Abstract
Background:
Technetium-99m diethylene triaminepentaacetic acid (Tc-99m DTPA) renal scintigraphy using modified Gate’s method is commonly adopted to calculate glomerular filtration rate (GFR).Objectives:
The purpose of this study was to find the correlations between GFRs, which are calculated by the in-house and non-in-house workstations from gamma cameras (GC) manufactured by different firms.Patients and Methods:
Medical records of patients receiving Tc-99m DTPA renal scan between January and December 2016 were analyzed. Patients were allocated randomly to a GC (1 or 2). The calculated GFRs were conducted by the in-house workstation (group A: workstation 1/ GC 1; group B: workstation2/ GC 2) and the non-in-house workstation from the GC (group C: workstation 2/ GC 1; group D: workstation 1/ GC 2). All patients had their creatinine levels checked to calculate the estimated GRF (eGFR) as the reference data. Comparison of correlation and difference between GFR calculations (group A, B, C and D), eGFRs and clinical parameters was analyzed.Results:
Forty patients (24 men, 16 women) were enrolled in this study. The average of eGFRs was 63.0 ± 38.5 mL/min/1.73m2. The GFRs (group A to D) calculated were all significantly correlated with the eGFR. However, there was a significant difference between group C and eGFR (P = 0.0021), and group D and eGFR (P = 0.0262). Eight patients (20%) had changed stage of chronic kidney disease when using non-in-house workstations, compared to in-house workstations from GC.Conclusion:
GFRs calculated by the non-in-house workstation were significantly different from eGFR. Use of workstation and GC from the same manufacturer would provide more accurate data in the clinical setting.Keywords
Tc-99m DTPA Glomerular Filtration Rate Different Workstations Gamma Camera Chronic Kidney Disease
1. Background
Chronic kidney disease (CKD) is characterized by progressive loss in renal function over a period of time. In Taiwan, the prevalence of CKD is high, and the number of people having CKD is expected to double by 2020 (1). Accurate measurement of glomerular filtration rate (GFR) is important to detect renal function and stratify the clinical stage of CKD. Early diagnosis can not only bring better quality of life but can also make early medical intervention possible to reduce medical waste.
There have been several methods to calculate GFR. Inulin clearance has been the widely accepted gold-standard method for measuring GFR (2). However, this method is unsuitable for routine use, due to its complex methodology. Plasma creatinine measurement is a simple method to determine renal function, but patients were not diagnosed to have renal function impairment until their creatinine level reached 1.6 mg/dL., which is clinically too late, because some patients already had stage 3, even stage 4 CKD (3).Renal scintigraphy with technetium-99m (Tc-99m) diethylene triaminepentaacetic acid (DTPA) using modified Gate’s method is another way to calculate GFR (4). The advantages include: (i) it is simple and consumes only 20 minutes, (ii) it can provide notable information such as determination of unilateral renal blood flow, distinguishing between pelvic ectasis and post-renal obstruction, and estimation of unilateral renal function. Hence, renal scintigraphy has been widely used. However, many studies have questioned the accuracy of modified Gate’s method in the measurement of GFR. De Santo et al. (5) compared the Tc-99m DTPA renal scintigraphy method with inulin clearance, and found that Gate’s method seems to underestimate GFR at high levels, and overestimate GFR at low levels of the same case. In Taiwan, researchers confirm that use of Tc-99m DTPA renal scintigraphy to measure GFR resulted in overestimation, compared to using inulin clearance, to measure GFR (6).
In our department, there are many patients who have undergone nuclear scintigraphy including Tc-99m DTPA renal scan. There are gamma cameras provided by different manufacturers to deal with these examinations. However, different gamma cameras have different counting efficiency, different workstations and even different equations to calculate the GFR. We have faced the situations where GFRs of patients would be calculated by the in-house workstation of the same brand gamma camera, or by the workstation provided by different manufacturers from the scintigraphic gamma camera. To the best of our knowledge, there is a paucity of reports discussing the discrepancy or consistency between GFRs from these situations.
2. Objectives
The aim of the current study was to find correlations between GFRs, which are calculated by the in-house workstation or other workstation from scintigraphic gamma cameras with different manufacturers.
3. Patients and Methods
3.1. Ethics Statement
The research review process was approved by the Institutional review board of Kaohsiung Medical University Hospital (KMUHIRB-E (II)-20160185). Written informed consent was waived because all the clinical data were retrospectively collected via medical chart review. However, the privileges of patients were informed upon clinical visit or admission.
3.2. Patients
The medical records of patients who underwent renal scintigraphy in our department were retrospectively reviewed. The indications for renal scintigraphy included renal function check in patients with chronic kidney disease, spinal cord injury and/or obstruction in the urinary tracts. The dates for data collection in the study were between January 2016 and December 2016. Patients were enrolled if they met the following criteria: (i) age > 20 years, (ii) had received a Tc-99m DPTA renal scintigraphy, and (iii) had laboratory test for plasma creatinine clearance done within 7 days apart from the renal scintigraphy. The exclusion criteria included history of previous renal transplantation.
3.3. Renal Scintigraphy and GFRs by Gate’s Method
Patients were encouraged to drink at least 300 ml of water 20 minutes before examination. First, we placed a syringe containing 6 mCi Tc-99m DTPA on a 30-cm thick styrofoam holder on the surface of the detector, and acquired 1 minute static image to get a pre-injection count. The patient then lay on the examination table in the supine position. After the bolus intravenous injection of Tc-99m DTPA, the dynamic imaging immediately started in a 128 × 128 frame matrix for the following 22 minutes divided into three phases. The first phase was of 32 seconds duration at a rate of 2 seconds per frame, the second of 320 seconds duration at a rate of 20 seconds, and the third phase was of 16 minutes duration at a rate of 30 seconds. Finally, the post-injection count was acquired again using the same parameters as for pre-injection.
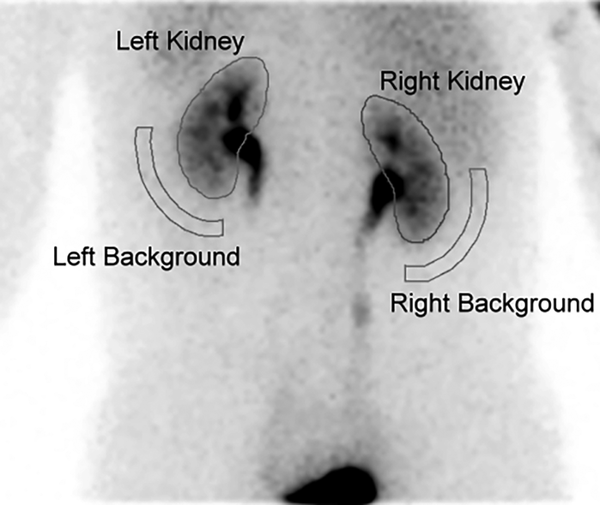
Patients were allocated to one of two scintigraphic gamma cameras from different manufacturers to get the renal scan: (i) Siemens E.CAM (Siemens, Erlangen, Germany), and (ii) GE Discovery NM 670 (GE Medical Systems, Waukesha, WI, USA). Each gamma camera was equipped with a low-energy, high-resolution collimator. A specific in-house workstation was used to calculate the patients’ GFRs: Siemens e.soft for Siemens E.CAM, and GE Xeleris for GE Discovery NM 670 gamma camera. The region of interest (ROI) for each kidney was drawn manually by an experienced nuclear medicine technician, who was blinded to the patients’ clinical history. The ROI for background subtraction was drawn automatically by placing a semilunar region around the outer-lower aspect of each kidney (Figure 1).
Demonstration of the selected region of interest (ROI) when calculating the glomerular filtration rate ( GFR) by Gate’s method from a 44-year-old man. The ROIs for each kidney were drawn manually via the posterior imaging acquisition. Background subtraction was done by placing a semilunar ROI in the outer-lower aspect of each kidney. The patient had a calculated GFR of 88 mL/min and placed clinically at stage II of chronic kidney disease (CKD).
The GFRs were calculated by the following equations with each workstation:
Total renal uptake percent (%) = [(CR - CRB) / e-μdR + (CL - CLB) / e-μdL]/(Pre iv - Post iv)
GFR = total renal uptake percent (%) × 100 × 9.8127 - 6.82519
Where Pre iv: pre-injection count, Post iv: post-injection count, CR: right kidney counts, CRB: right background counts, CL: left kidney counts, CLB: left background counts, dR: right kidney depth, dL: left kidney depth, μ: attenuation coefficient of Tc-99m in soft tissue (0.153 cm-1), e: Euler's number.
3.4. Estimated GFR (eGFR)
All patients had their plasma creatinine (Pcr) level checked. The Pcr levels were estimated in the laboratory (Department of Laboratory Medicine, Kaohsiung Medical University Hospital) with the normal reference range of 0.6 - 1.5 mg/dL on a Bechman-counter analyzer, using Jaffe’s method (7). We used the eGFR as the reference data in this study. The eGFR was a creatinine-based equation and was modified based on CKD patients in Chinese population. The eGFR was calculated using modified abbreviated modification of diet in renal disease equation (mMDRD) (8):
GFR estimated by mMDRD (mL/min/1.73m2) = 175 × (Pcr)- 1.234 × (Age)- 0.179 (× 0.79 if female)
Where Pcr was in unit of mg/dL; Age was in years.
3.5. Statistical Analysis
Continuous variables of collected data were presented as mean ± standard deviation (SD). The Kolmogorov-Smirnov test was used to test the normality of the different variables. The non-parametric statistical methods were used due to the relatively small sample size. A Spearman rank correlation test and a Mann-Whiteney U test were used to compare the correlations and the differences between variables, respectively. The agreement between calculated GFR through different workstations was accessed by Bland-Altman plot. All the analyses were performed using MedCalc Statistical Software version 17.9.7 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2017). All statistical tests were two-sided, and a two-tailed P < 0.05 was considered significant.
4. Results
4.1. Patients’ Characteristics
Table 1 lists the clinical characteristics of the total of 40 patients enrolled in this study. There were 24 men (60%) and 16 women (40%), with a mean age of 57.5 years (range, 20 - 84 years). The mean value of plasma creatinine was 1.9 ± 1.9 mg/dL. The average of eGFRs estimated by mMDRD equation was 63.0 ± 38.5 mL/ min/ 1.73m2. Overall, 9 (22.5%) of the 40 patients were at stage I, 10 (25.0%) at stage II, 13 (32.50%) at stage III, 4 (10.0%) at stage IV, and 4 (10.0%) were at stage V of CKD.
Characteristics of All 40 Patients Who Underwent Tc-99m DTPA Renal Scintigraphy
| Variable | Valuesa |
|---|---|
| Age | 57.5 ± 15.2 |
| Sex | |
| Male | 24 (60) |
| Female | 16 (40) |
| Body height (cm) | 163.1 ± 8.1 |
| Body weight (kg) | 65.0 ± 12.3 |
| BMI (kg/m2) | 24.4 ± 3.9 |
| Plasma creatinine (mg/dL) | 1.9 ± 1.9 |
| eGFR (ml/min/1.73m2) | 63.0 ± 38.5 |
| CKD stage | |
| I | 9 (22.5) |
| II | 10 (25.0) |
| III | 13 (32.5) |
| IV | 4 (10.0) |
| V | 4 (10.0) |
Using the Spearman rank analysis, the mMDRD was positively correlated with body height, body weight and body mass index (BMI). However, the correlations were not statistically significant.
4.2. Sub-Grouping Data and Comparison of the Characteristics Between Groups
The renal scintigraphic raw data was transferred into the workstations to calculate the GFR. The data were then grouped into 4 categories (Table 2). Groups A and B comprised GFRs calculated by in-house workstations from scintrgraphic gamma camera, i.e. GFRs calculated by workstation 1 from manufacturer 1 gamma camera (Group A), and GFRs calculated by workstation 2 from manufacturer 2 gamma camera (Group B). Groups C and D comprised of GFRs calculated by different workstations from scintrgraphic gamma camera, i.e. GFRs calculated by workstation 2 with the scintigraphic raw data acquired from manufacturer 1 gamma camera (Group C), and GFRs calculated by workstation 1 with the scintigraphic raw data acquired from manufacturer 2 gamma camera (Group D).
Subgrouping GFR Data by Different Scintigraphic Gamma Cameras and Workstations to Execute Gate’s Method
| Scintigraphic gamma camera | Workstation that process and calculate the GFR | |
|---|---|---|
| Workstation 1 | Workstation 2 | |
| Manufacturer 1 | Group A | Group C |
| Manufacturer 2 | Group D | Group B |
There were 24 patients having renal scintigraphy with gamma camera 1 (group A and C respectively), and 16 patients having renal scintigraphy with gamma camera 2 (group B and D respectively). The eGFR of patients scanned with gamma camera 1 and 2 were 55.6 ± 36.5 and 74.1 ± 40.0 ml/ min/ 1.73m2 respectively. There were no significant differences on gender (p = 0.7997), body height (P = 0.5164), body weight (P = 0.7093), creatinine level (P = 0.1511) and eGFR (P = 0.1434) among patients allocated to different scintigraphic gamma cameras.
4.3. Comparison of GFR Between Different Workstations
Among the 24 patients who had scintigraphic raw data from gamma camera 1, the GFR calculated by workstation 1 (group A) and workstation 2 (group C) were 43.8 ± 25.4 and 25.6 ± 14.4, respectively (P = 0.0109). The Bland-Altman plot revealed an agreement between these two workstations with the magnitude of difference changed in a linear fashion (P < 0.0001) (Figure 2A). The GFR calculated by workstation 1 (group A) and workstation 2 (group C) were both significantly correlated with the eGFR (P < 0.0001 for both groups A and C). However, while there was a significant difference between group C and eGFR (P = 0.0021), there was no statistical difference between group A and eGFR (P = 0.3753) (Table 3).
The Bland-Altman plot of the glomerular filtration rate (GFR) calculated through different workstations. A, Twenty-four patients scanned with gamma camera 1. The magnitude of difference between in-house workstation (group A) and non-in-house workstation (group C) changed in a linear fashion (P < 0.0001). Regression line is shown (y = -1.1975 + 0.5599x). B, Sixteen patients scanned with gamma camera 2. Similarly, the magnitude of difference between in-house workstation (group B) and non-in-house workstation (group D) changed in a linear fashion (P = 0.0001). Regression line is also shown (y = 9.9338 + 0.1176x).
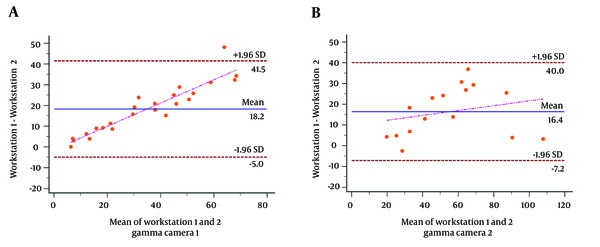
Among the 16 patients with scintigraphic raw data from gamma camera 2, the GFR calculated by workstation 2 (group B) and workstation 1 (group D) were 63.2 ± 27.5 and 46.8 ± 24.6, respectively (P = 0.0899). Similarly, the Bland-Altman plot revealed an agreement between these two workstations with the magnitude of difference changed in a linear fashion (P = 0.0001) (Figure 2B). The GFR calculated by workstation 2 (group B) and by workstation 1 (group D) were both significantly correlated with the eGFR (P = 0.0007 for group B; P = 0.0008 for group D). While there was a significant difference between group D and eGFR (P = 0.0262), there was no statistical difference between group B and eGFR (P = 0.3860) (Table 3).
4.4. Clinical Impacts of GFR Calculated from Different Workstations
Among the total of 40 patients, there were eight patients (20%) whose clinical stage of CKD changed if an in-house workstation was used, in comparison with the workstation provided by the scintigraphic gamma camera of a different manufacturer (Table 4). Among these eight patients, there were five patients scanned with gamma camera 1 and three patients scanned with gamma camera 2. Using the non-in-house workstation, five out of these patients (62.5%) were identified to underestimate the GFR, leading to an overestimated stage of CKD. However, there were not statistically significant differences between the GFR calculated by in-house and non-in-house workstations.
List of Eight Patients Whose Clinical Stage of CKD Changed by the In-House Workstation in Comparison with the Non-In-House Workstation
| Patient | Gender | Age | In-house workstation | Non-in-house workstation | P value | ||
|---|---|---|---|---|---|---|---|
| Calculated GFR | Stage of CKD | Calculated GFR | Stage of CKD | ||||
| GC: 1 | 0.4647 | ||||||
| 1 | M | 63 | 65.2 | 2 | 58.5 | 3 | |
| 2 | M | 64 | 61.5 | 2 | 57.9 | 3 | |
| 3 | F | 62 | 26.2 | 4 | 29.7 | 3 | |
| 4 | M | 68 | 15.3 | 4 | 14.6 | 5 | |
| 5 | M | 68 | 15.2 | 4 | 12.0 | 5 | |
| GC: 2 | 0.8273 | ||||||
| 6 | M | 59 | 83.2 | 2 | 97.1 | 1 | |
| 7 | F | 55 | 99.9 | 1 | 87.2 | 2 | |
| 8 | M | 60 | 21.9 | 4 | 31.1 | 3 | |
5. Discussion
This is a retrospective analysis study dealing with the comparison of GFR calculated by different workstations and by the mMDRD equation. In our patient groups, we found that GFR calculated by in-house workstation of the scintigraphic gamma camera and other manufacturer’s workstations both had good correlation with the eGFR. However, there were significant differences between GFR calculated from non-in-house workstation and the eGFR, while there was no significant difference between GFR calculated from in-house workstation and the eGFR.
Some studies found that GFR estimated from equations are closer to the true GFR (5). Abbreviated MDRD equation was the most acceptable measurement to estimate GFR (9). However, this equation was not suitable for Asians. Ma et al. modified this equation based on Chinese CKD patients, and implied that using mMDRD equation to estimate GFR was better than renal scintigraphy (8). Thus, we use the mMDRD equation as the reference method. However, this equation was developed for patients with chronic kidney disease, and might be inaccurate in patients without it. Besides, in some special groups as elders, children, underweight and overweight people, using the formula to estimate GFR renders it more prone to error (10).
In the current study, the patients were randomly allocated to gamma camera 1 and 2. There was no difference regarding gender, body height, body weight, creatinine level, and eGFR between these two patient groups. This ensured that the difference in GFR calculated by Gate’s method mainly resulted from the different workstations. Clinically, the different GFR calculated by different workstations could change the stage of CKD (20% of patients), in which 62.5% of patients were placed in a more clinically-deteriorated stage.
Some technical problems and unwanted factors were the sources of error in renal scintigraphy in the measurement of GFR. In Gate’s method, there were several conditions, like accurate counting rate in the kidneys and the background, net injection counts, linear attenuation coefficient and kidney depths, which may influence the GFR calculation (4).
The region of interest for kidneys was drawn manually. Some cases of disease status or patient movement during the image acquisition would head the edge of kidney indistinctness that produced the error of acquired counts. Single-Photon Emission Computed Tomography can help us define the kidney regions more accurately, but additional radiation to which patients would be exposed, should be taken into consideration. Different observers also produced errors. The automatic ROI setting may solve this problem in future (11).
Background subtraction is another problem that may induce errors in calculations. Using the modified Gate’s method, we drew the ROIs for the kidneys and background area, including kidneys and soft tissue around kidneys. In the previous studies, authors had mentioned that appropriate background subtraction could provide more accurate kidney counts (12, 13). When drawing the ROI of background around each kidney, excluding renal hilum could help obtain more accurate GFR (11).
The renal depth was also a variable in the modified Gate’s method. The formula of Tonnesen is commonly used to estimate renal depth in the modified Gate’s method (14). However, using the formula of Tonnesen to estimate renal depth was found to underestimate the renal depth, both in children (15) and adults (16). Results of patients with ectopic kidney or transplanted kidney using the formula to estimate renal depth were obviously invalid. Some researchers found that replacing the formula of Tonnesen that estimated renal depth with immediate measurement from bilateral views could improve the accuracy of Gate’s method for calculation of GFR (17).
Although the current study was relatively small with a retrospective design, the results underlined the difference in GFR from different workstations when calculated by Gate’s method. Further confirmatory experiments may be conducted with a prospective study design and larger study population.
In conclusion, our results suggested that the GFR calculated by the non-in-house workstation were significantly different from eGFR by mMDRD in comparison to GFR calculated by the in-house workstation from the same scintigraphic gamma camera. We hence suggest that using workstation and scintigraphic gamma camera from the same manufacturer would provide more accurate data in the clinical setting.
References
-
1.
Chen CY, Dai CS, Lee CC, Shyu YC, Huang TS, Yeung L, et al. Association between macular degeneration and mild to moderate chronic kidney disease: A nationwide population-based study. Medicine (Baltimore). 2017;96(11). e6405. [PubMed ID: 28296786]. [PubMed Central ID: PMC5369941]. https://doi.org/10.1097/MD.0000000000006405.
-
2.
Smith HW. The kidney: structure and function in health and disease. illustrated ed. New York: Oxford University Press; 1951.
-
3.
Hsu CC, Hwang SJ, Wen CP, Chang HY, Chen T, Shiu RS, et al. High prevalence and low awareness of CKD in Taiwan: a study on the relationship between serum creatinine and awareness from a nationally representative survey. Am J Kidney Dis. 2006;48(5):727-38. [PubMed ID: 17059992]. https://doi.org/10.1053/j.ajkd.2006.07.018.
-
4.
Gates GF. Glomerular filtration rate: estimation from fractional renal accumulation of 99mTc-DTPA (stannous). AJR Am J Roentgenol. 1982;138(3):565-70. [PubMed ID: 7039273]. https://doi.org/10.2214/ajr.138.3.565.
-
5.
De Santo NG, Anastasio P, Cirillo M, Santoro D, Spitali L, Mansi L, et al. Measurement of glomerular filtration rate by the 99mTc-DTPA renogram is less precise than measured and predicted creatinine clearance. Nephron. 1999;81(2):136-40. [PubMed ID: 9933747]. https://doi.org/10.1159/000045268.
-
6.
Chen LI, Kuo MC, Hwang SJ, Chen YW, Wu KD, Chen HC. Comparisons of technetium-99m diethylenetriaminepentaacetic acid plasma clearance and renal dynamic imaging with inulin clearance. Am J Kidney Dis. 2011;58(6):1043-5. [PubMed ID: 21995969]. https://doi.org/10.1053/j.ajkd.2011.08.026.
-
7.
Jaffe M. [Ueber den Niederschlag, welchen Pikrinsäure in normalem harn erzeugt und über eine neue reaction des kreatinins]. J Physiol Chem. 1886;10. German.
-
8.
Ma YC, Zuo L, Zhang CL, Wang M, Wang RF, Wang HY. Comparison of 99mTc-DTPA renal dynamic imaging with modified MDRD equation for glomerular filtration rate estimation in Chinese patients in different stages of chronic kidney disease. Nephrol Dial Transplant. 2007;22(2):417-23. [PubMed ID: 17053082]. https://doi.org/10.1093/ndt/gfl603.
-
9.
Levey AS. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11. A0828.
-
10.
Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16(3):763-73. [PubMed ID: 15659562]. https://doi.org/10.1681/ASN.2004070549.
-
11.
Inoue Y, Ohtake T, Homma Y, Yoshikawa K, Nishikawa J, Sasaki Y. Evaluation of glomerular filtration rate by camera-based method in both children and adults. J Nucl Med. 1998;39(10):1784-8. [PubMed ID: 9776288].
-
12.
Russell CD, Bischoff PG, Kontzen F, Rowell KL, Yester MV, Lloyd LK, et al. Measurement of glomerular filtration rate using 99mTc-DTPA and the gamma camera: a comparison of methods. Eur J Nucl Med. 1985;10(11-12):519-21. [PubMed ID: 3896814].
-
13.
Russell CD. Estimation of glomerular filtration rate using 99mTc-DTPA and the gamma camera. Eur J Nucl Med. 1987;12(11):548-52. [PubMed ID: 3552689].
-
14.
Tonnesen KH. Influence on the radiorenogram of variation in skin to kidney distance and the clinical importance hereof. Radionuclide Nephrol. 1975:79-86.
-
15.
Maneval DC, Magill HL, Cypess AM, Rodman JH. Measurement of skin-to-kidney distance in children: implications for quantitative renography. J Nucl Med. 1990;31(3):287-91. [PubMed ID: 2307998].
-
16.
Taylor A, Lewis C, Giacometti A, Hall EC, Barefield KP. Improved formulas for the estimation of renal depth in adults. J Nucl Med. 1993;34(10):1766-9. [PubMed ID: 8410296].
-
17.
Steinmetz AP, Zwas ST, Macadziob S, Rotemberg G, Shrem Y. Renal depth estimates to improve the accuracy of glomerular filtration rate. J Nucl Med. 1998;39(10):1822-5. [PubMed ID: 9776296].