Abstract
Background:
Nonalcoholic fatty liver disease (NAFLD) is a very common chronic disorder and obesity is thought to be the most common etiology of fatty infiltration of the liver.Objectives:
The objective of this study is to compare the effect of lifestyle intervention alone or in combination with vitamin E therapy in obese pediatric nonalcoholic fatty liver disease (NAFLD).Patients and Methods:
In the double-blind placebo study, 33 obese children with NAFLD from 2008 to 2009 were included. Lifestyle intervention (balanced calorie diet, 1300-1800 kcal/d and physical activity) was prescribed to all. The patients were concurrently randomized to receive vitamin E 400 mg/d (n = 17)] or placebo (n = 16).Results:
At the end of six months of therapy there was significant change in body mass index, serum aminotransferases, triglycerides, total cholesterol and low density lipoprotein-cholesterol levels in both groups (P <0.001), but the improvement in all these factors was only marginally different between the two groups. Alanine aminotransferase decreased to normal levels in 8 of 17 patients (47.05%) in the lifestyle and vitamin E group, and 7 of 16 patients (43.75%) in lifestyle and placebo group. Similarly, the improvement in the grade of steatosis on ultrasonography after intervention was the same in both groups.Conclusions:
lifestyle intervention with diet and physical exercise in obese children with NAFLD were induced weight loss and was associated with a significant improvement in liver function. Vitamin E did not seem to increase the efficacy of lifestyle intervention.Keywords
Children Lifestyle Fatty Liver Liver Diseases Obesity Vitamin E
1. Background
Nonalcoholic fatty liver disease (NAFLD) is a very common chronic disorder. The prevalence of fatty liver adjusted for age, gender, race, and ethnicity is estimated to range from 0.7% to 33% (1, 2). Obesity is thought to be the most common etiology of fatty infiltration of the liver. Some studies estimated that about half of the obese children may have fatty liver (3-5). The discrepancy among the studies is probably due to methods of sample selection. In recent years, likely due to an alteration in lifestyle and dietary habits, the incidence of fatty liver disease has increased in our country. Some children with NAFLD may have a more serious condition that could lead to severe liver scarring and cirrhosis (6). The diagnosis and treatment of NAFLD is expected to prevent the development of severe liver scarring later in life. In spite of the high prevalence of NAFLD and hepatic steatosis, and of the potential of NAFLD to lead to fibrosis and cirrhosis, no effective medical treatment is available. Treatment trials in pediatric NAFLD are insufficient, and as said, nothing thus far has proven to be effective. Lifestyle intervention has improved liver function in a small number of children with nonalcoholic fatty liver disease (7-9). During the last few years some therapeutic agents such as ursodeoxycholic, antioxidants and metformin have demonstrated to be useful in NAFLD (10-13). Vitamin E is a fat soluble that stored in the liver, fat tissues, heart and muscles. It also helps to prevent oxidation of polyunsaturated fatty acids in cell membranes and other body structures. There is conflicting data on the therapeutic efficacy of vitamin E in the literatures.
2. Objectives
In this study, we investigated the effects of the administration of vitamin E for six months on the serum levels of hepatic enzymes and liver lipid content evaluated by ultrasonography in obese children with NAFLD.
3. Patients and Methods
This randomized double-blind study was carried out on 33 obese children aged 4 to 15 years with NAFLD in Tabriz-Iran, from April 2008 to June 2009. This study was approved by the ethics committee of Tabriz University of Medical Sciences. Written consent was obtained from all parents of patients. Since the location of the study is a referral center, all obese children were referred to the endocrine clinic. The patients that meet inclusion criteria were recruited by census methods. Inclusion criteria were a body mass index higher than 97th percentile for age and sex, alanine aminotransferase (ALT), aspartate aminotransferase (AST) levels at least 1.5 times more than the upper limit of normality (5-40 IU/L) and signs of hepatic steatosis in ultrasonography. Patients with known presence of other liver diseases (Virus hepatitis, metabolic, genetic) and the ones using drugs to induce steatosis (prednisolone, valporate, amiodarone and methotrexate) were excluded. Eligible patients were randomized into two groups by a person who was not aware of the main objective by using software Random List. Group 1 (n = 17) was controlled by lifestyle intervention and vitamin E 400mg/d for six months, and group 2 (n = 16) was controlled by lifestyle intervention and a placebo whose package was similar to vitamin E, made by Pharmacology Faculty of Tabriz University of Medical Sciences. The lifestyle intervention program consisted of low calorie diet from 1300 to 1800 Kcal based on the individual requirements and physical exercises, including aerobic exercise and walking for 2 hours every day. ALT, AST, triglyceride (TG), total cholesterol (TCHO) and low density lipoprotein (LDL) cholesterol were measured at base line and repeated after six months. Liver ultrasonography was performed at the beginning and at the end of the study by one operator who was blinded to the groups. The researcher was also blinded to the groups.
Data were expressed as mean ± SD and number (%). The Kolmogorov - Smirnov statistic test was used for testing normality for continuous variables. Continuous variables were compared using U Mann Whitney and repeated measurement of ANOVA (RMA). Frequency of data were compared using the chi-square test. P <0.05 was considered statistically significant. The statistics package for social science (SPSS 16) was used for statistical analysis.
4. Results
The 33 patients (mean age 7.41 ± 3.21) who were divided in two groups and observed for six months had the same, baseline characteristics (Table1). The study groups were similar regarding their age, sex, BMI, TG, Total cholesterol, LDL-cholesterol, and hepatic enzyme serum levels.
Baseline Characteristics of between Groups
| Group1 (No. = 17) | Group2 (No. = 16) | P. value | |
|---|---|---|---|
| Age(y) Mean ± SD | 7.66 ± 3.10 | 7.14 ± 3.39 | 0.860 |
| Male/female ratio | 11/6 | 7/9 | 0.976 |
| BMI(kg/m2) | 32.66 ± 8.10 | 32.69 ± 6.04 | 0.385 |
| ALT(IU/L) | 75.29 ± 8.54 | 75.25 ± 8.86 | 0.655 |
| AST(IU/L) | 78.71 ± 8.48 | 76.12 ± 7.69 | 0.360 |
| TG(mg/dl) | 118.35 ± 4727 | 153.25 ± 91.96 | 0.055 |
| TCHO(mg/dl) | 169.24 ± 22.18 | 184.00 ± 29.82 | 0.247 |
| LDL-CHO(mg/dl) | 101.82 ± 15.76 | 115.50 ± 21.34 | 0.158 |
There was significant change in mean values of BMI, ALT, AST, Triglycerides, total cholesterol and LDL-cholesterol before and after intervention in both groups (p <0.0001) (Table 2). Although the decrease in the mean of these parameters in group 1 was more than group 2, there was not a significant difference between groups with the exception of LDL-cholesterol (p <0.04). Some predominant changes were observed between the values of hepatic density on ultrasonography at the beginning and the end of the study. There were changes in hepatic density in 3 of 17 cases in group of life style & vitamin E (Figure 1) and in 2 of 16 patients in group of life style and placebo (Figure 2) p = 0.68.
Comparison between Groups After and Before modification.
| Group | Before intervention (Mean ± SD) | After intervention (Mean ± SD) | P. Value | |
|---|---|---|---|---|
| BMI(kg/m2) | Group1 | 32.66 ± 8.1 | 30.81 ± 7.08 | 0.0001 |
| Group2 | 32.69 ± 6.00 | 31.04 ± 5.47 | 0.0001 | |
| ALT(IU/L) | Group1 | 75.29 ± 8.54 | 66.88 ± 7.57 | 0.0001 |
| Group2 | 75.25 ± 8.86 | 68.00 ± 8.29 | 0.0001 | |
| AST(IU/L) | Group1 | 78.71 ± 84.8 | 64.65 ± 7.68 | 0.0001 |
| Group2 | 76.12 ± 7.69 | 69.25 ± 7.87 | 0.0001 | |
| TCHO(mg/dl) | Group1 | 169.24 ± 22.18 | 157.53 ± 20.85 | 0.0001 |
| Group2 | 184.00 ± 29.82 | 165.75 ± 18.41 | 0.0001 | |
| LDLCHO(mg/dl) | Group1 | 101.82 ± 15.76 | 96.65 ± 15.37 | 0.0001 |
| Group2 | 115.50 ± 21.34 | 109.00 ± 18.51 | 0.0001 | |
| TG(mg/dl) | Group1 | 118.35 ± 47.27 | 104.00 ± 41.91 | 0.0001 |
| Group2 | 153.25 ± 91.86 | 139.31 ± 83.92 | 0.0001 |
Changes of Hepatic Density Following Intervention in Group 1
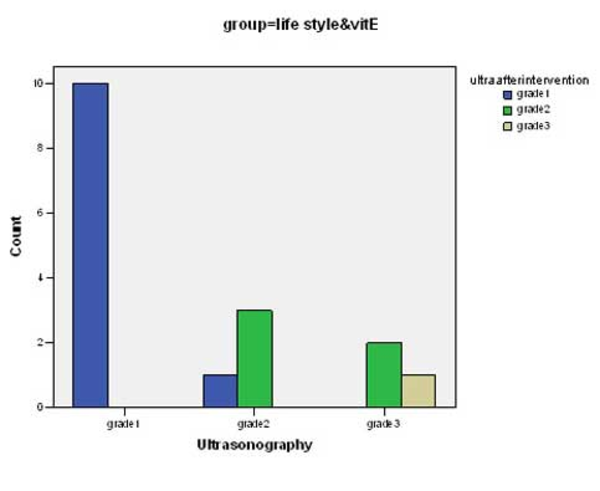
Changes of Hepatic Density Following Intervention in Group 2
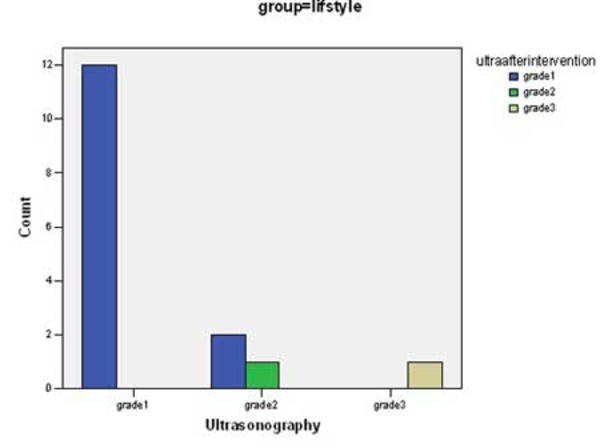
5. Discussion
Authors believe that the current study is the first one in determining the effect of lifestyle intervention and vitamin E therapy in children with NAFLD in our region. It has already been stated that the basic treatment of patients with obesity related to NAFLD would be weight loss. It is observed, however, that most of the individuals are not able to reach this objective consistently, needing a more effective treatment (10-13). Vitamin E is frequently used among patients with NAFLD. The useful effect of vitamin E in patients with steatohepatitis has been attributed to its antioxidant action. Oxidative stress plays an essential role in NAFLD pathogenesis. In the present study, a six-month lifestyle intervention with low calorie diet and increased physical activity was associated with a significant improvement of BMI, ALT, AST and serum lipid levels. Although group 1 received vitamin E, the mean reduction of all these parameters was a little more than group 2, (control) between group analysis revealed that a six-month therapy with the vitamin E was not better than a placebo for obese children with NAFLD. This finding confirms the results of previously published studies that have included a comparative control group (14, 15). In a study had been performed in Italy, diet and physical exercise in NAFLD children led to a significant improvement of liver function and glucose metabolism beyond any antioxidant therapy, but the dosage of vitamin E used in our study was lower than the dosage of the prescribed one in this study (16). Vajro et al. found no apparent beneficial effect of vitamin E supplementation in children who effectively lost weight, while the authors recommended the antioxidant prescription in those patients who did not lose weight. The authors did not observe any changes in liver brightness, despite the normalization of liver enzymes that already occurred after 2 months of therapy (17). In children, an open-label pilot trial of antioxidant therapy with doses of vitamin E ranging from 400 to 1200 IU daily was initiated to treat 11 obese patients with elevated aminotransferase levels without evidence of other liver disease (18). During a mean follow-up of 5.2 months, despite the insignificant decrease in body weight (BMI from 32.8 to 32.5 kg/m2) as well as compliance to the recommended diet, the author observed the normalization of aminotransferase levels during the treatment, but no improvement in liver brightness. Drug was justified according to the individual response. Patients were monitored after withdrawing vitamin E therapy, and they experienced an increase in liver enzyme concentrations (17). Kugelmans et al. found that vitamin E improved insulin sensitivity and several of its associated parameters, including ALT levels in overweight otherwise healthy subjects, but the effectiveness of treatment was not sustained over the time (19). In another investigation from China results showed that a brief period of therapy with 100 mg vitamin E and lifestyle intervention may have an effect on ALT levels and insulin resistance in children with NAFLD but the liver ultrasonography did not demonstrate any predominant change after a month intervention (20). In our study, the liver ultrasonography did not show any significant change after intervention. The liver steatosis stage of the vitamin group and the placebo group improved by a maximum one stage. It is likely that ultrasound is not sensitive enough to determine short improvement in steatosis.
Our study has some limitations; first, we did not evaluate circulating levels of vitamin E at the baseline and during the treatment. Second, all patients in our trial were subject to intervention with diet and physical activity however the degree of adherence to these strictures remained unclear. Also a small simple size did not allow us to draw firm conclusions from the efficacy of vitamin E treatment on NAFLD.
In conclusion, our results demonstrated that simple lifestyle intervention with diet and physical exercise in children with NAFLD can lead to a significant improvement of liver function and plasma lipid levels beyond any antioxidant therapy. Therefore, modification in lifestyle should represent the first step in the management of children with NAFLD. Therefore, additional studies are required.
Acknowledgements
References
-
1.
Raszeja-Wyszomirska J, Lawniczak M, Marlicz W, Miezynska-Kurtycz J, Milkiewicz P. [Non-alcoholic fatty liver disease--new view]. Pol Merkur Lekarski. 2008;24(144):568-71.
-
2.
Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115(5):e561-5. [PubMed ID: 15867021]. https://doi.org/10.1542/peds.2004-1832.
-
3.
Yiu D, Leung N. Epidemiological study: nonalcoholic fatty liver disease in Hong Kong Chinese. 2004.
-
4.
Tarantino G, Saldalamacchia G, Conca P, Arena A. Non-alcoholic fatty liver disease: further expression of the metabolic syndrome. J Gastroenterol Hepatol. 2007;22(3):293-303. [PubMed ID: 17295757]. https://doi.org/10.1111/j.1440-1746.2007.04824.x.
-
5.
Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221-31. [PubMed ID: 11961152]. https://doi.org/10.1056/NEJMra011775.
-
6.
Rafeey M, Mortazavi F, Mogaddasi N, Robabeh G, Ghaffari S, Hasani A. Fatty liver in children. Ther Clin Risk Manag. 2009;5(2):371-4. [PubMed ID: 2697538]. https://doi.org/10.2147/TCRM.S4467.
-
7.
Kinugasa A, Tsunamoto K, Furukawa N, Sawada T, Kusunoki T, Shimada N. Fatty liver and its fibrous changes found in simple obesity of children. J Ped Gastroenterol Nutr. 1984;3(3):408-14. [PubMed ID: 6737186]. https://doi.org/10.1097/00005176-198406000-00018.
-
8.
Reinehr T, Schmidt C, Toschke AM, Andler W. Lifestyle intervention in obese children with non-alcoholic fatty liver disease: 2-year follow-up study. Arch Dis Child. 2009;94(6):437-42. [PubMed ID: 19224892]. https://doi.org/10.1136/adc.2008.143594.
-
9.
Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J hepat. 1997;27(1):103-7. https://doi.org/10.1016/S0168-8278(97)80287-5.
-
10.
Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19(5):537-44. [PubMed ID: 14987322]. https://doi.org/10.1111/j.1365-2036.2004.01888.x.
-
11.
Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100(5):1082-90. [PubMed ID: 15842582]. https://doi.org/10.1111/j.1572-0241.2005.41583.x.
-
12.
Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39(3):770-8.
-
13.
Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology. 1990;99(5):1408-13.
-
14.
Manning PJ, Sutherland WH, Walker RJ, Williams SM, De Jong SA, Ryalls AR. Effect of high-dose vitamin E on insulin resistance and associated parameters in overweight subjects. Diabetes Care. 2004;27(9):2166-71.
-
15.
Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15(10):1667-72.
-
16.
Nobili V, Manco M, Devito R, Ciampalini P, Piemonte F, Marcellini M. Effect of vitamin E on aminotransferase levels and insulin resistance in children with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2006;24(11-12):1553-61.
-
17.
Vajro P, Franzese A, Valerio G, Iannucci MP, Aragione N. Lack of efficacy of ursodeoxycholic acid for the treatment of liver abnormalities in obese children. J Pediatr. 2000;136(6):739-43. https://doi.org/10.1016/S0022-3476(00)26774-7.
-
18.
Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136(6):734-8. [PubMed ID: 10839868]. https://doi.org/10.1067/mpd.2000.106566.
-
19.
Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38(2):413-9. [PubMed ID: 12883485]. https://doi.org/10.1053/jhep.2003.50316.
-
20.
Wang CL, Liang L, Fu JF, Zou CC, Hong F, Xue JZ, et al. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol. 2008;14(10):1598-602. [PubMed ID: 18330955]. https://doi.org/10.3748/wjg.14.1598.