1. Background
Chronic renal failure is a global public health problem (1) and a progressive and irreversible destructive disorder, the primary treatments for which are dialysis and kidney transplantation (2). Dialysis affects patients' lives, especially their social and mental health, in different ways, and they are exposed to various psychiatric complications. Depression is one of the most common psychological disorders in hemodialysis patients (1). Depression in dialysis patients is posed by many factors, including physical and emotional stress, medication side effects, functional limitations, dietary restrictions, and poor economic status (1, 3).
The prevalence of depression in hemodialysis patients is much higher than its prevalence in the general population (4). According to many studies, the prevalence of depression in dialysis patients is 3 - 4 times higher than that in the general population and 2 - 3 times higher than that in those with other chronic diseases (5). The overall prevalence of depression in hemodialysis patients is reported to be 5 - 70%, which varies in different regions (6). In Iran, the prevalence rate of depression in hemodialysis patients is reported to be 50 - 91% (1, 7-9). Evidence suggests that depression in hemodialysis patients is associated with a severe decline in quality of life and increased mortality (2, 4). Due to the nature of the disease and its routine treatment, hemodialysis patients usually are not concerned with controlling their depression (1).
The quality of dialysis is an important and effective factor in reducing these problems. If hemodialysis is not sufficient, the level of toxins in the blood and other life-threatening factors are not well-controlled, and patients' disability and mortality rates would increase (10, 11). Uremia is associated with inflammation and the release of inflammatory cytokines, which are detected to be upregulated in depression (12). Dialysis adequacy is one of the factors whose relationship with depression has recently been examined. Some other studies have also indicated that dialysis adequacy affects the survival of hemodialysis patients (13, 14). Urea reduction rate (URR) and Kt /V are the most common dialysis adequacy measurement indices. Various studies have suggested that 1.2 Kt/v and URR > 65% can improve the prognosis of hemodialysis patients (15). However, some studies have shown that dialysis adequacy can be inversely associated with the prevalence of depression (15, 16). However, there is no sufficient and robust evidence indicating the relationship between dialysis adequacy and psychological problems, especially depression (14), and the existing data are contradictory (12, 15).
It is of paramount importance to reach accurate knowledge about the prevalence of depression in these patients to prevent such problems, improve quality of life, and offer appropriate treatment. On the other hand, conflicting results have been achieved regarding the relationship between depression and dialysis adequacy (15). Accordingly, this study was to investigate the prevalence and severity of depression in hemodialysis patients in Ahvaz and its relationship with dialysis adequacy.
2. Methods
The present research was an analytical cross-sectional study performed on dialysis patients in Ahvaz hospitals in 2019. The study was conducted after obtaining permission from the Research Council and the Ethics Committee of the Ahwaz University of Medical Sciences (Code: IR.AJUMS.REC.1398.783). In the present study, all hemodialysis patients referred to the dialysis ward of Ahvaz teaching hospitals (namely Imam Khomeini, Golestan, and Razi Hospitals) in 2019 were considered as the research sample. Since the census method was used in this study, there was no need to determine the sample size. Patients who had been on dialysis for at least six months were included in the study after obtaining their written informed consent. In this study, all the provisions of the ethics statement in Helsinki research and the principles of patient information confidentiality were considered. Patients were ensured that their information was completely confidential and would only be used for research purposes.
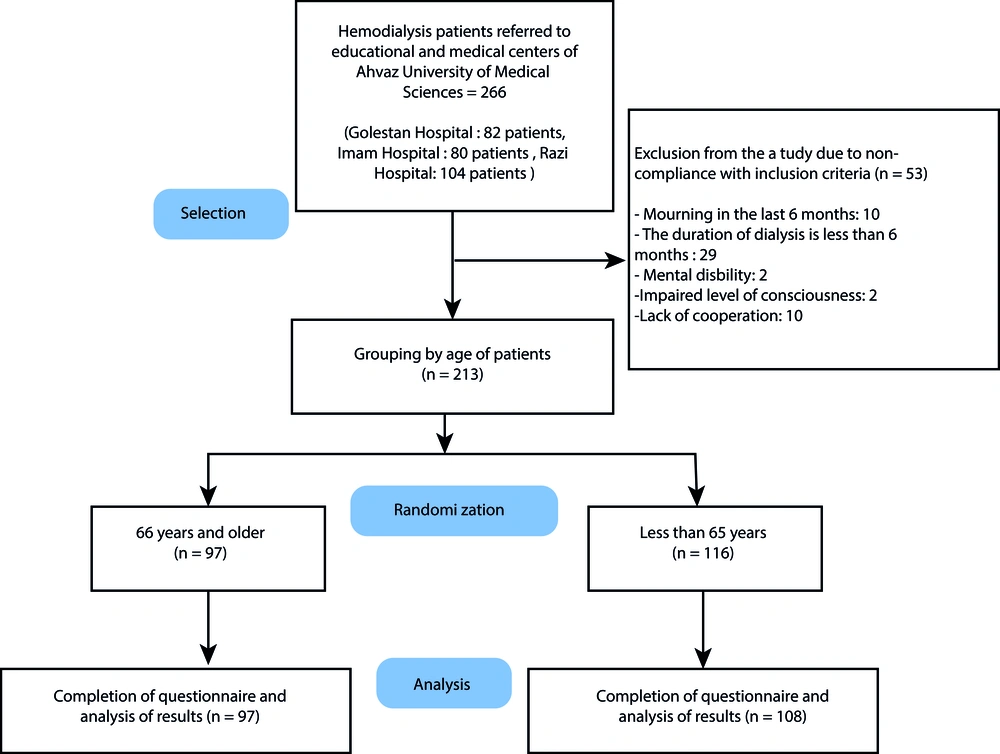
Exclusion criteria were a history of depression and a suicide attempt before hemodialysis treatment, the presence of grief over the last six months, mental disability, and impaired level of consciousness. Accordingly, 213 persons were preliminary included in the study. Further, the non-completed and distorted questionnaires were excluded from the study, resulting in 205 persons remained as the final sample size (Figure 1).
2.1. Data Collection
First, demographic and laboratory characteristics were collected by using a self-report questionnaire and reviewing patient medical reports. Patients' demographic information included age, gender, marital status, monthly income, level of education, place of treatment, occupation, health insurance, duration of illness, and history of dialysis in close family members. The Beck questionnaire was used to measure depression in hemodialysis patients aged below 65 years, and the Geriatric Depression Scale (GDS) was used for individuals aged above 65 years. These questionnaires were submitted to the patients during the dialysis procedures and were completed by the patients or their companions.
2.2. Dialysis Adequacy
The dialysis adequacy in all studied hospitals considering Kt/V index based on the percentage of urea reduction (URR) was calculated by the following formula:
Where, K was dialysis filtration; T represented dialysis duration, and V was the volume of urea distribution. The following formula calculated the URR:
To report dialysis adequacy, URR ≥ 65% and KT / V in patients undergoing dialysis three times a week was considered to be 1.2; hence, dialysis adequacy ≥ 1.2 is desirable (17).
2.3. Depression Assessment
The Beck Depression Inventory 2 (BDI-II) was used to determine depression and its severity in patients aged below 65 years. The scale contains 21 items, with four statements for each of the items, to incorporate the increasing severity of a particular symptom that might have been experienced over the past two weeks. Four items addressing weight loss, body image change, somatic preoccupation, and difficulty with work were removed from BDI-II, and four new items dealing with agitation, a feeling of worthlessness, difficulty with concentration, and energy loss, were added to this scale. Moreover, two other items dealing with sleep and appetite were re-worded. Accordingly, the respondent could determine if there has been a rise or a decrease in the intensity of symptom expressions.
Each item was scored based on a Likert scale ranging from 0 to 3. To obtain the total score of the questionnaire, the scores of all questions were calculated and added up as such the minimum, and maximum scores were 0 to 63, respectively. To grade the severity of the participants’ depression, the following cut-off points were set: 0 - 13 (no depression), 14 - 19 (mild depression), 20 - 28 (moderate depression), and 29 - 63 (severe depression).
Typically, patients with clinical depression were in the age range of 12 - 40 years. This questionnaire has a 97% correlation with Hamilton Rating Scale for Depression (HRSD), and its one-week reliability is 93%. The internal consistency of the scale is 91%. Studies on the validity of BDI-II in different countries, including Iran, have confirmed its acceptable validity (18). Moreover, the factor analysis and validity assessment results indicate its appropriateness in evaluating the results of different clinical trials. In the present study, Cronbach's alpha coefficient of BDI was 0.873, suggesting its acceptable internal consistency and high validity. When three researchers discussed and confirmed the questionnaire’s content, it was re-translated into Persian by a proficient bilingual person holding a master's degree in clinical psychology. BDI-II can be administered to adolescents, adults, and the elderly and completed during 5 - 10 minutes. Researchers reviewed and revised the final version of K-BDI-II.
The short form of the Geriatric Depression Scale (GDS) was used to evaluate depression in the patients aged above 65 years. This questionnaire was developed by Basavij (1993) to assess depression in the elderly and validated as a suitable test in various clinical and non-clinical settings. It has internal and external stability in the clinical diagnosis of depression, and its reliability is 85%, as determined by the test-retest method. In 1986, the 15-item short-form had 90% specificity and 80% sensitivity in diagnosing patients with depression. This test consists of 30 Yes/NO questions scored as follows: 0 - 9 (no depression), 10 - 19 (moderate depression), and 20 - 30 (severe depression). In the 15-item scale, the scores range from 0 to15; hence, the range of the above scores is divided by 2. The scores range from 0 to 15, with 0 - 4 indicating no depression; 5 - 9 presenting moderate depression, and 10-15 representing severe depression. Many studies have investigated the psychometrics of the GDS questionnaire in Iran (19), documenting the acceptable validity and reliability of this questionnaire. In the present study, Cronbach's alpha coefficient of this questionnaire was 0.808, indicating the internal consistency and high validity of its items.
2.4. Theoretical Definition of Depression
2.4.1. Definition of Depression Regarding DSM-V Criteria
According to the DSM-V criteria, depression encompasses indefinite depressive disorders (NOS) or disruptive mood disorders, major depressive disorders (MDD), and persistent depressive disorders (PDD). It also refers to suffering from more than five symptoms during the same two-week period, which are different from the previous functions. In this regard, depressed mood and/or loss of interest/pleasure must be present, and it excludes symptoms obviously attributable to another medical condition. Diagnostic criteria for the major depressive disorder are as follows:
(1) Depressed mood: Most of the day, nearly every day; either subjective (e.g., feels sad, empty, hopeless) or observed by others (e.g., appears tearful); May exhibited as irritable mood in children and adolescents.
(2) Loss of interest/pleasure: Markedly diminished interest/pleasure in all (or almost all) activities most of the day, nearly every day; either subjective or observed by others.
(3) Weight loss or gain: Significant weight loss (with no diet) or gain (change of >5% body weight per month), or a decrease or an increase in appetite nearly every day; may be a failure to gain expected weight in children.
(4) Insomnia or hypersomnia: Nearly every day, psychomotor agitation or retardation, nearly every day, and observable by others (not merely subjectively restless or slow).
(5) Fatigue: Loss of energy, nearly every day.
(6) Feeling worthless or excessive/inappropriate guilt: Nearly every day, guilt may be delusional, not merely self-reproached or guilt about being sick.
(7) Decreased concentration: Nearly every day, may be indecisiveness, either subjective or observed by others.
(8) Thoughts of death/suicide: Recurrent thoughts of death (not just fear of dying), recurrent suicidal ideation without a specific plan, suicide attempt, or a specific plan for suicide.
2.4.2. Definition of Depression Based on Beck Criteria
Beck (1967) defined depression as follows: Depression refers to a set of behaviors whose specific elements are slowness in movement and speech. Other symptoms include crying, sadness, lack of active responses, lack of interest, worthlessness, insomnia, and loss of appetite. According to Beck, clinical depression can be defined as a pathological disorder encompassing changes in five major behavioral areas. These changes may include all or any of the following symptoms:
(1) Self-dislike: Individuals may believe in worthlessness, experience self-hate, hold a negative attitude towards life, and experience persistent feelings of sadness and emptiness.
(2) Agitation: These feelings may make individuals have angry outbursts, feel irritable, and experience frustration in different situations.
(3) Loss of interest in hobbies you once enjoyed: Depression has a way of sapping the pleasure out of everything that brings individuals enjoyment. If an individual is withdrawing from normal activities that he/she used to look forward to, this may be a sign of depression.
(4) Changes in sleep: Disturbances in sleep patterns, such as insomnia or sleeping too much, are common symptoms of depression.
(5) Changes in appetite: Weight and appetite fluctuates appear in individuals with depression and can vary depending on their personality traits. Individuals may unintentionally have weight loss or gain or notice changes in eating habits.
(6) Loss of energy: Slowed thinking, speaking, or body movements can occur with depression and result in problems in concentrating, making decisions, and remembering.
(7) Unexplained physical problems: An individual may experience back pain or headaches with no other known causes. Moreover, depression and stress can have a negative impact on the immune system.
(8) Thoughts of death or self-harm: Depression is sometimes connected to the feelings of self-harm and suicide.
2.5. Statistical Analysis
SPSS software version 22 was used to analyze the collected data. Kolmogorov-Smirnov evaluated the normality of the data, and the homogeneity of variances was evaluated by Leven’s test. Give the non-normal distribution of the data, nonparametric tests were used to analyze the results in this study. Mann-Whitney U and Kruskal-Wallis nonparametric tests were used to compare the means of variables between the research groups, and Spearman and Chi-square (or Fisher's exact) correlation tests were used to determine the relationship between quantitative and qualitative variables, respectively. The significance level in the tests was set to be 0.05.
3. Results
3.1. Participants’ Demographic Characteristics
The present study included 205 hemodialysis patients with the mean age of 58.91 ± 14.89 years (range of 20 - 89 years). One hundred eight patients were aged below 65 years (47.78 ± 10.75), and 97 individuals were aged above 65 years (71.30 ± 6.91). Moreover, the mean duration of the disease was 4.46 ± 3.86 years (Table 1).
| Variables | Frequency (%) |
|---|---|
| Age | |
| 65 ≤ | 97 (47.3) |
| 65 > | 108 (52.7) |
| Gender | |
| Male | 141 (68.8) |
| Female | 64 (31.2) |
| Level of education | |
| Illiterate | 61 (29.8) |
| High school | 75 (36.6) |
| Diploma | 45 (22) |
| Associate degree | 8 (3.9) |
| Bachelor’s degree | 13 (6.3) |
| Master’s degree and higher | 3 (1.5) |
| Job | |
| Housewife / unemployed | 107 (50.7) |
| Employed | 47 (22.9) |
| Retired | 54 (26.3) |
| Marital status | |
| Single | 27 (13.2) |
| Married | 178 (86.8) |
| Family history | |
| Yes | 17 (8.3) |
| No | 188 (91.7) |
| Insurance | |
| Yes | 168 (82) |
| No | 37 (18) |
| Level of income | |
| Low | 105 (52.2) |
| Moderate | 84 (41) |
| High | 16 (7.8) |
| Dialysis adequacy | |
| 1.2 ≤ | 107 (52.2) |
| 1.2 > | 98 (47.8) |
3.2. Dialysis Adequacy
The mean Kt / V in the studied patients was 1.28 ± 0.60, and Kt / V was not significantly different between the two age groups aged below and above 65 years (1.28 ± 0.67 vs. 1.29 ± 0.51; P = 0.571). The mean URR in the studied patients was 46.98 ± 33.19. The mean URR was not significantly different between the age group under 65 years (47.03 ± 34.51 vs. 46.89 ± 31.48; P-value = 0.834). The dialysis adequacy was statistically different in patients of different hospitals (P = 0.020). The dialysis adequacy rates at Imam and Razi Hospitals were 1.30 ± 0.62 and 1.39 ± 0.61, respectively. Golestan hospital had the lowest Kt/V (1.12 ± 0.53), and the optimal Kt/V (1.2 ≤) was observed only in 35.6% of the patients in this medical center.
3.3. Prevalence of Depression in Dialysis Patients
The mean score of the BDI-II for individuals aged below 65 years was 1.6.86 ± 10.77, and the mean GDS in those aged above 65 years was 6.49 ± 3.67. According to the results, 81 hemodialysis patients (39.5%) had no depression, and 124 patients (60.5%) had depression. Different depression levels showed no significant difference between the two groups regarding the prevalence of depression (P = 0.070) (Table 2)
| Depression Level | Frequency (%) |
|---|---|
| BDI-depression inpatient patients aged below 65 years | |
| No depression | 49 (45.4) |
| Mild depression | 19 (17.6) |
| Moderate depression | 22 (20.4) |
| Severe depression | 18 (16.7) |
| GDS-depression in patients aged above 65 years | |
| No depression | 32 (33) |
| Moderate depression | 45 (46.4) |
| Severe depression | 20 (20.6) |
3.4. Relationship Between Depression with Different Variables in Hemodialysis Patients
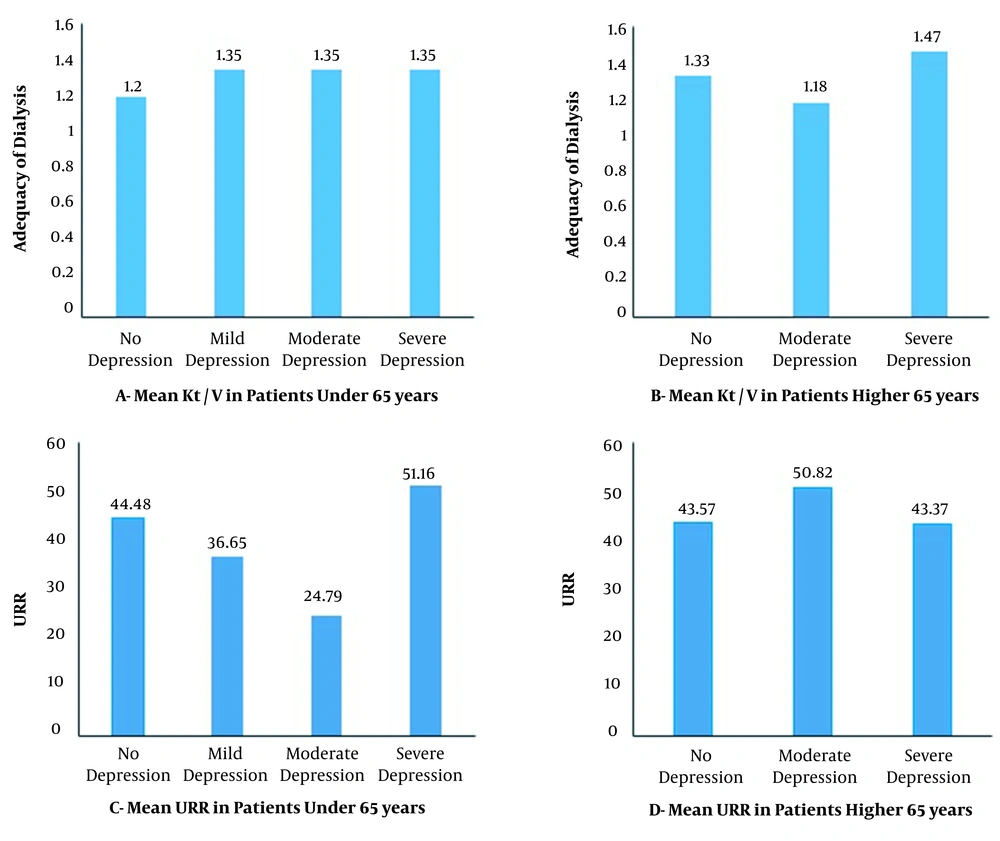
In neither of the age groups, there was no significant relationship between age, duration of illness, marital status of patients, level of education, occupation, and family history of dialysis in close family members, and health insurance with depression levels (P < 0.05). In the age group of > 65 years, there was a significant relationship between patients 'gender and depression levels (P = 0.030). In the age group < 65 years, there was a significant relationship between patients' income level and depression levels (P = 0.012) (Tables 3 and 4). There was also no significant relationship between dialysis adequacy and depression levels (Table 4 & Figure 2). Figure 2 shows the results of the mean URR and Kt/V regarding different depression levels in patients aged below and above 65 years.
| Variables | Aged Below 65 Years | P-Value | Aged Above 65 Years | P-Value |
|---|---|---|---|---|
| Gender | 0.925 | 0.030 | ||
| Male | 16.73 ±10.44 | 6.04 ± 3.78 | ||
| Female | 17.11 ± 11.55 | 7.60 ± 3.17 | ||
| Marital status | 0.608 | 0.279 | ||
| Single | 15.04 ± 7.95 | 8.00 ± 4.00 | ||
| Married | 17.29 ± 11.34 | 6.39 ± 3.65 | ||
| Level of education | 0.203 | 0.289 | ||
| Illiterate | 20.81 ± 10.89 | 6.91 ± 3.62 | ||
| High school | 16.34 ±10.06 | 6.54 ± 3.38 | ||
| Diploma | 16.69 ± 11.20 | 5.33 ± 4.18 | ||
| Associate degree | 20.60 ± 14.31 | 6.66 ± 1.15 | ||
| Bachelor | 11.50 ± 9.62 | 8.33 ± 1.15 | ||
| Master and higher | - | 2.22 ± 3.21 | ||
| Job | 0.620 | 0.072 | ||
| Housewife / unemployed | 17.01 ± 10.98 | 7.02 ± 3.42 | ||
| Employed | 18.00 ± 10.72 | 4.78 ± 3.58 | ||
| Retired | 15.00 ±10.54 | 6.75 ± 3.87 | ||
| Family history | 0.585 | 0.446 | ||
| Yes | 15.30 ± 11.42 | 7.28 ± 3.63 | ||
| No | 17.02 ± 10.75 | 68/3 ± 43/6 | ||
| Insurance | 0.321 | 0.950 | ||
| Yes | 16.37 ± 10.59 | 6.43 ± 3.63 | ||
| No | 19.47 ± 11.67 | 6.70 ± 3.90 | ||
| Level of income | 0.012 | 0.098 | ||
| Low | 19.21 ± 10.61 | 7.34 ± 3.80 | ||
| Moderate | 13.50 ± 9.99 | 5.55 ± 3.22 | ||
| High | 20.42 ± 12.44 | 5.77 ± 3.99 |
| Variables | P-Value | Spearman Correlation Coefficient |
|---|---|---|
| Age (y) | ||
| < 65 | 0.283 | -0.104 |
| > 65 | 0.573 | 0.058 |
| Duration of disease (y) | ||
| < 65 | 0.994 | 0.001 |
| > 65 | 0.964 | 0.005 |
| Kt/V (y) | ||
| < 65 | 0.944 | 0.007 |
| > 65 | 0.763 | 0.031 |
| URR (y) | ||
| < 65 | 0.684 | 0.040 |
| > 65 | 0.980 | 0.003 |
4. Discussion
Depression is the fourth leading cause of disability worldwide and one of the most common psychological disorders among hemodialysis patients. Various factors, including physical and emotional stress, medication side effects, functional limitations, dietary restrictions, and poor economic status, arouse depression in dialysis patients (1).
Many studies have investigated the prevalence of depression in hemodialysis patients in Iran; however, they have reported contradictory findings. Accurate knowledge and understanding of the depression prevalence in these patients are of great importance in preventing this disorder, improving the quality of their lives, and offering treatment. Moreover, further studies in different regions of the country at different periods would also contribute to understanding the epidemiology of this disease and adopting management strategies to prevent and reduce the incidence of depression in hemodialysis patients. Accordingly, the present study was to determine the prevalence of depression in hemodialysis patients in Ahvaz and its relationship with dialysis adequacy (2, 3, 5).
This study showed the high prevalence of depression in hemodialysis patients, indicating that 60.5% of the hemodialysis patients in Ahvaz were suffering from depression. In this regard, 54.6% of the hemodialysis patients aged below 65 years and 67.0% of the individuals aged above 65 years had different depression levels. Although the prevalence of depression levels was higher in patients aged above 65 years, no significant difference was observed between the two age groups.
Studies in Iran have reported the prevalence of depression in hemodialysis patients to be between 50 - 91%. Sanaei and Afshar surveyed 120 hemodialysis patients using the Beck questionnaire and reported the depression prevalence of 70% in these patients (9). In a meta-analysis of 1812 patients by Mirzaei and Akbari, the total prevalence of depression in the Iranian hemodialysis patients was 63% (8). In another meta-analysis by Ravaghi et al. on 2822 patients, the depression prevalence was 62% (1). These findings are consistent with those of the present study.
Recently, a meta-analysis by Abdi et al. was conducted on 2941 hemodialysis patients in Iran, and the prevalence of depression was 56.8% (7). This decrease in the prevalence of depression could be attributed to the increased public awareness and increased access to health care facilities. In total, these studies suggest that more than half of hemodialysis patients in Iran are suffering from depression, highlighting the need to identify depression in these patients to provide timely management and interventions.
The findings suggest that the prevalence of depression in dialysis patients is higher in Iran than in developed countries. According to many studies, there is an inverse relationship between a country’s level of progress and development and the prevalence rate of depression in hemodialysis patients as such, with an increase in the development rate of countries, the prevalence of depression decreases. Better health status, advanced medical equipment, better and more comfortable services for patients, psychological services, and emotional, social, and family support in developed countries can make differences between developing and developed countries regarding the prevalence of depression (1). The prevalence of depression in hemodialysis patients is lower in Iran compared to neighboring countries such as Saudi Arabia (68.6%), Iraq (80%), and Pakistan (75%) (20-22).
However, the prevalence of depression in hemodialysis patients is higher in Iran compared to Hungary (33%), China (29%), and Malaysia (36.6%) (23-25). Semaan et al. in a study in the United States detected 83 hemodialysis patients with ESRD and reported that 40.8% of these patients had depression (26). In Brazil, the prevalence of depression in hemodialysis patients (69 patients) was 42.7% (27). In another study in Brazil by de Brito et al. on 205 dialysis patients, the prevalence of depression using the Beck questionnaire was 41.7% (28). Other studies have reported the lower prevalence of depression (23.3 - 60.5%) (12).
Some studies have reported a very high prevalence of depression in hemodialysis patients. Nelson et al.'s study in India showed that 83.5% of patients had different depression levels (27.3% mild depression, 40.5% moderate depression, and 15.7% severe depression) (29). Khan et al., in their study in Malaysia on 213 hemodialysis patients, showed that 84.9% of patients had depression (4). Excessive drug use, economic burden imposed on patients and their families, and changes in social relationships may have caused the higher prevalence of depression among these patients.
The inconsistency of findings may be associated with differences in screening tools and depressive assessment methods, sample size, location, and community. Numerous intervening factors, including sociocultural differences, lifestyle, support services, care, and medical staff may also affect the findings. Moreover, another critical issue for hemodialysis patients is to receive social support. Patients with high social support experience less depression (30).
According to some studies in Iran, considering the cultural changes and social status for each age group, little social support is provided for patients with chronic diseases from the perspective of hemodialysis patients (31). This can be a significant risk factor for depression. In general, all studies have revealed that depressive symptoms should be considered in the evaluation and treatment of hemodialysis patients. Accordingly, this measure is now mandatory in all dialysis centers in the United States (29).
Some studies have suggested that dialysis adequacy can be inversely associated with the prevalence of depression. For example, Hung et al. (16) surveyed 146 hemodialysis patients and reported a weak relationship between depression and Kt / V. In another study, Klaric et al. (15) also showed a relationship between depression and dialysis adequacy in patients treated with peritoneal dialysis; however, no association was noticed in hemodialysis patients. According to these researchers, these findings could be due to the uneven distribution of Kt / V in hemodialysis patients. Montinaro et al. (12) also showed no difference between depressed and non-depressed patients regarding the mean Kt / V.
In our study, there was no significant relationship between Kt / V and URR with depression in none of the age groups. Small sample size and dialysis adequacy measurement only once may justify the lack of a significant relationship between depression and dialysis adequacy in the present study. Najafi et al. also reported that the mean dialysis adequacy was not significantly different between individuals with and without depression (14).
On the other hand, in a study by Al Awwa and Jallad (32), a negative relationship was observed between depressive symptoms and dialysis adequacy (Kt/V). In another study, there was an inverse relationship between dialysis adequacy and depression as such, those who had adequate dialysis adequacy had no or slight depression (33).
Different studies have adopted different methods to assess dialysis adequacy, and this can be a barrier to comparing different findings. On the other hand, there is no sufficient and robust evidence indicating a link between dialysis adequacy and psychological problems. Moreover, depression is a chronic condition, and given that dialysis adequacy indices can vary over time, they may not indicate dialysis adequacy at the same time as patients' mood (14). Furthermore, since many variables affect the outcomes, and given that the sample size has a large impact on this issue, contradictory findings are expected.
Many factors (namely blood flows, dialysis time, and access recirculation) affect dialysis adequacy. On the other hand, increased body mass, sodium removal, poor dialysis flow rate, blood tubing, and needle gauge size may also contribute to inadequate hemodialysis.
Anemia is prevalent in hemodialysis patients. On the other hand, anemia increased the risk of psychological problems, including depression. Analyzing the results may not be accurate without considering factors such as anemia.
Since mental disorders are especially prevalent in older individuals in public hospitals, they can also have a negative impact on the outcomes. Accordingly, to improve the survival and quality of life in these patients, detecting the most affected individuals and outcomes requires further studies (32).
This study was conducted in a multicenter manner, and the patients were selected from hospitals in different regions of Ahvaz, and this promotes the generalizability of the results. The present study was the first study evaluating and comparing the prevalence rates of depression in hemodialysis patients in two age groups aged below and above 65 years. Another strength of the present study was the use of a single method to calculate dialysis adequacy for all patients.
On the other hand, the present study also faced some limitations, including the use of a self-report questionnaire to assess depression. Moreover, no clinical and psychological interviews were performed to diagnose depression. In this study, dialysis adequacy was measured only once, and the mean values at different times were not calculated. The heterogeneity in the adaptation processes of the patients undergoing hemodialysis were another limitation of the present study. Individual differences between patients and psychological or emotional problems when answering the questionnaire may decrease the accuracy of response. Another limitation of the study is the small sample size because the low sample size can affect the study results and make the interpretation of the results be made with caution.
It should be noted that this study only included hemodialysis patients living in Ahvaz; hence, the generalization of the findings to the other regions of the country should be made with caution. Accordingly, better results can be achieved by conducting further studies with larger sample sizes and in different regions.
4.1. Conclusions
The analysis of the relationship between depression and dialysis adequacy is challenging since there are many factors affecting depression and dialysis adequacy as well as intervening, mediating, and moderating variables. In this regard, all patients undergoing routine hemodialysis should be screened for depression signs and symptoms. Prompt diagnosis and effective treatment of depression are essential for time management and interventions and improve quality of life, prognosis, and patient survival.