Abstract
Background:
Ureaplasma urealyticum (U. urealyticum) and Mycoplasma hominis (M. hominis) are two important pathogens in neonatal respiratory tract infections. As low gestational age and birth weight increase the colonization rate with these pathogens, and they are also risk factors of chronic lung disease (CLD), it is difficult to establish a statistically significant relation between these two factors.Objectives:
To determine the colonization rate of preterm ventilated neonates with U. urealyticum and M. hominis , and the relationship between infection and chronic lung disease in these neonates, a prospective study was performed.Patients and Methods:
Determining tracheal colonization rate of preterm neonates with ureaplasma and mycoplasmsa and its relation to CLD. In a cohort prospective study in Hazrat Rasoul Akram Hospital, with 62 ventilated neonates (< 35 weeks) in the first 24 h of life, where tracheal secretions were aspirated, transported, and cultured in a specific media. CLD was diagnosed as oxygen requirement at 28 days or 36 weeks post conceptional age.Results:
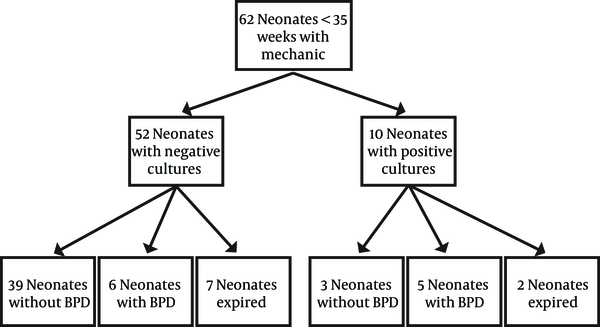
From 62 cultures, 7 were positive for U. urealyticum (11.3%, CI95%: 3.4-19.2%) and 3 were positive for M. hominis (4.8%, CI95%: 0-10.2%). From 53 neonates who were alive at 28 days after birth, 11 neonates needed oxygen (20.8%, CI95%: 9.8-31.7%). From 10 neonates with positive cultures, 2 (20%, CI95%: 0-44.8%) died, and from 8 alive neonates, 3 (37.5%, CI95%: 4.0-71.0%) survived without CLD. From 52 neonates with negative cultures, 7 (13.5%, CI95%: 4.2-22.7%) died, and from 45 alive neonates, 39 (86.7%, CI95%: 76.7-96.6%) survived without CLD, and there was a significant difference between neonatal groups with positive and negative tracheal culture progressing to CLD (62.5% vs. 13.3%) (P < 0.001) (Figure 1).Conclusions:
Intrauterine infection with U. urealyticum and M. hominis leads to negative neonatal outcome and increased rate of chronic lung disease in preterm neonates (< 35 weeks).Keywords
Ureaplasma urealyticum Mycoplasmsa hominis Lung Diseases Infant Premature
1. Background
Ureaplasma spp. can be found on the mucosal surfaces of the cervix or vagina of 40 to 80% of sexually mature asymptomatic women, whereas M. hominis may occur in 21 to 53% (1). Intrauterine infection is a major cause of preterm labor and different organisms can be cultured in approximately half of all preterm births, especially those occurring at less than 30 weeks of gestation, so increasing neonatal risk of congenital infections. Vertical transmission of ureaplasma spp. has been reported to range from 25 to 60% and isolation rates vary inversely with gestational age and maternal socioeconomic status, according to most studies (1, 2). Respiratory disease remains the most common cause of perinatal morbidity and mortality, especially in preterm neonates and ureaplasma spp and mycoplasma are known as pathogens in congenital pneumonia. Very little information exists to indicate what are the long-term consequences that may result from neonatal infection by ureaplasma spp. or M. hominis. Three independent reports associating the presence of ureaplasma spp. in the lower respiratory tract with progression to chronic lung disease (CLD), and even death in very low birth weight neonates were published in 1988 (3-5) . Pooled studies showed a significant association between ureaplasma colonization and development of CLD (P < 0.001) (3). But they did not show if medical treatment with erythromycinor or other macrolodes might reduce the CLD incidence, and most of their sample sizes were not big enough to conclude if the routine culture for ureaplasma and treatment of colonization with the aim of preventing CLD should be done routinely or not (6, 7). So the association between the infection by those pathogens and CLD needs more clinical investigation.
2. Objectives
The colonization rate of preterm neonates with U.urealyticum and M. hominis and their long term sequeal, such as CLD has been a matter of debate in recent years. A prospective study was performed to determine the relative incidence of tracheal cultures of preterm ventilated neonates with these organisms and development of CLD.
3. Patients and Methods
We have conducted a cohort prospective study in a neonatal intensive care unit (NICU) in Hazrat Rasoul Hospital in Tehran, Iran from September 2007 till March 2009. With all preterm neonates (< 35 weeks), where gestation needed mechanical ventilation, a sterile tracheal aspirate was taken in the first 24 hours after intubation and after infiltration in a transport media (phosphate-buffered saline), and it was cultured in highly specific media for ureaplasma and mycoplasma. Totally 80 samples were taken, from which 18 (22.5%) samples were not adequate (with different causes , 3 cases were dried samples, 4 were not transported with a transport media, 5 neonates had a gestational age more than 35 weeks, and 6 samples were taken from nasopharyngeal secretions instead of tracheal secrections.) and 62 samples were cultured. CLD or bronchopulmonary dysplasia was defined as oxygen requirement at 28 days after birth or 36 weeks post-conceptional age. Data analysis was done with Fisher exact test and T-test using SPSS 15.
4. Results
From 62 samples received by the laboratory, 10 samples (16.1%, CI95%: 7.0-25.3%) were positive, 7 (11.2%, CI95%: 3.4-19.2%) had U.urealyticum, and 3 (4.8%, CI95%: 0-10.2%) had MycoplasmsaHominis (Table 1). None were positive with both microorganisms.
From all 62 neonates, 18 (29%) weighted less than 1000 g, 29 (46.7%) weighted between 1000 to 1499 g, and 15 (24.1%) weighted between 1500 to 2500 g.
The Relationshipbetween Positive Tracheal Culture and Neonatal Characteristics
| Tracheal Culture Results | Positive, n = 10 | Negative, n = 52 | Total, n = 62 | P | |
|---|---|---|---|---|---|
| Gender | female | 4, 40 | 24, 46.1 | 28, 45.1 | 0.722 |
| male | 6, 60 | 28, 53.8 | 34, 54.8 | ||
| PROM | positive | 4, 40 | 15, 28.8 | 19, 30.6 | 0.418 |
| negative | 6, 60 | 37, 71.1 | 43, 69.3 | ||
| Maternal Steroid | yes | 7, 70 | 34, 65.3 | 41, 66.1 | 0.773 |
| no | 3, 30 | 18 34.6 | 21, 33.8 | ||
| Maternal Fever | yes | 3, 30 | 7, 13.4 | 10, 16.1 | 0.190 |
| no | 7, 70 | 45, 86.5 | 52, 83.8 | ||
| Route of Delivery | cesarean section | 8, 80 | 32, 61.5 | 40, 64.5% | 0.262 |
| vaginal delivery | 2, 20 | 20, 38.4 | 22, 35.5% | ||
| Final Outcome | alive to discharge | 8, 80 | 45, 86.5 | 53, 85.5% | 0.593 |
| death before discharge | 2, 20 | 7, 13.4 | 9, 14.5% | ||
| Needs Oxygen after 28 days | yes | 5, 62.5 | 6, 13.3 | 11, 20.7 | < 0.001 |
| no | 3, 37.5 | 39, 86.6 | 42, 79.2 | ||
| Gestational age (wks), Mean ± SD | 28.1 ± 1.8 | 31.5 ± 2.9 | 30.2± 3.3 | < 0.001 | |
| Weight( g), Mean ± SD | 1050.7 ± 354.4 | 1485.1 ± 425.7 | 1290.1±560.6 | 0.003 |
Mean ± SD of birth weight of neonates with positive cultures were 1050.7 ± 354.4 g and neonates with negative culture had a mean birth weight of 1485.1 ± 425.7 g that showed statistically significant difference (P = 0.003) (Table 1). There was also statistically significant difference in mean gestational age between neonates with positive and negative culture (28.1 ± 1.8 weeks vs. 31.5 ± 2.9) (P < 0.001) (Table 1). There was no statistically significant difference in other parameters such as maternal steroid administration in last week before delivery, maternal fever, route of delivery, prolonged premature rupture of membranes (more than 24 hours), or maternal antibiotic usage among these two groups (Table 1). Nine neonates died in the first month of life (5%). The other 53 alive neonates (85.4%) were followed till discharge from hospital, and 11 (20.7%) of them needed supplemental oxygen after 28 days of life (Figure 1). From 10 neonates with positive cultures, 2 (20%) died, and from 8 alive neonates, 5 (62.5%) had CLD and 3 (37.5%) survived without CLD. From 52 neonates with negative cultures, 7 (13.4%) died, and from 45 alive neonates, 6 (13.3%) had CLD and 39 (86.6%) survived without CLD (P < 0.001).
Summary of the Results and Outcome of 62 Neonates (< 35 Weeks) with Mechanical Ventilation
5. Discussion
The genital mycoplasmas and ureaplasmas represent a group of microorganisms that have been associated with different patterns of infection in pregnant mothers and their infants. With better laboratory detection and increasing knowledge about their significance in maternal and neonatal infections, this situation is now changing (8), as high as 40 to 80% of women have been reported to be colonized with genital mycoplasmas (9). Their role in infertility, post-partum endometritis, chorioamnionitis, preterm labor, and spontaneous abortion has been extensively investigated. In Iran, Badami reported M.hominis was isolated from 35% and U.urealyticum was isolated from 33% of infertile females compared with 7.2% of normal population (10), and Najar Peerayeh reported a 37.4% detection rate with PCR for mycoplasmas, from which 22.5% were positive with U.uralyticum in infertile Iranian women (11).
The fetus can be infected via three different routes: vertical transmission from infected amniotic fluid, hematogenous spread from the placenta, and birth through infected vaginal canal. Vertical transmission rates among neonates born to colonized women are 25-60%. Colonization rate of the neonates is adversely related to their gestational age and birth weight and has been shown to be 60% in neonates under 1000 g, 15% in neonates 1000-1500 g, and 10% in term neonates (1). Ureaplasma has been implicated in neonatal morbidity and mortality including congenital pneumonia, preterm delivery, low birth weight, and intrauterine growth retardation, and it is thought to infect or colonize up to 37% of newborns. Heggie (12) et al. showed from tracheal aspirate specimens, U. urealyticum was recovered from 17% (37) and M. hominis from 2%(4). Although neonates with positive results were less mature than their cohorts with negative results, there were no substantive differences in clinical outcomes between the two groups. In India, 20%(20) of the study neonates were colonized with U. urealyticum and they showed the mean gestational age of the neonates in the colonized group was less than that of non-colonized neonates (P < 0.05) (13). We have shown a prevalence rate of 11% for ureaplasma and 5% for mycoplasma with more infection rate in less premature neonates as well. None of the 20 babies colonized with U. urealyticum in Pandey study developed CLD as compared with two (2.5%) of the non-colonized group, and they suggested colonization of the airways with U. urealyticum had no significant role in development of CLD in Indian preterm infants. The association between presence of ureaplasma and the development of CLD remains controversial and hotly debated. In 1995, a meta-analysis by Wang et al. included 1479 babies from 17 studies (14), reporting a significant association between CLD diagnosed at 28 days of life and ureaplasma colonization, with an overall relative risk of 1.72 (CI95%: 1.5–1.96). In a cohort of 126 preterm deliveries, Kafetzis et al. (15) found a significant increase in CLD as well as mortality among ureaplasma colonized infants. Van Waarde et al. (16) found that Ureaplasma was significantly associated with both CLD and lower gestational age, but logistic regression analysis failed to show a correlation between ureaplasma colonization and CLD. Schelonka et al. (17) found an odds ratio (OR) of 2.83 (CI95%: 2.29–3.51) for the relationship between the presence of ureaplasma and CLD in a meta-analysis of 23 studies, and Goldenberg et al. (18) confirmed a probable association between infection and CLD as well. Waites studied the colonization rate of U. urealyticum and a significant trend toward higher neonatal morbidities such as longer ventilation and hospital stay and CLD (3). The etiology of bronchopulmonary dysplasia (BPD) is multifactorial and complex. Development of BPD is associated with prenatal and postnatal factors that lead to an arrest of lung development. Prenatally, maternal chorioamnionitis is associated with later development of BPD, with U.urealyticum being the most common association with chorioamnionitis at less than 30 weeks of gestational age (19). Postnatal insults include oxygen toxicity, barotrauma and volutrauma from mechanical ventilation, and sepsis. These additional insults lead to activation of the inflammatory cascade and significantly increase the risk of BPD. In recent years, an appreciation for the role of inflammation as a consequence of perinatal infection emerged as an important factor in the pathogenesis of BPD. Some proinflammatory cytokines [interleukin-1β (IL-1β), tumor necrosis factor alpha, and IL-6 and 8] are shown to have an important role in different neonatal pathologies such as cerebral palsy (20) and CLD (21). Dyke et al. found that the route of delivery might have a role in risk of developing BPD, and those who have been delivered by cesarean section and had positive gastric aspirate with ureaplasma had a higher rate of progressing to BPD (21). We have shown a greater incidence of BPD in culture positive neonates (50%) in comparision to culture negative infants (11.5%), but they did not show any significant difference in their route of delivery or weight or other demographic factors. As there are conflicting results regarding infection-BPD association, some studies have reported the results of antibiotic therapy in colonized infants. Two small randomized clinical trials of erythromycin therapy in high risk neonates with tracheobronchial colonization of ureaplasma failed to show any difference between treated and non-treated neonates in the development of BPD (22, 23). It is evident from different studies that in a group of preterm infants, colonization with genital mycoplasmas have negative effects in their outcome, where it is not known which infant progresses to CLD at this time and some neonates might not show benefit from antibiotic therapy for their infection, and this matter needs further investigation.
Acknowledgements
References
-
1.
Chua KB, Ngeow YF, Lim CT, Ng KB, Chye JK. Colonization and transmission of Ureaplasma urealyticum and Mycoplasma hominis from mothers to full and preterm babies by normal vaginal delivery. Med J Malaysia. 1999;54(2):242-6. [PubMed ID: 10972036].
-
2.
Dinsmoor MJ, Ramamurthy RS, Gibbs RS. Transmission of genital mycoplasmas from mother to neonate in women with prolonged membrane rupture. Pediatr Infect Dis J. 1989;8(8):483-7. [PubMed ID: 2771527].
-
3.
Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18(4):757-89. [PubMed ID: 16223956]. https://doi.org/10.1128/CMR.18.4.757-789.2005.
-
4.
Sanchez PJ, Regan JA. Ureaplasma urealyticum colonization and chronic lung disease in low birth weight infants. Pediatr Infect Dis J. 1988;7(8):542-6. [PubMed ID: 3174298].
-
5.
Wang EE, Frayha H, Watts J, Hammerberg O, Chernesky MA, Mahony JB, et al. Role of Ureaplasma urealyticum and other pathogens in the development of chronic lung disease of prematurity. Pediatr Infect Dis J. 1988;7(8):547-51. [PubMed ID: 2845345].
-
6.
Jonsson B, Rylander M, Faxelius G. Ureaplasma urealyticum, erythromycin and respiratory morbidity in high-risk preterm neonates. Acta Paediatr. 1998;87(10):1079-84. [PubMed ID: 9825977].
-
7.
Lyon AJ, McColm J, Middlemist L, Fergusson S, McIntosh N, Ross PW. Randomised trial of erythromycin on the development of chronic lung disease in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;78(1):F10-4. [PubMed ID: 9536833].
-
8.
Mirnejad R, Amirmozafari N, Kazemi B. Simultaneous and rapid differential diagnosis of Mycoplasma genitalium and Ureaplasma urealyticum based on a polymerase chain reaction-restriction fragment length polymorphism. Indian J Med Microbiol. 2011;29(1):33-6. [PubMed ID: 21304192]. https://doi.org/10.4103/0255-0857.76521.
-
9.
Maxwell NC, Nuttall D, Kotecha S. Does Ureaplasma spp. cause chronic lung disease of prematurity: ask the audience? Early Hum Dev. 2009;85(5):291-6. [PubMed ID: 19144476]. https://doi.org/10.1016/j.earlhumdev.2008.12.002.
-
10.
Badami N, Salari MH. Rate of Chlamydia trachomatis, Mycoplasma hominis and Ureaplasma urealyticum in infertile females and control group. Iran J Public Health. 2001;30(1-2):57-60.
-
11.
Peerayeh SN, Samimi R. Detection of Ureaplasma Urealyticum in Clinical Samples from Infertile Women by Polymerase Chain Reaction. IJPT. 2007;6(1).
-
12.
Heggie AD, Jacobs MR, Butler VT, Baley JE, Boxerbaum B. Frequency and significance of isolation of Ureaplasma urealyticum and Mycoplasma hominis from cerebrospinal fluid and tracheal aspirate specimens from low birth weight infants. J Pediatr. 1994;124(6):956-61. [PubMed ID: 8201486].
-
13.
Pandey A, Dhawan B, Gupta V, Chaudhry R, Deorari AK. Clinical significance of airways colonization with Ureaplasma urealyticum in premature (<34 wk) neonates. Indian J Med Res. 2007;125(5):679-84. [PubMed ID: 17642504].
-
14.
Wang EE, Ohlsson A, Kellner JD. Association of Ureaplasma urealyticum colonization with chronic lung disease of prematurity: results of a metaanalysis. J Pediatr. 1995;127(4):640-4. [PubMed ID: 7562292].
-
15.
Kafetzis DA, Skevaki CL, Skouteri V, Gavrili S, Peppa K, Kostalos C, et al. Maternal genital colonization with Ureaplasma urealyticum promotes preterm delivery: association of the respiratory colonization of premature infants with chronic lung disease and increased mortality. Clin Infect Dis. 2004;39(8):1113-22. [PubMed ID: 15486833]. https://doi.org/10.1086/424505.
-
16.
Van Waarde WM, Brus F, Okken A, Kimpen JL. Ureaplasma urealyticum colonization, prematurity and bronchopulmonary dysplasia. Eur Respir J. 1997;10(4):886-90. [PubMed ID: 9150329].
-
17.
Schelonka RL, Katz B, Waites KB, Benjamin DK, Jr. Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr Infect Dis J. 2005;24(12):1033-9. [PubMed ID: 16371861].
-
18.
Goldenberg RL, Andrews WW, Goepfert AR, Faye-Petersen O, Cliver SP, Carlo WA, et al. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198(1):43 e1-5. [PubMed ID: 18166302]. https://doi.org/10.1016/j.ajog.2007.07.033.
-
19.
Klein LL, Gibbs RS. Use of microbial cultures and antibiotics in the prevention of infection-associated preterm birth. Am J Obstet Gynecol. 2004;190(6):1493-1502. [PubMed ID: 15284720]. https://doi.org/10.1016/j.ajog.2004.03.014.
-
20.
Wu YW, Colford JM, Jr. Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284(11):1417-24. [PubMed ID: 10989405].
-
21.
Dyke MP, Grauaug A, Kohan R, Ott K, Andrews R. Ureaplasma urealyticum in a neonatal intensive care population. J Paediatr Child Health. 1993;29(4):295-7. [PubMed ID: 8373676].
-
22.
Mabanta CG, Pryhuber GS, Weinberg GA, Phelps DL. Erythromycin for the prevention of chronic lung disease in intubated preterm infants at risk for, or colonized or infected with Ureaplasma urealyticum. Cochrane Database Syst Rev. 2003;4.
-
23.
Ballard HO, Anstead MI, Shook LA. Azithromycin in the extremely low birth weight infant for the prevention of bronchopulmonary dysplasia: a pilot study. Respir Res. 2007;8:41. [PubMed ID: 17550598]. https://doi.org/10.1186/1465-9921-8-41.