suggested that MPMNs are more likely reactive in nature (4). It has been suggested that MPMNs are associated with benign pulmonary disease, congestive heart failure, and neoplasm such as pulmonary adenocarcinoma (2, 4, 6-8). Radiologic findings of MPMNs reported to date have been of non - specific small nodules with possible cystic change often simulating pulmonary hematogeneous metastasis in malignant condition or adenocarcinoma of the lung (1, 2, 9). We present a case of MPMNs presenting unreported radiologic findings.
2. Case Presentation
A 45 - year - old woman, referred from another hospital to our hospital, presented with multiple peribronchial consolidations in both lungs on a chest - computed tomography (CT) scan. She initially had dyspnea. She had no past history of surgery or underlying chronic medical disease. Her laboratory exam and culture of sputum and blood showed no specific finding.
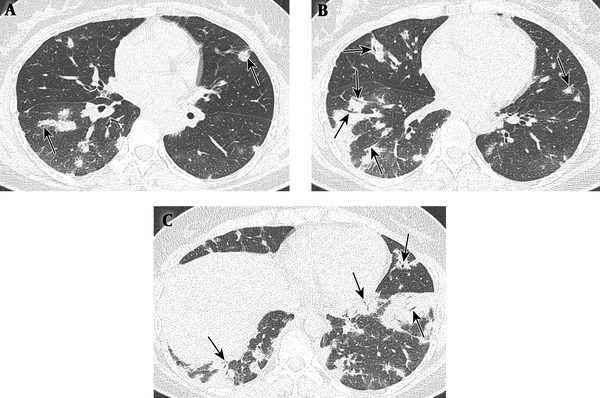
The chest CT scan from the other hospital showed multiple patchy and nodular areas of peribronchial consolidations containing patent air bronchogram with relatively homogeneous attenuation in both lungs with lower lung predominance (Figure 1). After taking antibiotic medication for 20 days, she still complained of dyspnea. Follow - up chest CT scan revealed no significant interval change of the lesions. Pneumonia was excluded due to lack of pneumonic symptoms and laboratory findings with unchanged chest CT scan and patent air bronchogram. Differential consideration included cryptogenic organizing pneumonia and multicentric consolidative primary lung malignancies such as adenocarcinomas or lymphomas. For further evaluation, bronchoscopic washing and brushing cytology, and transbronchial lung biopsy were performed at anterior and posterior basal segments of the right lower lobe of the lung. Microscopic features revealed multiple small meningothelioid nests in peribronchial interstitium. The tumor cells were positive for epithelial membrane antigen (EMA) and vimentin, but negative for cytokeratin (CK), synaptophysin, CD56, CD1a, thyroid transcription factor - 1 (TIF - 1), CD68, and S - 100 (Figure 2).
A 45-year-old woman with dyspnea. A - C, Lung window images (A - C, upper to lower) of initial chest CT. There are multiple patchy and nodular areas of peribronchial consolidations with relatively homogeneous attenuation and internal patent air bronchogram (arrows) in both lungs, with lower lung predominance.
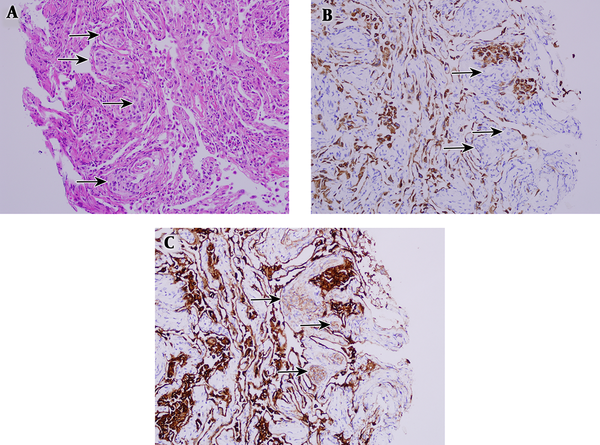
Microscopic findings. A, Transbronchial lung biopsy specimen shows the typical histological features of a minute pulmonary meningothelial - like lesion. Several areas of interstitial proliferation of cytologically bland oval to blunted spindle cells are shown (arrows). (hematoxylin - eosin, original magnification × 40). B, Tumor cells were not immunoreactive for cytokeratin (CK) (arrows, original magnification × 200). C, Tumor cells were immunoreactive for epithelial membrane antigen (EMA) (arrows, epithelial membrane antigen) (original magnification × 200).
3. Discussion
MPMNs have a prevalence of 0.07 to 13.8% at autopsy or surgical resection, but these lesions are rarely pathologically confirmed by transbronchial lung biopsy (2-5). To our knowledge, only two cases of single MPMN have been described in a transbronchial biopsy specimen (1, 10). The clinicopathologic significance of MPMNs is unclear due to the small numbers of reported studies. Multiple lesions are defined as diffuse pulmonary meningotheliomatosis or MPMN - omatosis (11). There were no significant differences found between single and multiple MPMN(s) in regards to age, sex, or location; however, there were in regards to nodule size. Each MPMN was significantly larger in multiple MPMN cases than in single MPMN cases (8).
The etiology of the disease is unknown. MPMNs occur most frequently in the sixth decade and predominantly in women (3, 8). The lesions are most often found in the upper lobe and right lung (3, 12). Diameters range from 100 µm to 3 mm.
MPMNs have been associated with various pulmonary abnormalities such as pulmonary thromboembolism, respiratory bronchiolitis - associated interstitial lung disease, desquamative interstitial pneumonia, and atypical adenomatous hyperplasia (6, 12). Additionally, association with congestive heart failure (7, 8, 12) or pulmonary neoplasm such as adenocarcinoma has been suggested (2, 4, 8).
CT findings of MPMNs describe them as a single small nodule, micronodules, and multiple small nodules. Multiple nodules can mimic pulmonary metastasis in a patient with underlying malignancy. Some cavitary changes or central lucency of multiple nodules have been reported in rare cases of the disease (1, 2). Other uncommon findings of MPMNs are ground glass opacities. Kuroki et al. suggested that this pathophysiology likely resulted from spread of the lesion along the alveolar walls (9).
To our knowledge, a pattern of peribronchial consolidations containing patent air bronchogram of MPMN has never been described in the English research so far. There are many differential diagnoses for multiple peribronchial consolidations containing patent air bronchogram including both of benign lesions, such as multifocal bronchopneumonias or cryptogenic organizing pneumonias, and pneumonic malignant lesions, such as primary pulmonary adenocarcinomas or pulmonary lymphomas (13). Adenocarcinoma lineage, especially epidermal growth factor receptor (EGFR) -mutation- positive adenocarcinomas commonly show patent air bronchogram with lepidic growth spreading along the alveolar wall (14). Therefore, in patients with lung cancer including EGFR - mutation - positive lung cancer, MPMNs should be excluded for the final diagnosis of lung to lung metastases regardless of imaging findings indicating multiple small nodules or multiple peribronchial consolidations.
Patients with MPMNs are usually asymptomatic (3). However, the patient in this report was symptomatic and she complained of dyspnea. We presumed this symptom was related to atypical extensive peribronchial involvement of MPMNs that were presented as multiple peribronchial consolidations, rather than multiple small nodules. As a result, the term of MPMN is not appropriate in this case, but rather “diffuse pulmonary meningotheliomatosis” is more correct.
In conclusion, MPMNs should be considered in the differential diagnosis of peribronchial consolidations with patent air bronchogram to prevent potential diagnostic pitfall. For the same reason, they should also be considered in the imaging findings suggestive of pneumonic type lung malignancies after excluding pneumonia, particularly when the possibility of malignancy is weak with low fluorodeoxyglucose (FDG) uptake and normal tumor markers.
Acknowledgements
References
-
1.
Bernabeu Mora R, Sanchez Nieto JM, Hu C, Alcaraz Mateos E, Gimenez Bascunana A, Rodriguez Rodriguez M. Diffuse pulmonary meningotheliomatosis diagnosed by transbronchial lung biopsy. Respiration. 2013;86(2):145-8. [PubMed ID: 23796886]. https://doi.org/10.1159/000350430.
-
2.
Kraushaar G, Ajlan AM, English JC, Muller NL. Minute pulmonary meningothelial-like nodules: a case of incidentally detected diffuse cystic micronodules on thin-section computed tomography. J Comput Assist Tomogr. 2010;34(5):780-2. [PubMed ID: 20861786]. https://doi.org/10.1097/RCT.0b013e3181e47c28.
-
3.
Gaffey MJ, Mills SE, Askin FB. Minute pulmonary meningothelial-like nodules. A clinicopathologic study of so-called minute pulmonary chemodectoma. Am J Surg Pathol. 1988;12(3):167-75. [PubMed ID: 2830799].
-
4.
Niho S, Yokose T, Nishiwaki Y, Mukai K. Immunohistochemical and clonal analysis of minute pulmonary meningothelial-like nodules. Hum Pathol. 1999;30(4):425-9. [PubMed ID: 10208464].
-
5.
Mukhopadhyay S, El-Zammar OA, Katzenstein AL. Pulmonary meningothelial-like nodules: new insights into a common but poorly understood entity. Am J Surg Pathol. 2009;33(4):487-95. [PubMed ID: 19047895]. https://doi.org/10.1097/PAS.0b013e31818b1de7.
-
6.
Spain DM. Intrapulmonary chemodectomas in subjects with organizing pulmonary thromboemboli. Am Rev Respir Dis. 1967;96(6):1158-64. [PubMed ID: 4293645]. https://doi.org/10.1164/arrd.1967.96.6.1158.
-
7.
Korn D, Bensch K, Liebow AA, Castleman B. Multiple minute pulmonary tumors resembling chemodectomas. Am J Pathol. 1960;37:641-72. [PubMed ID: 13753180]. [PubMed Central ID: PMC1942284].
-
8.
Mizutani E, Tsuta K, Maeshima AM, Asamura H, Matsuno Y. Minute pulmonary meningothelial-like nodules: clinicopathologic analysis of 121 patients. Hum Pathol. 2009;40(5):678-82. [PubMed ID: 19144385]. https://doi.org/10.1016/j.humpath.2008.08.018.
-
9.
Kuroki M, Nakata H, Masuda T, Hashiguchi N, Tamura S, Nabeshima K, et al. Minute pulmonary meningothelial-like nodules: high-resolution computed tomography and pathologic correlations. J Thorac Imaging. 2002;17(3):227-9. [PubMed ID: 12082375].
-
10.
Agozzino M, Inzani F, Cavallero A, Arbustini E, Meloni F, Oggionni T, et al. Minute pulmonary meningothelial-like nodules in the transbronchial biopsy of a lung transplant recipient. J Heart Lung Transplant. 2006;25(1):148-50. [PubMed ID: 16399548]. https://doi.org/10.1016/j.healun.2005.07.016.
-
11.
Ionescu DN, Sasatomi E, Aldeeb D, Omalu BI, Finkelstein SD, Swalsky PA, et al. Pulmonary meningothelial-like nodules: a genotypic comparison with meningiomas. Am J Surg Pathol. 2004;28(2):207-14. [PubMed ID: 15043310].
-
12.
Churg AM, Warnock ML. So-called "minute pulmonary chemodectoma": a tumor not related to paragangliomas. Cancer. 1976;37(4):1759-69. [PubMed ID: 177176].
-
13.
Jung JI, Kim H, Park SH, Kim HH, Ahn MI, Kim HS, et al. CT differentiation of pneumonic-type bronchioloalveolar cell carcinoma and infectious pneumonia. Br J Radiol. 2001;74(882):490-4. [PubMed ID: 11459727]. https://doi.org/10.1259/bjr.74.882.740490.
-
14.
Liu Y, Kim J, Qu F, Liu S, Wang H, Balagurunathan Y, et al. CT Features Associated with Epidermal Growth Factor Receptor Mutation Status in Patients with Lung Adenocarcinoma. Radiology. 2016;280(1):271-80. [PubMed ID: 26937803]. [PubMed Central ID: PMC4934516]. https://doi.org/10.1148/radiol.2016151455.
