Abstract
Keywords
1. Introduction
Since the present discussion is about exercise effects on arteries, it should be reminded that the structure and function of the central and peripheral arteries differ in different physiological and pathological adaptations in life. The different adaptations are also evident through exercise stimulus (1); therefore, the current study specifically focuses on central arteries: aorta and carotid.
Central arterial stiffness (CAS) is an independent predictor of cardiovascular mortality (2), and it is associated with many other risk factors (3, 4). The benefic reduction in CAS through endurance exercise interventions is highlighted in a variety of human population (5). A number of studies show that CAS is reduced in physically-active individuals compared to their sedentary counterparts and this reduction is more pronounced in endurance-trained individuals (6-12). Nevertheless, CAS is increased in marathon and ultramarathon runners compared to active individuals (13, 14) suggesting that the CAS adaptation can be represented by a U-curve, according to endurance exercise level (Figure 1).
Proposed Scheme for Adaptations of Central Arterial Stiffness According to Endurance Training Level
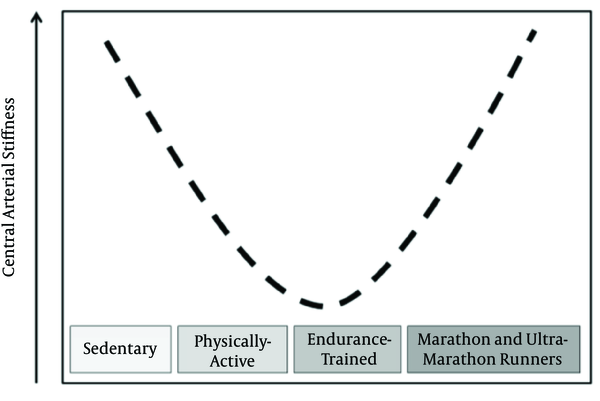
Corroborating the chronic adaptations, many studies have showed an endurance exercise session reduce (15-17) or do not change of CAS acutely (18, 19). Furthermore, similar to the chronic effects, three studies showed increase in CAS after endurance exercise session when it was more strenuous (20-22). It is noteworthy that the malefic effects of endurance training over CAS are neglected, and the increased CAS in very highly endurance-trained individuals as well as its acute increments should be investigated. The central arteries of these individuals are stiffer, even though they might have other optimal physiological adaptations. However, if it is a maladaptation or a protection to endure such strenuous training sessions will be discussed in the next paragraphs.
2. Arguments
2.1. Risks of Increased Central Arterial Stiffness
2.1.1. Acute Risks
Albert et al. (23), in a 12-year retrospective study, found that out of 122 sudden deaths (among 21,481 males) 18.8% happened during or 30 minutes after vigorous efforts. Acutely, exercise really increases the risk of cardiovascular events and the risk of sudden cardiac death might be increased in athletes from various modalities, due to higher frequency of exposure to strenuous exercise sessions (24, 25). Several physiological changes trigger sudden deaths during vigorous efforts, whether it would be related to known diseases or not. Among them, the following might be mentioned: increase in sympathetic outflow for heart and blood vessels, increasing electric instability and myocardial contraction strength; rupture of atherosclerotic plaques and appearance of embolisms through increase in blood pressure (BP), circulating catecholamines and vasomotor oscillations and rupture of aneurysms due to abrupt changes in BP (26).
Obviously, avoiding the CAS increase during acute efforts is not enough to protect from sudden death risk; therefore, it might reduce part of it. The stiffer the central arteries, smaller is the wall deformation and worse is the baroreceptor sensitivity. It enables higher BP variability, which in turn increases sympathetic outflow and circulating catecholamine levels, leading to a cardiovascular risk environment (23, 24).
There are two other factors that evince acute increase in CAS leading to immediate vulnerable state to myocardium. First, elevation in the left ventricle afterload requires higher myocardial efforts. Second, during vigorous efforts the increase in heart rate reduces diastolic duration, which concomitantly increases afterload, and impairs coronary irrigation.
Besides the immediate vulnerable state, the acute effects of exercise might also represent an opportunity to chronic adaptations. In the same way post exercise hypotension might predict chronic BP reduction (27), there are good reasons to believe that the sum of acute effects on CAS could lead to chronic adaptations in arterial wall.
It is interesting to notice that the rationale for this hypothesis mainly comes from the studies finding an increase in CAS following resistance training. Many researchers assume that acute changes in BP during exercise are the mechanical stimulus responsible for arterial wall remodeling they found following a period of training. To date, only Ozaki et al. (28) supported this relationship between acute stimulus and chronic adaptation, showing the higher BP peak during resistance exercise in the group increasing CAS following the role period of training. In this way, the acute intermittent elevations in BP that occur during resistance exercise, might overload the viscoelastic tissue of the arterial walls, and consequently alter the arterial wall structure and/or load-bearing properties (29).
Traditionally, during endurance exercise, BP does not reach such high levels as in resistance exercise. Otherwise, the two studies increasing CAS acutely after endurance exercise noticed that this increase was due to maximum BP levels during exercise sessions. Hu et al. (20) explained that it was due to very high intensities hit by one of the trial groups. Michaelides et al. (21), in spite of defending other mechanisms to explain CAS increase, also showed an association between higher BP values with higher duration of exercise session (post 24 hours) in sedentary elderly with coronary arterial disease. Other physiological mechanisms could prompt acute increase in CAS at strenuous exercise sessions such as: increase in inflammation and oxidative stress that in turn reduces the nitric oxide bioavailability and vasodilation (21, 30); and/or increases factors that modulate extracellular matrix turnover (31). Thus, independent of what is the stimulus for increased CAS after exercise session; this acute increase in CAS may mediate chronic adaptations. Even so, it is a huge matter of debate that needs to be proved in future researches.
2.1.2. Chronic Risks
Besides the acute stimulus to arouse chronic adaptations through exercise training, CAS would also be increased by other chronic physiological adaptation mediations. The hypervolemia and bradycardia induced by strenuous exercise training might overload arterial wall, leading to fatigue and fragmentation of elastin fibers (32), and consequently CAS increase. Exercise trainings that overpass the limits of individuals might incite several stimuli such as released chemotactic factors, onset of inflammatory process, increased sympathetic output and also prompt other types of unbalances, and in turn increase CAS.
Beyond the harmful consequences of acute increase in CAS, chronically, CAS is known as an independent predictor of cardiovascular mortality (2). Increased CAS is associated with the left ventricle hypertrophy, reduction in baroreceptor sensitivity, increased aneurism formation and stroke incidence, besides contribution to hypertension, myocardial infarction, congestive heart failure, dementia and atherosclerosis (33). Among all these cardiovascular risk factors, following exercise (resistance exercise), at least left ventricle hypertrophy is associated with CAS increase (34-37).
It should also be considered that the BP elevation is the higher risk factor for premature cardiovascular, cerebrovascular, renovascular, and other vascular diseases worldwide (38). It is possible, these cardiovascular risks related to high BP depend on the arterial stiffness and hypertension is only one of the consequences (39).
The accumulating evidence regarding the relationship between increased CAS and mortality risk is based on epidemiologic studies (i.e. individuals with higher CAS are prone to increased risk of mortality compared to the individuals with lower values) and not many studies are looking for consequences of CAS changes following exercise programs, mainly in apparently healthy people such as the exercise trained ones. It is interesting to notice that regarding CAS increase by resistance exercise in two studies (40, 41) when participants had baseline CAS values greater than normal reference (4), CAS increased following training, while it did not change in other studies (42-45) in which the participants’ pre-training CAS values were within the normal range. In this way, neither resistance training nor strenuous endurance training should be prescribed for all. Instead of that, the accurate intensity volume and training variables should be planned individually as a personalized exercise program according to cardiovascular risks.
2.2. Benefits of Exercise Training
Even though the risks of high CAS were completely highlighted, the consequences of increased CAS through exercise training need to be discussed. Perhaps, such increased vascular risks are offset by other beneficial cardiovascular adaptations, at least following endurance training, since this type of exercise has increased life expectancy also in endurance athletes (46, 47). Anyway, the associations between the dose of exercise (i.e. intensity and volume during training and competition) and benefit of the adaptations acquired by exercise trained individuals, need to be better investigated. Furthermore, the increase in CAS through exercise training might be a protection to the high elevations in BP that arteries have to bear during this kind of strenuous exercise.
Numerous position stands recommend exercise training for diverse preventive and therapeutic healthy benefits, for different populations and different pathological conditions. They also show that acute risks from exercise sessions do not overcome exercise benefits, since people exercising more days per week reduce the risk of myocardial infarction (24, 48). Likely, it is due to prevention of lethal arrhythmia and ischemia by improved autonomic control and consequently reduction of electrical instability (48, 49). Nevertheless, it cannot be rejected that the aforementioned protective effect hypothesis could not be applicable to exercise trained individuals, mainly marathon and ultramarathon runners.
3. Conclusion
Increase in CAS, generated by illness or exercise training, could be a stimulus to increase left ventricular load and myocardial oxygen demands, and in turn compromising coronary irrigation, besides the fatigue on its own elastic components favor arterial dissection and rupture. Thus, CAS increases following exercise training despite being a possible protective mechanism during exercise, it can be pathophysiological during rest. In addition, as the risk to increase CAS seems to be higher in people with higher baseline CAS values, individuals should make a complete clinical check-up when they decided to face the strenuous training routines. A new matter of debate is suggested: personalized exercise programs might be prescribed according to individual cardiovascular risks including CAS, and the guidelines for exercise prescriptions proposed by healthy organizations need to attend these special cases.
Acknowledgements
References
-
1.
Sardeli AV, Gaspari AF, Chacon-Mikahil MP. About the article: Effect of combined aerobic and resistance training versus aerobic training on arterial stiffness. Int J Cardiol. 2015;184:519-20. [PubMed ID: 25767007]. https://doi.org/10.1016/j.ijcard.2015.03.019.
-
2.
Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318-27. [PubMed ID: 20338492]. https://doi.org/10.1016/j.jacc.2009.10.061.
-
3.
van Sloten TT, Sedaghat S, Laurent S, London GM, Pannier B, Ikram MA, et al. Carotid stiffness is associated with incident stroke: a systematic review and individual participant data meta-analysis. J Am Coll Cardiol. 2015;66(19):2116-25. [PubMed ID: 26541923]. https://doi.org/10.1016/j.jacc.2015.08.888.
-
4.
Reference Values for Arterial Stiffness C. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values'. Eur Heart J. 2010;31(19):2338-50. [PubMed ID: 20530030]. https://doi.org/10.1093/eurheartj/ehq165.
-
5.
Montero D, Vinet A, Roberts CK. Effect of combined aerobic and resistance training versus aerobic training on arterial stiffness. Int J Cardiol. 2015;178:69-76. [PubMed ID: 25464222]. https://doi.org/10.1016/j.ijcard.2014.10.147.
-
6.
Gates PE, Tanaka H, Graves J, Seals DR. Left ventricular structure and diastolic function with human ageing. Relation to habitual exercise and arterial stiffness. Eur Heart J. 2003;24(24):2213-20. [PubMed ID: 14659773].
-
7.
Kakiyama T, Matsuda M, Koseki S. Effect of physical activity on the distensibility of the aortic wall in healthy males. Angiology. 1998;49(9):749-57. [PubMed ID: 9756427].
-
8.
Otsuki T, Maeda S, Iemitsu M, Saito Y, Tanimura Y, Ajisaka R, et al. Relationship between arterial stiffness and athletic training programs in young adult men. Am J Hypertens. 2007;20(9):967-73. [PubMed ID: 17765138]. https://doi.org/10.1016/j.amjhyper.2007.05.001.
-
9.
Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102(11):1270-5. [PubMed ID: 10982542].
-
10.
Nualnim N, Barnes JN, Tarumi T, Renzi CP, Tanaka H. Comparison of central artery elasticity in swimmers, runners, and the sedentary. Am J Cardiol. 2011;107(5):783-7. [PubMed ID: 21247521]. https://doi.org/10.1016/j.amjcard.2010.10.062.
-
11.
Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236-41. [PubMed ID: 11358934].
-
12.
Terenzi TJ. An alteration in arterial compliance associated with elevated aerobic fitness. J Manipulative Physiol Ther. 2000;23(1):27-31. [PubMed ID: 10658873].
-
13.
Vlachopoulos C, Kardara D, Anastasakis A, Baou K, Terentes-Printzios D, Tousoulis D, et al. Arterial stiffness and wave reflections in marathon runners. Am J Hypertens. 2010;23(9):974-9. [PubMed ID: 20489686]. https://doi.org/10.1038/ajh.2010.99.
-
14.
Burr JF, Drury CT, Phillips AA, Ivey A, Ku J, Warburton DE. Long-term ultra-marathon running and arterial compliance. J Sci Med Sport. 2014;17(3):322-5. [PubMed ID: 23707138]. https://doi.org/10.1016/j.jsams.2013.04.018.
-
15.
Collier SR, Diggle MD, Heffernan KS, Kelly EE, Tobin MM, Fernhall B. Changes in arterial distensibility and flow-mediated dilation after acute resistance vs. aerobic exercise. J Strength Cond Res. 2010;24(10):2846-52. [PubMed ID: 20885204]. https://doi.org/10.1519/JSC.0b013e3181e840e0.
-
16.
Heffernan KS, Collier SR, Kelly EE, Jae SY, Fernhall B. Arterial stiffness and baroreflex sensitivity following bouts of aerobic and resistance exercise. Int J Sports Med. 2007;28(3):197-203. [PubMed ID: 17024636]. https://doi.org/10.1055/s-2006-924290.
-
17.
Kingwell BA, Berry KL, Cameron JD, Jennings GL, Dart AM. Arterial compliance increases after moderate-intensity cycling. Am J Physiol. 1997;273(5 Pt 2):H2186-91. [PubMed ID: 9374752].
-
18.
Heffernan KS, Jae SY, Echols GH, Lepine NR, Fernhall B. Arterial stiffness and wave reflection following exercise in resistance-trained men. Med Sci Sports Exerc. 2007;39(5):842-8. [PubMed ID: 17468584]. https://doi.org/10.1249/mss.0b013e318031b03c.
-
19.
Ranadive SM, Fahs CA, Yan H, Rossow LM, Agiovlasitis S, Fernhall B. Comparison of the acute impact of maximal arm and leg aerobic exercise on arterial stiffness. Eur J Appl Physiol. 2012;112(7):2631-5. [PubMed ID: 22083536]. https://doi.org/10.1007/s00421-011-2238-z.
-
20.
Hu M, Yan H, Ranadive SM, Agiovlasitis S, Fahs CA, Atiq M, et al. Arterial stiffness response to exercise in persons with and without Down syndrome. Res Dev Disabil. 2013;34(10):3139-47. [PubMed ID: 23883823]. https://doi.org/10.1016/j.ridd.2013.06.041.
-
21.
Michaelides AP, Soulis D, Antoniades C, Antonopoulos AS, Miliou A, Ioakeimidis N, et al. Exercise duration as a determinant of vascular function and antioxidant balance in patients with coronary artery disease. Heart. 2011;97(10):832-7. [PubMed ID: 21357374]. https://doi.org/10.1136/hrt.2010.209080.
-
22.
Rossow L, Fahs CA, Guerra M, Jae SY, Heffernan KS, Fernhall B. Acute effects of supramaximal exercise on carotid artery compliance and pulse pressure in young men and women. Eur J Appl Physiol. 2010;110(4):729-37. [PubMed ID: 20589389]. https://doi.org/10.1007/s00421-010-1552-1.
-
23.
Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343(19):1355-61. [PubMed ID: 11070099]. https://doi.org/10.1056/NEJM200011093431902.
-
24.
Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes N3, et al. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115(17):2358-68. [PubMed ID: 17468391]. https://doi.org/10.1161/CIRCULATIONAHA.107.181485.
-
25.
Corrado D, Basso C, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden cardiac death? J Cardiovasc Med (Hagerstown). 2006;7(4):228-33. [PubMed ID: 16645394]. https://doi.org/10.2459/01.JCM.0000219313.89633.45.
-
26.
London GM, Guerin AP. Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am Heart J. 1999;138(3 Pt 2):220-4. [PubMed ID: 10467216].
-
27.
Kiviniemi AM, Hautala AJ, Karjalainen JJ, Piira OP, Lepojarvi S, Ukkola O, et al. Acute post-exercise change in blood pressure and exercise training response in patients with coronary artery disease. Front Physiol. 2014;5:526. [PubMed ID: 25628572]. https://doi.org/10.3389/fphys.2014.00526.
-
28.
Ozaki H, Yasuda T, Ogasawara R, Sakamaki-Sunaga M, Naito H, Abe T. Effects of high-intensity and blood flow-restricted low-intensity resistance training on carotid arterial compliance: role of blood pressure during training sessions. Eur J Appl Physiol. 2013;113(1):167-74. [PubMed ID: 22618304]. https://doi.org/10.1007/s00421-012-2422-9.
-
29.
Dobrin PB. Mechanical factors associated with the development of intimal and medial thickening in vein grafts subjected to arterial pressure. A model of arteries exposed to hypertension. Hypertension. 1995;26(1):38-43. [PubMed ID: 7607730].
-
30.
Clapp BR, Hingorani AD, Kharbanda RK, Mohamed-Ali V, Stephens JW, Vallance P, et al. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64(1):172-8. [PubMed ID: 15364625]. https://doi.org/10.1016/j.cardiores.2004.06.020.
-
31.
Koskinen SO, Hoyhtya M, Turpeenniemi-Hujanen T, Martikkala V, Makinen TT, Oksa J, et al. Serum concentrations of collagen degrading enzymes and their inhibitors after downhill running. Scand J Med Sci Sports. 2001;11(1):9-15. [PubMed ID: 11169229].
-
32.
Heffernan KS. How healthy were the arteries of Phidippides? Clin Cardiol. 2012;35(2):65-8. [PubMed ID: 22125198]. https://doi.org/10.1002/clc.21009.
-
33.
Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107(1):139-46. [PubMed ID: 12515756].
-
34.
Sugawara J, Komine H, Miyazawa T, Imai T, Fisher JP, Ogoh S. Impact of chronic exercise training on the blood pressure response to orthostatic stimulation. J Appl Physiol (1985). 2012;112(11):1891-6. [PubMed ID: 22422799]. https://doi.org/10.1152/japplphysiol.01460.2011.
-
35.
Kawano H, Tanaka H, Miyachi M. Resistance training and arterial compliance: keeping the benefits while minimizing the stiffening. J Hypertens. 2006;24(9):1753-9. [PubMed ID: 16915024]. https://doi.org/10.1097/01.hjh.0000242399.60838.14.
-
36.
Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, et al. Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation. 2004;110(18):2858-63. [PubMed ID: 15492301]. https://doi.org/10.1161/01.CIR.0000146380.08401.99.
-
37.
Bertovic DA, Waddell TK, Gatzka CD, Cameron JD, Dart AM, Kingwell BA. Muscular strength training is associated with low arterial compliance and high pulse pressure. Hypertension. 1999;33(6):1385-91. [PubMed ID: 10373221].
-
38.
Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88(5 Pt 1):2460-70. [PubMed ID: 8222141].
-
39.
Dawber TR, Thomas HJ, McNamara PM. Characteristics of the dicrotic notch of the arterial pulse wave in coronary heart disease. Angiology. 1973;24(4):244-55. [PubMed ID: 4699520].
-
40.
Collier SR, Kanaley JA, Carhart RJ, Frechette V, Tobin MM, Hall AK, et al. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens. 2008;22(10):678-86. [PubMed ID: 18432253]. https://doi.org/10.1038/jhh.2008.36.
-
41.
Cortez-Cooper MY, DeVan AE, Anton MM, Farrar RP, Beckwith KA, Todd JS, et al. Effects of high intensity resistance training on arterial stiffness and wave reflection in women. Am J Hypertens. 2005;18(7):930-4. [PubMed ID: 16053989]. https://doi.org/10.1016/j.amjhyper.2005.01.008.
-
42.
Cortez-Cooper MY, Anton MM, Devan AE, Neidre DB, Cook JN, Tanaka H. The effects of strength training on central arterial compliance in middle-aged and older adults. Eur J Cardiovasc Prev Rehabil. 2008;15(2):149-55. [PubMed ID: 18391640]. https://doi.org/10.1097/HJR.0b013e3282f02fe2.
-
43.
Casey DP, Beck DT, Braith RW. Progressive resistance training without volume increases does not alter arterial stiffness and aortic wave reflection. Exp Biol Med (Maywood). 2007;232(9):1228-35. [PubMed ID: 17895531]. https://doi.org/10.3181/0703-RM-65.
-
44.
Heffernan KS, Fahs CA, Iwamoto GA, Jae SY, Wilund KR, Woods JA, et al. Resistance exercise training reduces central blood pressure and improves microvascular function in African American and white men. Atherosclerosis. 2009;207(1):220-6. [PubMed ID: 19410255]. https://doi.org/10.1016/j.atherosclerosis.2009.03.043.
-
45.
Maeda S, Otsuki T, Iemitsu M, Kamioka M, Sugawara J, Kuno S, et al. Effects of leg resistance training on arterial function in older men. Br J Sports Med. 2006;40(10):867-9. [PubMed ID: 16920770]. https://doi.org/10.1136/bjsm.2006.029538.
-
46.
Reimers CD, Knapp G, Reimers AK. Does physical activity increase life expectancy? A review of the literature. J Aging Res. 2012;2012:243958. [PubMed ID: 22811911]. https://doi.org/10.1155/2012/243958.
-
47.
Ruiz JR, Fiuza-Luces C, Garatachea N, Lucia A. Reduced mortality in former elite endurance athletes. Int J Sports Physiol Perform. 2014;9(6):1046-9. [PubMed ID: 24584695]. https://doi.org/10.1123/ijspp.2013-0492.
-
48.
Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116(5):572-84. [PubMed ID: 17638929]. https://doi.org/10.1161/CIRCULATIONAHA.107.185214.
-
49.
Hull SJ, Vanoli E, Adamson PB, Verrier RL, Foreman RD, Schwartz PJ. Exercise training confers anticipatory protection from sudden death during acute myocardial ischemia. Circulation. 1994;89(2):548-52. [PubMed ID: 8313542].