Abstract
Background:
Extubation readiness is assessed by spontaneous breathing trials (SBTs); however, there is a lack of universally agreed protocols for their accurate performance and reporting in pediatric intensive care units (PICUs).Objectives:
We aimed to evaluate extubating bundles, including modified SBT, in predicting successful extubation in critically-ill children with planned extubation.Method:
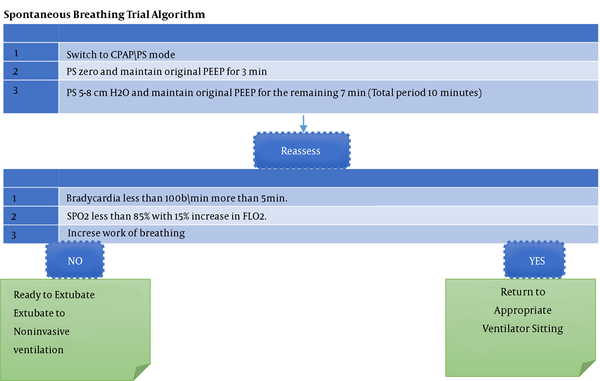
This prospective cross-sectional study was based on the collection of data from 150 critically-ill children admitted to the PICU at Minia University Hospital. From January 2019 to June 2020, those children admitted to the PCIU and subjected to mechanical ventilation (MV), and extubation were enrolled. When the clinical team decided a child was ready for extubation based on the extubating bundle, a modified SBT (10 min) was used. It was started with switching to the CPAP\PS mode, followed by PS zero, and maintaining the original PEEP for 3 min. Finally, PS was kept at 5 - 8 cm H2O, and the original PEEP was maintained for the remaining 7 min (total period of 10 min).Results:
The extubation bundle with modified SBT could predict extubation success with 89% sensitivity and 89.9% positive predictive value (PPV). There were no significant differences in age, weight, gender, and length of intubation between children with failed SBT and those who were successfully extubated. In 41 cases, SBT failure occurred in 3 ‐ 5 min, while nine cases showed failure in 6 ‐ 10 min.Conclusions:
Extubation bundle with modified SBT before elective extubation is indicated for children. Guidelines for extubation among critically-ill children are needed to reduce unnecessary exposure to mechanical ventilation's adverse effects. Further multicenter research is required to enhance outcomes and decline the burden of these patients.Keywords
Spontaneous Breathing Trial Extubation PICU Mechanical Ventilator
1. Background
Mechanical ventilation (MV) is a crucial intervention for pediatric patients with a critical illness and respiratory failure. Annually, hundreds of thousands of pediatric patients receive MV (1, 2). Prolonged ventilation is associated with increased length of stay, hospital costs (3), and adverse events, including decreased respiratory muscle strength, (4) ventilator-associated pneumonia, and mortality (5). Failed extubation, which occurs in approximately 15% of the cases (3, 6, 7), has its own detrimental effects on patient outcomes and cost (3, 7). Centers for Medicare and Medicaid Services has defined prolonged mechanical ventilation (PMV) as more significant than three weeks of MV for at least six hours per day (8).
It is estimated that about 4 - 13% of mechanically ventilated cases need PMV (9, 10), resulting in between 7250 and 11,400 patients undergoing PMV at any one time (9). Given the risks of prolonged mechanical ventilation, physicians require mechanical ventilation exposure readiness for extubation on a regular basis (11). Currently, the choice to extubate is according to clinical judgment based on ventilatory support, blood gas readings, and the patient's general clinical stability (3, 4). Spontaneous breathing trials (SBTs) have been more popular in recent years for determining extubation readiness (12, 13). Spontaneous breathing trials involve a 3- to 10-minute period of spontaneous breathing using endotracheal continuous positive airway pressure (ET-CPAP), during which a mix of clinical events decides whether the patient passes or fails (apneas, bradycardias, and desaturations). Only two short studies have been conducted in this regard so far (14-16). According to the morbidities correlated with MV's long duration in critically-ill children, a series of objective extubation indicators should be developed to avoid reintubation (17).
In our PICU, prior research indicated that up to 35% of severely ill children on MV require reintubation following extubation. Extubation is the procedure through which the endotracheal tube is removed (ETT). It is the last step in a patient's MV discontinuation. Extubation failure is correlated with increased mortality, morbidity, hospitalization, and need for ventilation (15). Hence, developing algorithms to predict successful extubation efforts can help decline the mortality and morbidity associated with inadvertent extubation. Extubation should not be performed until the patient's medical state has been stabilized. A weaning trial has been successful when the airway is patent, and no potential difficulties in reintubation have been identified. Most patients are extubated during daytime hours, although nocturnal extubation is appropriate in exceptional circumstances. Patients cannot be extubated unless the condition for intubation is improved and the clinical criteria for weaning have been met. Pediatric intensive care unit patients should not be extubated unless a successful weaning trial has been passed.
2. Objectives
This work assesses the application of an extubation bundle, such as a modified spontaneous breathing trial (10 min), to decrease reintubation rate in critically-ill children who are mechanically ventilated and are extubated to noninvasive ventilation in PICU.
3. Methods
We followed a prospective cross-sectional design to collect the data of critically-ill children who were mechanically ventilated at PICU of a pediatrics hospital. The study was approved by the Ethics Committee, and it was performed during January 2019 to June 2020. When the medical professionals determine that a patient is ready for extubation, they do a modified SBT (10 min) (Tables 1 and 2, Figure 1).
Extubation Bundle (Weaning from Conventional Ventilation)
| Clinical Criteria Used to Determine Readiness for Trials of Spontaneous Breathing | |
|---|---|
| Goal | |
| 1 | SBT is an excellent tool to assess patient readiness for extubation |
| 2 | Continuously assess readiness to wean ventilation |
| 3 | Consider extubation when the patient can demonstrate adequate respiratory drive |
| Ventilator Status | |
| 1 | Blood gas parameters in target ranges (pH > 7.25) |
| 2 | PaO2/FiO2 ≥ 150 |
| 3 | or SpO2 ≥ 90 - 95) percentage on FiO2 ≤ 3 0 percent and positive end-expiratory pressure (PEEP) ≤ 6 cm H2O |
| 4 | Set the respiratory rate < 45 |
| 5 | Tidal volume 4 ‐ 5 mL/kg |
| 6 | Mean airway pressure < 7 |
| Patient Status | |
| 1 | The cause of the respiratory failure has improved |
| 2 | Able to initiate an inspiratory effort |
| 3 | Hemodynamic stability (no or low dose vasopressor medications) |
| 4 | Hemoglobin ≥ 7 mg/dL |
| 5 | Stable vital signs. |
| 6 | Blood pressure stable without inotropes |
| 7 | Stable temperature (Core temperature ≤ 38 to 38.5°Centigrade) |
| 8 | Mental status awakes and alert or easily arousal |
| 9 | Consider the presence of gag reflex |
| 10 | Sedation reviewed |
Spontaneous breathing trial algorithm
Extubation Bundle Elements
| Extubation Bundle Elements | |||||
|---|---|---|---|---|---|
| Discuss readiness for extubation | Children had adequate respiratory drive | Mean airway pressure < 9 cm H2O | FiO2 < 30% | VT < 5 mL/kg | Modified SBT done |
| Weaned from sedation | |||||
The study was conducted in the PICU to minimize extubation failure. Our PICU is a tertiary-level care department with 18 beds (intensive & intermediate care). During the study period, 457 children were admitted to our PICU,166 of whom required MV, parents of seven patients refused to participate in the study, and nine patients died during the course of their illness and before extubation. The study included 150 critically-ill children of both genders admitted to our PICU for various medical disorders. All patients aged more than one month and less than 18 years, admitted to PICU, and submitted to MV through an endotracheal tube for more than 24 hours were eligible for inclusion. Patients likely to require tracheostomy, those with accidental extubation or self-extubation, and patients not meeting the eligibility criteria for extubation were excluded. Neumovent ventilator was used for mechanical ventilation. The occurrence of extubation failure and clinical and treatment-related factors were assessed. Extubation was judged effective when the children could remain without invasive ventilator assistance for 24 h; extubation failure was defined as the necessity for reintubation for any reason within 24 h of extubation. Extubation failure was not defined as accidental extubation followed by prompt reintubation or the use of noninvasive ventilator assistance. The medical staff decided the appropriate time for extubation according to clinical evaluations and a extubation bundle, which was locally designed (Tables 1 and 2).
We considered the etiology of the participants’ respiratory failure, the patients' prognosis, the predicted progress of the condition, and the lack of significant causes to continue MV. We began planning extubation on the first day of intubation. Unless management plans altered, any patient who successfully completed the modified SBT was extubated. Extubation was not recommended in patients who were inappropriate for starting a ventilator liberation plan or those with failed spontaneous breathing trial.
Patients with the following conditions should not be considered liberation: Acute respiratory failure requiring active management, shallow rapid breaths with increased respiratory rate and decreased tidal volumes, extensive secretions, deteriorating chest imaging, any evidence of circulatory instability, a Glasgow Coma Scale (GCS) score of less than 8, any disorder of the nervous system impairing the patient's capacity to breathe spontaneously, any acute brain injury (like the use of an invasive intracranial pressure measurement device in the presence of elevated intracranial pressure), which was the main cause of intubation, schemes for re-surgery need during the next 24 h, and requiring general anesthesia. Extubation was avoided if the patient needed to receive another intubation for surgery and might remain on the ventilator for a few further days depending on the results of the procedure.
3.1. Assessment of Weaning Readiness
In the assessment, we considered all the aforementioned contraindications, as well as improvement of clinical aspects in identifying the cause of respiratory failure, oxygenation, ventilation factors, mental condition, secretions, cardiovascular status, and patient-specific weaning factors.
While executing the modified SBT, all the intubation equipment was readily accessible, including two to three sizes of ETTs, a bag-mask with a positive end-expiratory pressure (PEEP) valve, airway bougies, tube exchangers, a traditional direct laryngoscopy, a video laryngoscope, a flexible bronchoscope, induction medications, and suction catheters.
Oxygenation equipment, such as a nasal cannula, oxygen mask, venturi mask, high-flow oxygen system, or continuous positive airway pressure (CPAP)/bi-level-positive airway pressure (BPAP), was readily available following extubation. Additionally, after extubation in the PICU, a respiratory therapist and bedside nurse were present with the pediatric intensivist. An anesthesiologist was present for the extubation of a problematic airway.
Preparation for extubation was initiated on the intubation day and was carried on during the acute care of the original issue that resulted in respiratory failure. Every day, all the ventilated PICU patients were evaluated for weaning readiness. Pediatric intensivists weighed the advantages and disadvantages of early weaning against the burden caused by failed extubation.
Before extubation, the following steps were taken: (1) The first step was to screen for any potential barriers to starting the liberation pathway. (2) If no contraindications existed, the case should be evaluated using weaning variables. (3) If a case was determined to be a suitable candidate, we initiated the modified SBT. (4) Throughout the modified SBT and at the conclusion of the modified SBT, we evaluated for modified SBT failure/passage. (5) If the patient passed the modified SBT, extubation was performed.
3.2. Modified SBT
The weaning trial was initiated at the conclusion of the first spontaneous breathing experiment. The modified SBT took ten minutes to complete. Several strategies could be employed to complete the modified SBT, including the T-tube (T-piece) trial, pressure support ventilation, automatic tube compensation, continuous positive airway pressure, and automated weaning. We conducted the modified SBT using pressure support ventilation and continuous positive airway pressure.
3.3. Extubation Suitability
If the patient passed the modified SBT, they were reassessed for extubation suitability. Numerous evaluations were conducted at the beginning of the weaning trial and as part of the patient's daily evaluations of preparedness to wean. The major component of this examination was determining the case's capacity to defend and maintain a patent airway, the level of consciousness needed to be adequate or greater than 8 on the GCS scale, the patient's cough should be vigorous, and the amount and thickness of the patient's respiratory secretions were determined.
Following a successful modified SBT weaning, the choice to continue with extubation was made. All the required devices for extubation were available, as was additional equipment in case extubation did not go as planned. All the patients who were successfully weaned were monitored closely for extubation failure and required to be re-intubated within 72 h.
Weaning failure indicated that the case was unable to endure the modified SBT, in contrast to extubation failure, which occurred when the patient passed the spontaneous breathing trial and then failed to extubate successfully. When ventilated children were ready for extubation, a modified SBT was conducted; if unsuccessful, SBT was repeated daily until successful.
Both the intubation and extubation procedures were recorded in the patients' electronic medical records, including dates and timings, as well as any issues or difficulties encountered. The data were recorded using hospital standard charts. The variables analyzed included the following: age, weight, gender, detailed medical history, and clinical examination date of intubation and extubation, length of MV (days), extubation failure, and reintubation.
Depending on the outcome of extubation attempt, the children were categorized into two groups. Failed extubation (reintubation within 24 h) was the primary outcome. The reintubation indications were as follows: (a) at least two episodes of apnea resulted in need for intervention in8 hours, (b) respiratory acidosis (PaCO2 > 65 mmHg and pH < 7.25), and (c) FiO2 0.60 to keep SpO2 in the predefined spectrum (90 - 95%). The secondary outcome was defining the range of the modified SBT.
3.4. Sample Size and Data Analysis
Previous studies in PICUs indicated that up to 63% of severely ill children on MV require reintubation following extubation (18). We hypothesized that the application of an extubation bundle, such as a modified spontaneous breathing trial (10 min), to decrease reintubation rate in critically-ill children who were mechanically ventilated and extubated to noninvasive ventilation in PICU could account for a 40.13 % reduction in the incidence of extubation failure. To demonstrate a difference in extubation failure from 63% to 33.3%, a sample size of 150 was required (power of 80%, β of 20%, and α of 5%). Eleven patients were added to patient group to account for any differences in extubation failure rates in our unit, compared with the values reported previously. Therefore, 150 patients were required for this study. Descriptive statistics were applied to investigate the distribution of variables. T-test was applied to compare continuous variables, while the chi-square test was used for categorical variables. The criteria of sensitivity, specificity, positive predictive Value, negative predictive value, and diagnostic accuracy were reported for both groups. Data were analyzed using Excel and OpenEpi, Version 3, open-source calculator. Statistical significance was set at P < 0.05 (Table 3).
Results from OpenEpi, Version 3, Open-Source Calculator‐Diagnostic Test
| Parameter | Estimate (%) | Lower ‐ Upper 95% CIs | Method |
|---|---|---|---|
| Sensitivity | 89 | (88.39, 97.43¹) | Wilson Score |
| Specificity | 80 | (71.89, 88.21¹) | Wilson Score |
| Positive predictive value | 89.9 | (85, 94.02¹) | Wilson Score |
| Negative predictive value | 78 | (81.05, 94.71¹) | Wilson Score |
| Diagnostic accuracy | 90.2 | (85.84, 93.33¹) | Wilson Score |
| Likelihood ratio of a positive test | 5.105 | (4.513 ‐ 5.774) | |
| Likelihood ratio of a negative test | 0.06181 | (0.04807 ‐ 0.07948) | |
| Diagnostic odds | 82.58 | (33.75 ‐ 202.1) | |
| Cohen's kappa (Unweighted) | 0.7803 | (0.6554 ‐ 0.9052) | |
| Entropy reduction after a positive test | 33.23 | ||
| Entropy reduction after a negative test | 31.74 | ||
| Bias index | 0.0326 |
4. Results
In our study, there were no statistically significant differences in age, weight, gender, and length of intubation between children who failed and those who were successfully extubated (Table 4). On admission to PICU, 60 (40%) patients were diagnosed with pneumonia, and 36 (24%) were diagnosed as CNS infection (Table 5). Extubation bundles with modified SBT could predict extubation success with 89% sensitivity and 89.9% PPV among critically-ill children who were mechanically ventilated and extubated to noninvasive ventilation in the PICU (Table 6). In 41 cases, SBT failure happened in 3 ‐ 5 min, while nine cases showed failure in 6 ‐ 10 min (Table 7).
Demographic and Clinical Laboratory Data for the Study Groups (n = 150)
Diagnosis of Groups Being Studied on Admission to the PICU (n = 150)
| Diagnosis | No. (%) |
|---|---|
| Pneumonia | 60 (40) |
| CNS infection | 36 (24) |
| Viral encephalitis | 33 (22) |
| Gastro-enteritis | 24 (16) |
| Meningococcemia | 3 (2) |
Sensitivity and Septicity for Modified SBT in Predicting Successful Extubation in the Groups Under Study (n = 150)
| Extubation | ||||
|---|---|---|---|---|
| Successful | Unsuccessful | Total | ||
| Passed | 89 | 10 | 99 | PPV:89.9% |
| Failed | 11 | 40 | 51 | NPV:78% |
| Total | 100 | 50 | 150 | |
| Sensitivity: a/(a+c) = 89% | Specificity: d/(b + d) = 80% | Accuracy: (a+d)/(a+b+c+d) = 84.5% | ||
Failed SBT in the Groups Being Studied
| Failure in 3 ‐ 5 min | Failure in 6 ‐ 10 min | |
|---|---|---|
| Groups are studied (N = 50) | 41 | 9 |
5. Discussion
The current study intended to investigate the value of modified SBT as a part of the extubation bundle to increase extubation success among critically-ill children who were mechanically ventilated in a PICU. In our study, extubation bundles with modified SBT could predict extubation success with 89% sensitivity and 89.9% PPV among critically-ill children who were mechanically ventilated and extubated to noninvasive ventilation in the PICU. We arbitrarily selected 10 min during modified SBT, but the duration of standard SBT can range between 30 min and 2 h (8, 9).
Extubating mechanically ventilated patients at the appropriate time is a major issue in critical care (19, 20). To improve the outcome of mechanically ventilated patients in the PICU, the reliable prediction of post-extubation suffering and the early diagnosis of the causes of failure of a trial of pressure support ventilation or a trial of fully unsupported respiration (T-tube) are critical (21). The conventional criteria for readiness to wean off MV are relatively easy to use, but their sensitivity and specificity are relatively poor (22). The current approach in PICU ventilator management is to use the term 'liberation' rather than 'weaning', as the target is to immediately remove the cases from the ventilator rather than gradually weaning them over several days to weeks.
In long-term acute care settings, weaning is more common (23, 24), and much research has indicated a considerable reduction in PICU length of stay and ventilation days when particular protocols and policies are followed. However, some studies argue against the use of protocolized weaning. In practice, such procedures expedite decision-making and promote faster liberation. There must be a respiratory therapy-driven removal plan for all patients. Ventilator liberation is a lengthy process that involves numerous variables that must be monitored carefully. The coordination between various service providers is critical. Communication among team members is critical to ensure that everyone understands their responsibilities. Having a team-based, interprofessional perspective (e.g., physicians, nurses, respiratory therapists, and pharmacists) to extubation care is the most effective way to ensure a favorable outcome, regardless of how events unfold during extubation (25, 26). In a study by Teixeira et al. studying 533 patients who followed a standard weaning protocol, the incidence of failure of mechanical ventilation extubation was 13.3% (27). In another study by Vidotto et al. on 317 postoperative patients, the incidence of weaning failure was 20% (28). This is in controversy to Ferrari et al. study, where a 63% failure rate was reported; this is explained by the non-uniform rule in study population selection with different causes for mechanical ventilation and different ventilation periods before starting the weaning process, which may affect the outcome of the weaning process (18).
From a practical perspective, the incidence of weaning failure in the four studies was relatively high, which gives strength to the theory stating that the present weaning tools are still far from ideal in predicting the success rate of weaning. As it was hypothesized, a prolonged duration of SBT in children and adults facilitated atelectasis and improved breathing in critically-ill children (29, 30). Most pediatric patients required PICU admission were under the age of five years, and approximately half of the extubations were in infants at a higher risk for extubation failure (29, 31, 32). However, in our study, age was not shown to affect extubation failure rate significantly. In addition, it is proposed that males are at an increased risk for extubation failure (31). In our study, no significant differences were found in terms of sex. In this study, the most frequent criterion for intubation was respiratory disorders causing acute respiratory failure. This finding is in line with some other study results (33-35). Nevertheless, we also had a considerable cohort of cases with neurological problems who required MV. We also had cases with declined consciousness, status epilepticus, or necessitating MV. In this study, we used a subjective classification of diagnoses (rather than objective), as the primary indication of intubation must be deduced from a retrospective review of patient notes. Meanwhile, according to the findings, the diagnosis did not have a considerable impact on extubation failure. Nevertheless, investigating more patients may provide more useful information. The other potential confounders were weight and duration of PICU admission; however, we did not observe a significant impact on extubation failure regarding these variables.
The sample size of this study was determined according to the overall impact of the guidelines (instead of sole components); thus, investigating more patients as well as considering other criteria may affect extubation outcome. The management of comorbidities before extubation and hemodynamic stability were the other two criteria of the guidelines that presented protective properties against extubation failure. A study with a larger cohort of participants would provide more valuable information.
5.1. Limitations of the Study
The current study has two limitations. First, the endotracheal intubation period was not long enough. Second, through encouraging extubation sooner than the caregiver might have decided, the number of cases who needed reintubation probably increased. It is recommended that other studies in this field be carried out with larger sample sizes. Also, according to PMV side effects, the best way to prevent delay or early disconnection of MV and misdiagnosis or over use in these subjects is to use guidelines. It is helpful to use guidelines in many cases of medical challenges, for instance, in preventing the overuse of antibiotics and medical modalities and facilitating diagnosis and management of difficult medical situations such as the presented issue (PMV) (36).
5.2. Conclusions
An extubation bundle with modified SBT before elective extubation is recommended to be used in predicting successful extubation in children. Guidelines for extubation among critically-ill children are needed to reduce unnecessary exposure to mechanical ventilation's adverse effects. Further multicenter research can enhance the outcomes and decline the burden of these patients.
References
-
1.
Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O'Brien CR, Rubenfeld GD. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355(1):41-50. [PubMed ID: 16822995]. https://doi.org/10.1056/NEJMsa053993.
-
2.
Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38(10):1947-53. [PubMed ID: 20639743]. https://doi.org/10.1097/CCM.0b013e3181ef4460.
-
3.
Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112(1):186-92. [PubMed ID: 9228375]. https://doi.org/10.1378/chest.112.1.186.
-
4.
De Jonghe B, Bastuji-Garin S, Durand MC, Malissin I, Rodrigues P, Cerf C, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35(9):2007-15. [PubMed ID: 17855814]. https://doi.org/10.1097/01.ccm.0000281450.01881.d8.
-
5.
Zeggwagh AA, Abouqal R, Madani N, Zekraoui A, Kerkeb O. Weaning from mechanical ventilation: a model for extubation. Intensive Care Med. 1999;25(10):1077-83. [PubMed ID: 10551962]. https://doi.org/10.1007/s001340051015.
-
6.
Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Valverdu I, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332(6):345-50. [PubMed ID: 7823995]. https://doi.org/10.1056/NEJM199502093320601.
-
7.
Seymour CW, Martinez A, Christie JD, Fuchs BD. The outcome of extubation failure in a community hospital intensive care unit: a cohort study. Crit Care. 2004;8(5):R322-7. [PubMed ID: 15469575]. [PubMed Central ID: PMC1065021]. https://doi.org/10.1186/cc2913.
-
8.
MacIntyre NR, Epstein SK, Carson S, Scheinhorn D, Christopher K, Muldoon S, et al. Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest. 2005;128(6):3937-54. [PubMed ID: 16354866]. https://doi.org/10.1378/chest.128.6.3937.
-
9.
Nevins ML, Epstein SK. Weaning from prolonged mechanical ventilation. Clin Chest Med. 2001;22(1):13-33. https://doi.org/10.1016/s0272-5231(05)70023-x.
-
10.
Lone NI, Walsh TS. Prolonged mechanical ventilation in critically ill patients: epidemiology, outcomes and modelling the potential cost consequences of establishing a regional weaning unit. Crit Care. 2011;15(2):R102. [PubMed ID: 21439086]. [PubMed Central ID: PMC3219374]. https://doi.org/10.1186/cc10117.
-
11.
Jensen EA, DeMauro SB, Kornhauser M, Aghai ZH, Greenspan JS, Dysart KC. Effects of Multiple Ventilation Courses and Duration of Mechanical Ventilation on Respiratory Outcomes in Extremely Low-Birth-Weight Infants. JAMA Pediatr. 2015;169(11):1011-7. [PubMed ID: 26414549]. [PubMed Central ID: PMC6445387]. https://doi.org/10.1001/jamapediatrics.2015.2401.
-
12.
Al-Mandari H, Shalish W, Dempsey E, Keszler M, Davis PG, Sant'Anna G. International survey on periextubation practices in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed. 2015;100(5):F428-31. [PubMed ID: 26063193]. https://doi.org/10.1136/archdischild-2015-308549.
-
13.
Beltempo M, Isayama T, Vento M, Lui K, Kusuda S, Lehtonen L, et al. Respiratory Management of Extremely Preterm Infants: An International Survey. Neonatology. 2018;114(1):28-36. [PubMed ID: 29656287]. https://doi.org/10.1159/000487987.
-
14.
Kamlin CO, Davis PG, Morley CJ. Predicting successful extubation of very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2006;91(3):F180-3. [PubMed ID: 16410255]. [PubMed Central ID: PMC2672700]. https://doi.org/10.1136/adc.2005.081083.
-
15.
Chawla S, Natarajan G, Gelmini M, Kazzi SN. Role of spontaneous breathing trial in predicting successful extubation in premature infants. Pediatr Pulmonol. 2013;48(5):443-8. [PubMed ID: 22811341]. https://doi.org/10.1002/ppul.22623.
-
16.
Shalish W, Latremouille S, Papenburg J, Sant'Anna GM. Predictors of extubation readiness in preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2019;104(1):F89-97. [PubMed ID: 29519808]. https://doi.org/10.1136/archdischild-2017-313878.
-
17.
Biban P, Gaffuri M, Spaggiari S, Silvagni D, Zaglia F, Santuz P. Weaning newborn infants from mechanical ventilation. J Pediatr Neonatal Individ Med. 2013;2(2):e020225.
-
18.
Ferrari G, De Filippi G, Elia F, Panero F, Volpicelli G, Apra F. Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit Ultrasound J. 2014;6(1):8. [PubMed ID: 24949192]. [PubMed Central ID: PMC4057909]. https://doi.org/10.1186/2036-7902-6-8.
-
19.
Rose L, Blackwood B, Egerod I, Haugdahl HS, Hofhuis J, Isfort M, et al. Decisional responsibility for mechanical ventilation and weaning: an international survey. Crit Care. 2011;15(6):R295. [PubMed ID: 22169094]. [PubMed Central ID: PMC3388643]. https://doi.org/10.1186/cc10588.
-
20.
Mcconville JF, Kress JP. Weaning Patients from the Ventilator. N Engl J Med. 2013;368(11):1067-9. https://doi.org/10.1056/NEJMc1300398.
-
21.
Silva S, Ait Aissa D, Cocquet P, Hoarau L, Ruiz J, Ferre F, et al. Combined Thoracic Ultrasound Assessment during a Successful Weaning Trial Predicts Postextubation Distress. Anesthesiology. 2017;127(4):666-74. [PubMed ID: 28650414]. https://doi.org/10.1097/ALN.0000000000001773.
-
22.
Stawicki S. Mechanical ventilation: Weaning and extubation. Int J Acad Med. 2017;3(3). https://doi.org/10.4103/ijam.Ijam_87_16.
-
23.
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033-56. [PubMed ID: 17470624]. https://doi.org/10.1183/09031936.00010206.
-
24.
Penuelas O, Thille AW, Esteban A. Discontinuation of ventilatory support: new solutions to old dilemmas. Curr Opin Crit Care. 2015;21(1):74-81. [PubMed ID: 25546535]. https://doi.org/10.1097/MCC.0000000000000169.
-
25.
Blackwood B, Alderdice F, Burns K, Cardwell C, Lavery G, O'Halloran P. Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta-analysis. BMJ. 2011;342:c7237. [PubMed ID: 21233157]. [PubMed Central ID: PMC3020589]. https://doi.org/10.1136/bmj.c7237.
-
26.
Kollef MH, Shapiro SD, Silver P, St John RE, Prentice D, Sauer S, et al. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997;25(4):567-74. [PubMed ID: 9142019]. https://doi.org/10.1097/00003246-199704000-00004.
-
27.
Teixeira C, Maccari JG, Vieira SR, Oliveira RP, Savi A, Machado AS, et al. Impact of a mechanical ventilation weaning protocol on the extubation failure rate in difficult-to-wean patients. J Bras Pneumol. 2012;38(3):364-71. [PubMed ID: 22782607]. https://doi.org/10.1590/s1806-37132012000300012.
-
28.
Vidotto MC, Sogame LC, Gazzotti MR, Prandini M, Jardim JR. Implications of extubation failure and prolonged mechanical ventilation in the postoperative period following elective intracranial surgery. Braz J Med Biol Res. 2011;44(12):1291-8. [PubMed ID: 22030868]. https://doi.org/10.1590/s0100-879x2011007500146.
-
29.
Baisch SD, Wheeler WB, Kurachek SC, Cornfield DN. Extubation failure in pediatric intensive care incidence and outcomes. Pediatr Crit Care Med. 2005;6(3):312-8. [PubMed ID: 15857531]. https://doi.org/10.1097/01.PCC.0000161119.05076.91.
-
30.
Farias JA, Frutos F, Esteban A, Flores JC, Retta A, Baltodano A, et al. What is the daily practice of mechanical ventilation in pediatric intensive care units? A multicenter study. Intensive Care Med. 2004;30(5):918-25. [PubMed ID: 15029473]. [PubMed Central ID: PMC7095496]. https://doi.org/10.1007/s00134-004-2225-5.
-
31.
Randolph AG, Wypij D, Venkataraman ST, Hanson JH, Gedeit RG, Meert KL, et al. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA. 2002;288(20):2561-8.
-
32.
Fontela PCS, Piva JP, Garcia PC, Bered PCL, Zilles K. Risk factors for extubation failure in mechanically ventilated pediatric patients. Pediatr Crit Care Med. 2005;6(2):166-70. https://doi.org/10.1097/01.Pcc.0000154922.65189.48.
-
33.
Silva DCBD, Shibata ARO, Farias JA, Troster EJ. How is mechanical ventilation employed in a pediatric intensive care unit in Brazil? Clinics. 2009;64:1161-6.
-
34.
Zamzam MA, Abd El Aziz AA, Elhefnawy MY, Shaheen NA. Study of the characteristics and outcomes of patients on mechanical ventilation in the intensive care unit of EL-Mahalla Chest Hospital. Egypt J Chest Dis Tuberc. 2015;64(3):693-701. https://doi.org/10.1016/j.ejcdt.2015.04.001.
-
35.
Vincent JL, Akca S, De Mendonca A, Haji-Michael P, Sprung C, Moreno R, et al. The epidemiology of acute respiratory failure in critically ill patients(*). Chest. 2002;121(5):1602-9. [PubMed ID: 12006450]. https://doi.org/10.1378/chest.121.5.1602.
-
36.
Siroosbakht S, Rezakhaniha B. A Survey of Pediatricians’ Views and Practices Regarding Parents’ Request for Prescribing Antibiotics: A Qualitative Study. Arch Pediatr Infect Dis. 2019;7(3). e91217. https://doi.org/10.5812/pedinfect.91217.