Abstract
Background:
Cystic fibrosis is a complex progressive disease which assessing its progression and severity is essential. For this purpose there are scoring systems available to evaluate the disease severity.Objectives:
The aim of the present study was to determine the clinical status of CF patients using shwachman score system in the pediatric pulmonary ward of Masih Daneshvari Hospital.Patients and Methods:
A cross-sectional study was conducted to evaluate the clinical status with shwachman score system. 23 patients ranging from 5 to23 years were enrolled in this study. All data was extracted objectively from Shwachman-Kulczycki scoring system.Results:
The overall mean Shwachman-Kulczycki score was 53.48 ± 13.8. Total scores of < 40 (severe), 41-55, 56-70, and 71-85 were detected in 1.7%, 39.1%, 30.4% and 8.7% of patients respectively. None of the patients were categorized in the excellent range. We found no statistically significant correlation between mortality rate and clinical score (p = 0.136). However, the results showed a statistically significant correlation between mortality rate and Shwachman score, (p = 0.02).Conclusions:
Shwachman-Kulczycki score is an easily applied scoring system which can reflect the clinical status of patients objectively. However, concurrent use of other evaluating tools such as pulmonary function test (PFT) and Computed tomography scoring systems provide a more robust monitoring and a reliable evaluation tool.Keywords
1. Background
Cystic fibrosis (CF) is the most fatal genetic disorder among white populations (1, 2). CF is a multi-system disease with various symptoms which cause many different complications. Despite all the improvements in the treatment of CF patients which have led to increase in life expectancy for them, still chronic respiratory failure has remained the cardinal cause of death for most CF patients (3, 4). Much of the improvement is because of the development of CF scoring systems which emphasizes assessing the disease progression by regular evaluations (5, 6). Clinical evaluation has always been considered by physicians’ points of view (7, 8). Scoring systems for evaluation of the severity of CF have been used for over 50 years and Shwachman and Kulczycki scoring system was the first clinical score published (9).
2. Objectives
This study evaluates the clinical outcomes of 23 patients from pediatric pulmonary ward of Masih Daneshvari Hospital using the Shwachman and Kulczycki scoring system.
3. Patients and Methods
23 CF patients with an age range of 5 to 23 years were enrolled in this cross-sectional study. The study was conducted on patients admitted in Pediatric Pulmonary ward of Masih Danehsvari Hospital, Tehran, Iran. The diagnosis of CF was defined by two positive results of sweat tests along with clinical manifestations. Clinical status of the patients was scored by a pediatric pulmonologist, using a modified Shwachman-Kulczycki technique. This scoring system determines the clinical severity of cystic fibrosis using four different parameters: general activity, physical examination, nutrition status and radiological findings. Each of the parameters is scored from 5 (severely impaired) to 25 (normal), which results in a total score categorized as excellent (86-100), good (71-85), mild (56-70), moderate (41-55) and severe (<40). (Table 1). Spearman and Kendall’s correlation was applied to determine the association between age and Shwachman score.
| Total Score | Points | General Activity | Physical Examination | Nutrition | X Ray Findings |
|---|---|---|---|---|---|
| Excellent (86-100) | 25 | Normal | Normal | Normal | Normal |
| Good (71-85) | 20 | Lack of endurance | Rare cough, no clubbing, minimal hyperinflation | Stools slightly abnormal | Minimal accentuation of bronchovascular markings, early hyperinflation |
| Mild (56-70) | 15 | Tires easily after exertion | Occasional cough wheeze, increased respiratory rate early clubbing | Stools often abnormal, minimal abdominal distension, reduced muscle mass | Mild hyperinflation, patchy atelectasis, increased bronchoalveolar markings |
| Moderate (41-55) | 10 | Home teacher dyspnea after short walk | Frequent cough, clubbing, moderate hyperinflation crackles and wheezes | weight and height below the 3rd centile, offensive stools, reduced muscle mass | Moderate hyperinflation, widespread atelectasis and areas of infection |
| Severe <40 | 5 | Orthopnea stays in chair or bed | Tachypnea, tachycardia, extensive crackles, cyanosis, severe clubbing | Marked malnutrition, rectal prolapse, etc. | Severe hyperinflation, lobar atelectasis and bronchiectasis. nodules/cysts, cardiac enlargement |
4. Results
23 CF patients (9 females and 14 males) with an age range of 5 to23 years and a mean age of 13.42 were studied. Sadly, 6 of the 23 patients died during the study. The overall mean Shwachman-Kulczycki score was 53.48 ± 13.8. Total scores of <40(severe), 41-55, 56-70, and 71-85 were detected in 1.7%, 39.1%, 30.4% and 8.7% of patients respectively. (Figure 1) Hence, none of the patients were categorized in the excellent range.
The Summary of the Total Score Among Patients
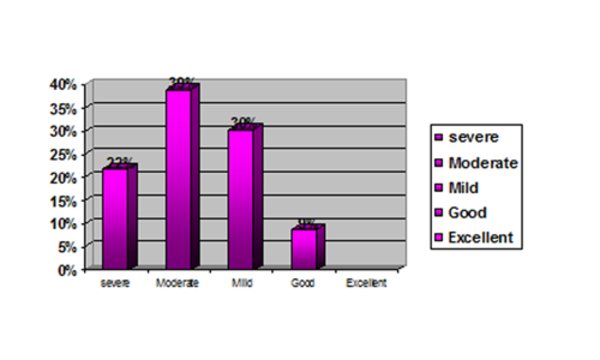
Regarding general activity, 13% of the patients presented with the lowest score (5), while 39.1% presented with a high score (20), (mean score: 15). For physical examination, 8.7% presented the lowest score (5), while 17.4% presented a high score of 20, (mean score: 13.47). For nutrition, the lowest score was 10 and 47.8% of patients presented a score of 10 and 8.7% presented with the high score of 20, (mean score: 13.04). In X-ray findings, 4.3% presented a score of 5 and the same percentage of patients presented a score of 20. However, none of the patients in any of the Shwachman-Kulczycki score parameters presented the highest score, (25). Table 2 summarizes the number of patients, minimum, maximum and the mean age of patients in different groups due to their total Shwachman scores.
The results indicated that Shwachman-Kulczycki score showed no correlation with the patients age (p = 0.136). All of the Shwachman-Kulczycki score parameters had a significant correlation with the total score (p = 0), but physical examination was the one that contributed the most to the total score (p = 0, r = 0.850). Six of the 23 patients died due to pulmonary insufficiency followed by respiratory infection. In the deceased group, the mean scores were 9.16, 9.16, 10.83 and 9.16 in general activity, physical examination, nutrition and X-ray findings, respectively. Among the remaining patients, the mean score was 17.05 in general activity, 15 in physical examination, 13.82 in nutrition and 12.94 in X-ray findings (Table 3). The results showed that all the patients who died had lower Shwachman-Kulczycki scores compared to the remaining patients.
Total Shwachman Score Regarding Patient age
| Total Score | Number of Patients | Minimum age, y | Maximum age, y | Mean age, y | SD |
|---|---|---|---|---|---|
| Severe | 5 | 11 | 23 | 15.6 | 4.77 |
| Moderate | 9 | 5 | 19 | 14.3 | 5.47 |
| Mild | 7 | 5 | 18 | 11.1 | 4.52 |
| Good | 2 | 10 | 14 | 12 | 2.82 |
Mean Score of Died and Alive Patients
| Death | No. | Mean | |
|---|---|---|---|
| No | general activity | 17 | 17.0588 |
| physical examination | 17 | 15.0000 | |
| nutrition | 17 | 13.8235 | |
| X ray findings | 17 | 12.9412 | |
| total score | 17 | 58.8235 | |
| Yes | general activity | 6 | 9.1667 |
| physical examination | 6 | 9.1667 | |
| nutrition | 6 | 10.8333 | |
| X ray findings | 6 | 9.1667 | |
| total score | 6 | 38.3333 | |
5. Discussion
As the physicians are in favor of using other clinical parameters in the evaluation and follow-up the patients with cystic fibrosis, clinical scoring systems which could be helpful, should be employed (7, 8, 10). Shwachman score was the first clinical scoring system for evaluating the progression of CF and has still remained the mostly used system (11). Although the publication of Shwachman score dates back over 50 years ago, we found no previous report of applying this scoring system in Iranian hospitals. In the present study, the clinical status of our 23 CF patients by means of four parameters listed in Shwachman score was evaluated. The mean age of patients categorized in severe (total score < 40), moderate (total score of 41-55) mild (total score of 56-71) and good (total score of 71-85) groups were 15.6, 14.3, 11.1 and 12, respectively. The mean age progressed in severe, moderate and mild groups respectively. The reason why the mean age in the group of good was higher than the mild, might be due to the few number of patients (2 patients) categorized in this group. In this study, the results demonstrated no correlation between total score and patients age, (p = 0.136). This could be contributed to the impaired clinical status of most patients at the time of diagnosis due to a lack of neonatal screening tests and higher average age at diagnosis in our patients. Another reason for this non-correlation is the small sample size. In a study conducted on 117 cases in Austria, patients were divided into three groups based on their age; group 1 (0-5y), group 2 (6-16 y) and group 3 (> 17 y) (12). The overall Shwachman-Kulczycki clinical score was 61.7 ± 8.8. This clinical scoring system failed to indicate any significant difference between groups 1 and 2, however both had almost the same mean score of 63 and a statistically significant decrease in Shwachman score was observed for 17 years old patients and older (Group 3). As our results indicated, none of the patients, qualified for the excellent group and none of the patients got the highest score for any of the Shwachman-Kulczycki score parameter. This indicates that the clinical status of most of the studied patients was impaired at the time of diagnosis and we can also claim that clinical outcomes in CF patients are mainly influenced during the early stages of life. In this study, the total score correlated significantly with the score of each of its parameters separately (p = 0), and physical examination was the parameter which most influenced the total score, (p = 0, r = 0/85). During this cross-sectional study, 6 of 23 patients died. The scores for each parameter and the total score of all the dead patients were lower than those who were still alive. Thus, the results demonstrated a significant correlation between mortality and Shwachman score, (p = 0.02). Overall, Shwachman-Kulczycki score based on objective information is an easily applied system to evaluate the severity of disease. This study had its own limitations such as small sample size and its retrospective nature. Although this scoring system to some extent could reflect the clinical status of the patients and identified the difference between the deceased patients and those who survived, concurrent use of these evaluation systems will provide clinicians with more robust and reliable monitoring and evaluating tools, demonstrating their correlations.
Acknowledgements
References
-
1.
Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173(5):475-82. [PubMed ID: 16126935]. https://doi.org/10.1164/rccm.200505-840OE.
-
2.
Moss RB. Long-term benefits of inhaled tobramycin in adolescent patients with cystic fibrosis. Chest. 2002;121(1):55-63. [PubMed ID: 11796432]. https://doi.org/10.1378/chest.121.1.55.
-
3.
Jaffe A, Bush A. Cystic fibrosis: review of the decade. Monaldi Arch Chest Dis. 2001;56(3):240-7. [PubMed ID: 11665504].
-
4.
Ramsey BW. Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med. 1996;335(3):179-88. [PubMed ID: 8657217]. https://doi.org/10.1056/NEJM199607183350307.
-
5.
Goss CH, Mayer-Hamblett N, Kronmal RA, Ramsey BW. The cystic fibrosis therapeutics development network (CF TDN): a paradigm of a clinical trials network for genetic and orphan diseases. Ad Drug Deli Rev. 2002;54(11):1505-28. https://doi.org/10.1016/S0169-409X(02)00163-1.
-
6.
PB. D. Cystic fibrosis in 20th century.Doershuk C, editor. Cystic fibrosis in the 20th century : people, events, and progress. Cleveland, Ohio: AM Pub; 2001.
-
7.
Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154(5):1229-56. [PubMed ID: 8912731].
-
8.
Katz JN, Horwitz RI, Dolan TF, Shapiro ED. Clinical features as predictors of functional status in children with cystic fibrosis. J Ped. 1986;108(3):352-8. https://doi.org/10.1016/S0022-3476(86)80872-1.
-
9.
Shwachman H, Kulczycki LL. Long-term study of one hundred five patients with cystic fibrosis; studies made over a five- to fourteen-year period. AMA J Dis Child. 1958;96(1):6-15. [PubMed ID: 13544726].
-
10.
Dankert-Roelse JE, te Meerman GJ. Long term prognosis of patients with cystic fibrosis in relation to early detection by neonatal screening and treatment in a cystic fibrosis centre. Thorax. 1995;50(7):712-8. [PubMed ID: 7570403]. https://doi.org/10.1136/thx.50.7.712.
-
11.
Taussig LM, Kattwinkel J, Friedewald WT, di Sant'Agnese PA. A new prognostic score and clinical evaluation system for cystic fibrosis. J Ped. 1973;82(3):380-90. https://doi.org/10.1016/S0022-3476(73)80110-6.
-
12.
Helbich TH, Heinz-Peer G, Eichler I, Wunderbaldinger P, Gotz M, Wojnarowski C, et al. Cystic fibrosis: CT assessment of lung involvement in children and adults. Radiology. 1999;213(2):537-44.