Abstract
Background:
Recombinant activated factor VII (rFVIIa; NovoSeven® RT, Novo Nordisk, Bagsvaerd, Denmark) is a synthetic pro-coagulation factor derived from hamster kidney cells.Objectives:
The purpose of this study was to evaluate the prescribing patterns of recombinant factor VIIa (rFVIIa) at a single, tertiary care pediatric hospital by indication of usage and dose administered.Materials and Methods:
Retrospective data was queried from the centralized pharmacy medication computer system. All patients receiving rFVIIa between September 2009 and September 2012 were included in the analysis.Results:
Over the three year period 887 doses of rFVIIa were administered to 186 patients. Only 4% of patients received rFVIIa for an FDA approved indication. The most common indication for off-label usage was refactory bleeding either during or following cardiothoracic surgery, accounting for 83% of all doses administered off-label. Despite being only a small portion of all patients receiving rFVIIa, the group receiving rFVIIa for an FDA approved indication received the majority of doses dispensed (72.6% of all doses). A significant difference (P < 0.0001) was noted in the dose administered to patients in the on-label versus off-label groups. Patients in the on-label group received a median dose of 113.6 mcg/kg (IQR 54.5 mcg/kg - 172.7 mcg/kg) versus a median dose of 74.4 mcg/kg (IQR 20.0 mcg/kg - 128.8 mcg/kg) in the off-label group.Conclusions:
Prospective pediatric studies are needed to evaluate rFVIIa efficacy and safety in populations receiving the medication for off-label indications given increasing concerns involving the potential adverse effect profile of the medication as well as to develop evidence based dosing parameters for these indications.Keywords
1. Background
Recombinant activated factor VII (rFVIIa; NovoSeven® RT, Novo Nordisk, Bagsvaerd, Denmark) is a synthetic pro-coagulation factor derived from hamster kidney cells. It functions via two separate mechanisms of action: 1) binding tissue factor (TF) released from subendothelium at sites of vascular injury, with subsequent activation of the common coagulation cascade and generation of activated factor X (FXa), activated factor V (FVa) and thrombin 2) binding activated platelets with surface bound FXa and FVa resulting in thrombin generation. Both mechanisms result in fibrin meshwork formation and clot stabilization at the region of endothelial disruption (1, 2).
Food and Drug Administration (FDA) approved indications for rFVIIa use include the management of bleeding in patients with; a) hemophilia A or B with inhibitors b) congenital factor VII deficiency. The drug was also approved for surgical bleeding prophylaxis in patients with hemophilia A or B (3). Due to its potent pro-coagulant effects, rFVIIa has been utilized off-label to treat life-threatening hemorrhage related to cardiothoracic surgery, intracranial hemorrhage, trauma, and liver disease. rFVIIa usage for the management of refractory bleeding during cardiothoracic surgery following cardiopulmonary bypass (CPB) has increased dramatically due to a lack of other suitable pharmacologic agents. Yet, randomized controlled studies demonstrating efficacy for this off label indications are limited (4, 5).
The recommended rFVIIa dose for treatment of hemophilia A or B is 90 mcg/kg repeated every 2 hours as needed, while congenital factor VII deficiency has a lower recommended dose of 15-30 mcg/kg repeated every 4 to 6 hours (3). There are no large efficacy studies evaluating rFVIIa dosage for off-label administration of rFVIIa in adults, much less children. Consequently, appropriate dosing regimens for off-label usage have not been well established with a wide range described in the literature (2). For example, doses of 5 – 160 mcg/kg as a single dose or 45 – 400 mcg/kg as a total rFVIIa dose have been reported for management of refractory bleeding following cardiothoracic surgery (4, 6). Age related differences in pharmacokinetics must also be taken into consideration.
2. Objectives
The goal of this study was to evaluate the institutional prescribing patterns of rFVIIa at a single tertiary pediatric hospital both in terms of indication for use and dose administered. We hypothesized that the majority of patients would receive rFVIIa for off-label indications, and that there would be no clear pattern of dose selection.
3. Materials and Methods
This was a retrospective cohort study. Data was collected via a medication use report obtained from the centralized pharmacy medication computer system. Investigational review board (IRB) approval was obtained for this study. All patients receiving rFVIIa between September 1, 2009 and September 30, 2012 at our institution were included in the data collection process. Variables collected included patient weight, indication for use, prescribing physician, rFVIIa dose (cumulative and per kilogram dose), and number of doses received. Descriptive statistics were used to describe prescribing and dosing patterns. Average dosage for on-label versus off-label indications was reported as a median with interquartile range (IQR) due to the nonparametric distribution of the data. A Wilcoxon ranked sum comparison test was used to compare the on-label versus off-label groups. A P value of 0.05 was considered significant for all statistical analyses. SAS On-demand 4.3 (SAS Institute, Cary, NC) was used to perform all statistical analyses.
4. Results
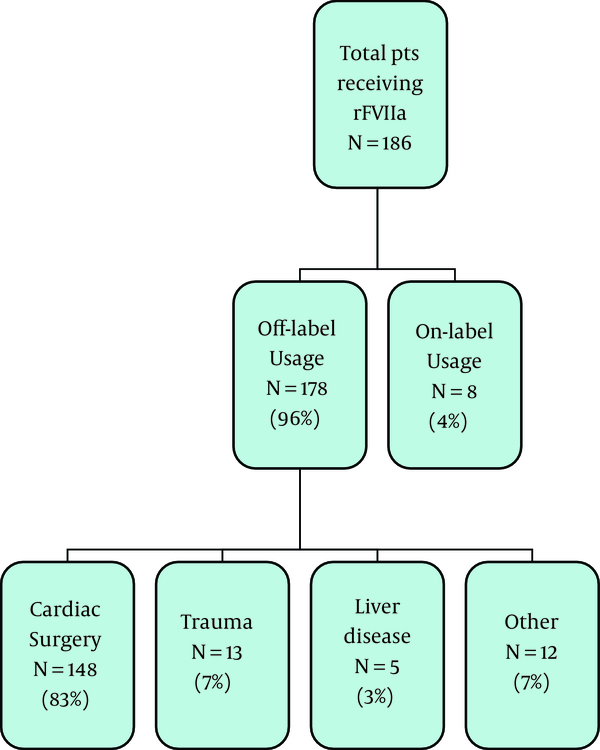
Over the three-year study period, 887 doses of rFVIIa were dispensed by pharmacy to a total of 186 patients. The majority of these patients (n = 178, 96%) received rFVIIa for an indication that was considered off-label. Only 8 of the patients (4%) received the medication for an FDA approved indication. Figure 1 summarizes the relative indications that rFVIIa was administered for over the three year period. Refractory bleeding either during or following cardiothoracic surgery was the most common off-label indication accounting for 83% of all patients receiving rFVIIa off-label. Although patients receiving rFVIIa for an FDA approved indication represented a small percentage of the patient population, they received the majority of the doses administered. The on-label group received 644 doses (72.6% of all doses). Individuals in the on-label group received between 1 and 242 doses of rFVIIa over the three-year period. The majority of the patients in the off-label group received one to two doses only. Focusing on the cardiothoracic surgery group specifically, 70% of those patients received only one dose of rFVIIa. Only 11% of the cardiac subgroup received three or more doses of rFVIIa, with a total of five doses being the most administered to any one individual patient.
Percentage of Patients Receiving rFVIIa by Primary Diagnosis
4.1. rFVIIa Dosing
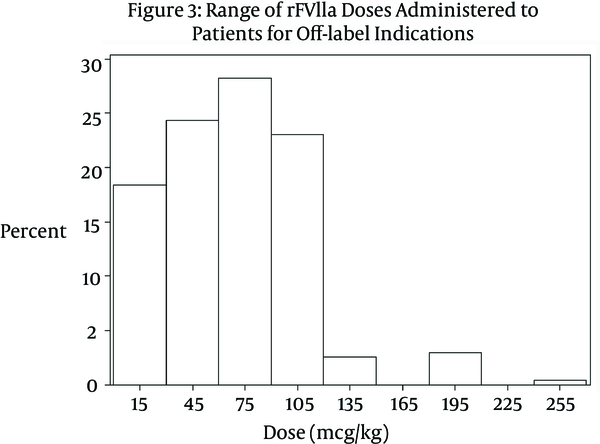
A significant difference was noted between the doses received by patients in the on-label group compared to those in the off-label group (P < 0.0001). The median dose received by patients in the on-label group was 113.6 mcg/kg (IQR 54.5 mcg/kg - 172.7 mcg/kg). The range of dosing was 15.6 mcg/kg – 222 mcg/kg per dose (Figure 2). Patients in the off-label group received on average a much smaller dose. The median dose in the off-label group was 74.4 mcg/kg (IQR 20.0 mcg/kg - 128.8 mcg/kg). The range of doses in the off-label group was 8.46 mcg/kg – 250 mcg/kg per dose (Figure 3). Table 1 compares variables related to total dose received between the two groups. Approximately 35% of patients in the off-label group (n = 63) received a dose ≤ 40 mcg/kg. In the cardiothoracic surgery subgroup, the median dose administered was 74.1 mcg/kg (IQR 38.0 mcg/kg – 91.0 mcg/kg).
Range of rFVIIa Doses Administered to Patients with FDA Approved Indications

Range of rFVIIa Doses Administered to Patients for Off-label Indications
Comparison of Variables Related to Dose for Patients Receiving rFVIIa Based on Indication of Use a,b
| Variable, Median (IQR) | Off-Label Patients (n = 178) | On-Label Patients (n = 8) | P Value |
|---|---|---|---|
| Weight in kg | 12 (7.4 - 22) | 27 (13.6 - 44) | < 0.0001 |
| Dose in mcg/kg | 74.4 (20 - 128.8) | 113.6 (54.5 - 172.7) | < 0.0001 |
| Doses per patient | 1 (1 - 1) | 13 (3 - 184) | < 0.0001 |
5. Discussion
This study examines the institutional prescribing and dosing patterns for rFVIIa within a large tertiary children’s hospital. The overwhelming majority of patients receiving rFVIIa were treated for off-label indications, consistent with previous reported studies (6). However, most doses were prescribed for on-label indications. Patients in the on-label group received higher median doses than recommended by the manufacturer. This is likely due to the fact that six of the patients in this group had a diagnosis of hemophilia with inhibitors. The presence of inhibitors requires gradual dose escalation over time. A wide range of doses were administered to the off label group, similar to previously described dosing for off-label use (2, 4).
Prospective pediatric studies evaluating rFVIIa efficacy and safety are required given the emerging adverse effect profile of the drug. Adults receiving rFVIIa for off-label indication have a higher rate of serious adverse events when compared to those treated for on-label indications. Thromboembolic complications (stroke, myocardial infarction, and other arterial thrombosis) occur at higher rates with off-label use, A review of the FDA’s Adverse Event Reporting System (AERS) found 168 reports of thromboembolic events associated with rFVIIa usage between 1999-2004 (7). Multiple meta-analyses have shown a trend towards thromboembolic events when rFVIIa is administered to cardiothoracic surgery patients (8, 9). The potential for thromboembolic adverse events resulted in an FDA black box warning be added to the package insert in 2005. Recently, retrospective studies in children have shown the thromboembolic event to be as high as 5.4% following rFVIIa administration (10, 11). The rate could be much higher though as these studies were limited by their retrospective design and did not follow subjects for prolonged periods of time following rFVIIa administration. Future prospective pediatric studies must also focus on the potential relationship between adverse events and dose administered.
Similar clinical effectiveness trials examining optimal dosing strategies for off label rFVIIa use in children are needed given the important age related differences in rFVIIa pharmacokinetics. Children exhibit increased clearance of rFVIIa compared to adults, and this effect may be more pronounced in neonates (12, 13). In a study comparing adults and children with hemophilia A receiving rFVIIa, children received significantly higher doses with no observed difference in efficacy. Pychynska-Pokorska showed that the dosage of rFVIIa required for efficacy in managing post-CPB bleeding refractory to other interventions may change with age. Neonates received a mean dosage of 131.7 ± 69.8 mcg/kg versus a dosage of 44.6 ± 15.3 mcg/kg for children older than a year of age (12). This data suggests that rFVIIa dosage for off label indications may vary inversely with age even within the pediatric subgroup. Within our study population, children receiving rFVIIa for management of post-CPB bleeding received on average a lower dose than those children receiving the medication for an on-label indication.
The cost of rFVIIa remains significant more than a decade after initial approval by the FDA. Total sales accounted for 1.5 billion USD of revenue in 2012. Acquisition costs may vary somewhat institution to institution, but the cost of rFVIIa is generally greater than 1 USD per mcg dispensed. In our study 65% of patients receiving rFVIIa for an off-label use received a dose greater than 40 mcg/kg. If these patients had received a dose of 40 mcg/kg instead, the costs savings to the inpatient pharmacy would have been more than $240,000 over a 3-year period. Thus determining minimum dosing guidelines may also have important healthcare economic implications.
Our study has a number of limitations based primarily on the retrospective study design. Data available for collection was limited to standard data retained in the pharmacy dispensing system and available on a medication use report. Data was collected from a single pediatric center. The use of rFVIIa for management of refractory bleeding for cardiothoracic surgery may have been overly represented in our results due to the high volume of congenital cardiac surgical procedures performed at our institution. In the future prospective studies focusing on the efficacy of different dosing regimens need to be performed in order to provide more cost-effective care.
This study highlights the need for the development of guidelines for the administration and dosing off rFVIIa in children. Increasing off-label administration of this medication has important implications in terms of both patient morbidity and cost. Ultimately, rFVIIa may prove to be a cost effective therapy for off label indication. However, given the dearth of data regarding rFVIIa efficacy and dosing in children, prospective studies are needed before the routine off label administration of this potent pro-coagulant and pro-thrombotic agent can be recommended.
References
-
1.
Hedner U. Factor VIIa and its potential therapeutic use in bleeding-associated pathologies. Thromb Haemost. 2008;100(4):557-62. [PubMed ID: 18841276].
-
2.
Logan AC, Goodnough LT. Recombinant factor VIIa: an assessment of evidence regarding its efficacy and safety in the off-label setting. Hematology Am Soc Hematol Educ Program. 2010;2010:153-9. [PubMed ID: 21239786]. https://doi.org/10.1182/asheducation-2010.1.153.
-
3.
[package insert] Coagulation Factor VIIa (Recombinant). Bagsvaerd, Denmark: Novo Nordisk; 2006.
-
4.
Guzzetta NA, Russell IA, Williams GD. Review of the off-label use of recombinant activated factor VII in pediatric cardiac surgery patients. Anesth Analg. 2012;115(2):364-78. [PubMed ID: 22652310]. https://doi.org/10.1213/ANE.0b013e31825aff10.
-
5.
Scott JP, Costigan DJ, Hoffman GM, Simpson PM, Dasgupta M, Punzalan R, et al. Increased recombinant activated factor VII use and need for surgical reexploration following a switch from aprotinin to epsilon-aminocaproic acid in infant cardiac surgery. J Clin Anesth. 2014;26(3):204-11. [PubMed ID: 24809789]. https://doi.org/10.1016/j.jclinane.2013.10.015.
-
6.
Witmer CM, Huang YS, Lynch K, Raffini LJ, Shah SS. Off-label recombinant factor VIIa use and thrombosis in children: a multi-center cohort study. J Pediatr. 2011;158(5):820-825 e1. [PubMed ID: 21146180]. https://doi.org/10.1016/j.jpeds.2010.10.038.
-
7.
O'Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA. 2006;295(3):293-8. [PubMed ID: 16418464]. https://doi.org/10.1001/jama.295.3.293.
-
8.
Yank V, Tuohy CV, Logan AC, Bravata DM, Staudenmayer K, Eisenhut R, et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med. 2011;154(8):529-40. [PubMed ID: 21502651]. https://doi.org/10.7326/0003-4819-154-8-201104190-00004.
-
9.
Ponschab M, Landoni G, Biondi-Zoccai G, Bignami E, Frati E, Nicolotti D, et al. Recombinant activated factor VII increases stroke in cardiac surgery: a meta-analysis. J Cardiothorac Vasc Anesth. 2011;25(5):804-10. [PubMed ID: 21596585]. https://doi.org/10.1053/j.jvca.2011.03.004.
-
10.
Blatny J, Mathew P, Monagle P, Ovesna P, Fiamoli V. Safety and efficacy of recombinant activated factor VII in nonhemophilia children with severe or life-threatening bleeding: a report from the SeveNBleeP registry. Blood Coagul Fibrinolysis. 2014;25(4):326-32. [PubMed ID: 24389586]. https://doi.org/10.1097/MBC.0000000000000036.
-
11.
McQuilten ZK, Barnes C, Zatta A, Phillips LE, Haemostasis Registry Steering C. Off-label use of recombinant factor VIIa in pediatric patients. Pediatrics. 2012;129(6):e1533-40. [PubMed ID: 22641758]. https://doi.org/10.1542/peds.2011-2561.
-
12.
Pychynska-Pokorska M, Pagowska-Klimek I, Krajewski W, Moll JJ. Use of recombinant activated factor VII for controlling refractory postoperative bleeding in children undergoing cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2011;25(6):987-94. [PubMed ID: 21835642]. https://doi.org/10.1053/j.jvca.2011.05.012.
-
13.
Hedner U, Kristensen H, Berntorp E, Ljung R, Petrini P, Tengborn L. Pharmacokinetics of rFVIIa in children. Haemophilia. 1998;4(3):355.