Abstract
Background:
Smoking is known to be the main risk factor in systemic inflammation and insulin resistance.Objectives:
In the present study, the researchers aimed at comparing serum interleukin 6 as a pro-inflammatory cytokine between smokers and non-smokers and determining acute and recovery response of IL-6 to a single bout aerobic exercise in 2 groups.Methods:
For this purpose, 15 adult male non-trained smokers (n = 15) and non-smokers (n = 15) matched for age (35.5 ± 5.8 years) and body mass index (BMI) (31.30 ± 4.5 kg/m2) were selected to participate in this study. Subjects of the 2 groups completed aerobic exercise consisting of 40 minutes of running on a flat surface with no slope at 70%maximal heart rate. Venous blood samples were collected before, immediately, 60 minutes and 24 hours after the exercise test of the 2 groups. Data were analyzed by one-way analysis of variance with repeated measures in each group.Results:
Based on statistical data, serum Interleukin (IL)-6 level in smoker subjects was significantly higher than non-smokers at baseline. A significant increase was observed in serum IL-6, immediately after exercise test in smokers yet remained unchanged in nonsmokers. Interleukin-6 concentration decreased 1 and 24 hours after exercise compared with baseline in the 2 groups.Conclusions:
Acute increase and recovery decrease in serum IL-6 response to exercise suggests an anti-inflammatory effect of moderate aerobic exercise on smokers.Keywords
1. Background
Several factors are effective in improvement of respiratory function and respiratory dysfunction. These factors are involved in oxygen transfer pattern and carbon dioxide excretion from body tissues. In the meantime, tobacco use and particularly smoking are important factors involved in dysfunction in oxygen transport function via the respiratory system. Scientific resources confirm the central role of cigarette smoking in chronic diseases (1). Similar to some other anomalies or diseases, such as beta thalassemia major (2), it seems that cigarette smoking affects quality of life (QOL) negatively. Many scientific studies reported the incidence of chronic diseases, such as cardiovascular diseases, type 2 diabetes, atherosclerosis, hypertension, osteoporosis and respiratory diseases (e.g. chronic obstructive pulmonary and asthma), to be due to deleterious effects of cigarette smoking on systemic inflammation (3). These studies showed that long-term cigarette smoking is associated with increase in inflammatory markers, such as C-reactive protein (CRP), Interleukin-6-type cytokines (IL-6), and resistin that collectively cause incidence and increase severity of the above-mentioned diseases (1, 4). Various studies have shown that serum levels of IL-6 in elderly smokers are higher than their non-smoker peers (5). In a recent study, the IL-6 level in smokers was 47% higher than non-smokers (6).
On the other hand, it has been found out that training programs have different effects on levels of these inflammatory markers and other cardiovascular risk factors (7, 8). Nevertheless, there are numerous reports on smoking-induced changes in the inflammatory and immune system (9). However, the mechanism of short-term physiological response to different interventions in smokers has not been fully understood. Nevertheless, exercise is known as an effective regulator of the immune system (10). On the other hand, there are numerous reports on the role of exercise as an anti-inflammatory therapeutic agent (11-13). Accordingly, exercise-induced inflammatory responses can be useful in the long-term. It has been suggested that acute increases in Interleukin (IL)-6 following prolonged exercise are associated with the induction of a transient anti-inflammatory state (e.g. increases in IL-10) that is partly responsible for the health benefits of regular exercise (14). Although the mechanisms responsible for these changes are not fully understood, some recent studies reported improvement in inflammatory cytokines or lipid profile markers in response to physical activity in smoking-associated chronic diseases, such as diabetes, asthma, and cardiovascular disease (7, 8, 15-17). For example, in a study by Lopes et al. (2016), despite decrease in leptin, insulin resistance and CRP, serum IL-6 remained without change by 12 weeks of combined training without caloric restriction (18). In contrast, Gondim et al. (2015) reported a significant decrease in serum IL-6 and resistin after 6 or 12 weeks of aerobic training (19). On the other hand, response of these inflammatory or anti-inflammatory cytokines or lipid profile to either a long-term or one-session exercise was investigated in some of these studies. Limited studies have aimed at determining acute and recovery response of these variables to one-session exercise training among smokers.
2. Objectives
Therefore, the present study aimed at determining acute and recovery response (after one hour and after 24 hours) of IL-6 to 40-minute running exercise in smokers compared with non-smoker peers.
3. Methods
As mentioned above, in addition to comparing serum IL-6 between current smoker and non-smoker males, acute and recovery response of serum IL-6 to an aerobic exercise was investigated in smokers and compared with non-smokers in this semi-experimental study. Subjects were non-trained healthy adult smokers (n = 15) and non-smokers (n = 15) matched for age, gender (men) and BMI, and were selected by accessible sampling in this study.
Ethical consideration: The study protocol was approved by the ethics committee of Islamic Azad University, Iran (ethic code: 73/454791) and informed consent was obtained from all subjects before recruitment in the project.
Inclusion or Exclusion criteria: To understand the medical history, subjects were asked to complete questionnaires on general health, smoking, alcohol consumption, and present medications. The inclusion criterion for the smoker group was smoking history of at least 10 cigarettes a day for 3 years (current smokers). None of the subjects used drugs or therapies for obesity, and none had a past history of disease or injury that would prevent daily exercise. Patients with known history of respiratory infections, neuromuscular disease, cardiopulmonary disease, and type II diabetes or other chronic diseases were excluded. Participants were non-athletes and non-alcoholics. All subjects had not participated in regular exercise/diet programs for the preceding 6 months.
Anthropometrical measures: all smokers and non-smokers underwent anthropometric measurements. Body mass index (BMI) was calculated by dividing body mass (kg) by height in meters squared (m2). The weight and height of the participants were measured by the same person when the participant had thin clothes on and was wearing no shoes. Abdominal obesity and hip circumference were determined in the standing position at the end of normal expiration.
Exercise protocol, blood analysis and assay: All participants refrained from any severe physical activity 48 hours before blood sampling. Aerobic exercise lasted 40 minutes at 70% of maximal heart rate, involving running on a flat surface with no slope. Target heart rate was controlled with polar telemetry of each subject. Blood samples were collected before, immediately after exercise, and after 60 minutes and 24 hours of recovery in the 2 groups. Blood samples were dispensed to EDTA-coated tubes and centrifuged for 10 minutes in order to separate the serum. Serum was used to measure IL-6 by the Enzyme-linked Immunosorbent Assay (ELISA) method for quantitative detection of human IL-6 (Austria). The Intra-assay coefficient of variation and sensitivity of the method were 3.4% and 5.2 pg/mL, respectively, for IL-6.
Data collection: data were analyzed by the computer using the statistical package for social sciences (SPSS) for Windows, version 11.5. Normal distribution of data was analyzed by the Kolmogorov-Smirnov normality test. Two-way repeated measure Analysis of Variance (ANOVA) was used to effectively assess changes in serum IL-6 by the exercise test in the 2 groups. A p value of less than 0.05 was considered as statistically significant.
4. Results
The physical characteristics of the study participants in the smoker and non-smoker groups are described in Table 1. Means and standard deviations were calculated for all variables. Significant differences were not found in body weight, body mass index, and other anthropometrical markers between the 2 groups at baseline (P > 0.05).
| Age, y | Height, cm | Weight, kg | AC, cm | BMI, kg/m2 | BF, % | |
|---|---|---|---|---|---|---|
| Smoker | 35 ± 5 | 176 ± 6.8 | 97 ± 9.8 | 101 ± 8.8 | 31.31 ± 4.3 | 29.3 ± 3.5 |
| Non-smoker | 36 ± 5.8 | 177 ± 5.3 | 36 ± 5.8 | 100 ± 9.7 | 31.28 ± 3.3 | 29.8 ± 4.1 |
No significant differences were found between smokers and non-smokers with regards to serum IL-6 at baseline (P = 0.362). Based on the repeated measures method, the results suggest that although serum IL-6 remained unchanged immediately after the exercise test in non-smokers, a significant increase was observed in smokers compared with baseline (P = 0.021).
Regarding the difference in acute response of serum IL-6 to exercise test between the 2 groups, the change pattern was similar at 60 minutes and 24 hours of recovery between the 2 groups. Therefore, a significant decrease was found in serum IL-6 at 60 minutes and 24 hours post-exercise compared with pre-exercise (baseline) in the 2 groups (see Table 2 and Figure 1).
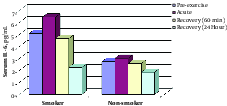
5. Discussion
Comparison of IL-6 baseline levels in smokers and non-smokers in this study revealed higher levels of this inflammatory mediator in smokers compared to non-smokers. Accordingly, IL-6 levels in male smokers are twice the levels in non-smokers. Some studies reported that levels of inflammatory cytokines, such as TNF-α and CRP, are higher in smokers than non-smokers (20, 21). Acute and response measuring of IL-6 as important pro-inflammatory cytokines to exercise test in cigarette smokers was the strength of the present study. In the present study, serum IL-6 was increased immediately after the exercise test and returned to baseline after 1 to 24 hours of recovery. Consistent with these findings, Ulven et al. (2015) reported an acute increase in IL-6 and other cytokines’ (such as TNF-α and IL-1B) response to 1-hour cycling at 70% of VO2max in healthy males (22). However, Benini et al. (2015) reported no acute and 30-minute recovery changes in IL-6 to a moderate intensity resistance exercise in healthy females (23).
Long-term tobacco use is associated with a wide range of inflammatory processes and changes in immune system function. These are key factors in pathogenesis of cardiovascular disease, diabetes and chronic obstructive pulmonary disease (24). Up-regulation of inflammatory cytokines in smokers leads to systemic inflammation, which is an independent factor involved in chronic diseases (25).
Cigarette smoking increases inflammation and decreases immune system function, which consequently increases the prevalence and severity of chronic diseases (26). In addition, exercise reduces the severity of these diseases in an effective manner (27, 28). According to the above-mentioned issues, it is important to determine response of effective components in inflammation and immune system to exercise among smokers. Several recent studies have shown that active people and those participating in exercise activities are less inclined to smoke cigarettes compared to sedentary individuals (29). Furthermore, a positive relationship was reported between physical activity, physical fitness, and lung capacity (30, 31). Accordingly, some studies confirmed beneficial effects of short-term and long-term exercise on inflammatory profile in different population groups (7, 8). Although some studies rejected the effect of physical activity on pulmonary growth and function (32), many scientific studies have shown that pulmonary function is lower in adult athlete smokers than non-smoker athletes (33).
Most studies regarding long-term training programs reported acute beneficial effects of physical activity on the above variables. On the other hand, no response or confounding responses were reported in studies examining acute response of the studied cytokines to one-session exercise (34, 35). Nevertheless, findings of this study showed that IL-6 levels significantly increase compared to baseline levels immediately after a relatively long-term aerobic exercise in male smokers. However, IL-6 levels significantly decreased compared to baseline levels after one-hour of recovery. Accordingly, a downward trend was observed in IL-6 levels 24 hours after exercise. Although various IL-6 levels were measured in both smokers and non-smokers at every phase of the project, the findings showed identical patterns of acute and recovery changes in this inflammatory mediator in response to aerobic exercise in both groups. In accordance with these observations, in a study by Tegan et al. (2017), no significant difference was observed for IL-6 after 40 minutes of cycling at 50% peak aerobic exercise between smokers and non-smokers (36).
In summary, a relatively long-term moderate-intensity aerobic exercise session has similar effects on IL-6 levels immediately to 24 hours after recovery in both smokers and non-smokers. In the present study, IL-6 levels significantly increased immediately after exercise. In other words, a moderate-intensity aerobic exercise led to an acute increase in IL-6 levels in male smokers. In this context, scientific studies have shown that acute increase in IL-6 levels after long-term exercise is associated with unstable anti-inflammatory states. This is due to the beneficial effects of physical activity (14). Accordingly, increase in IL-6 levels in response to acute exercise is associated with increase in IL-10 as an inflammatory cytokine (14). In another study, an intense aerobic training session in form of repeated cycling exercise at 2-minute intervals along with 1-minute break time led to acute increase in cytokines 60 minutes after exercise in obese and thin children (37). On the other hand, Kastelin et al. (2016) showed no significant difference in IL-6 response to one-session exercise between smokers and non-smokers (36). At the end, it should be noted that the small sample size for smoker and non-smokers groups was a limitation of the present study. In addition, lack of measuring other inflammatory or anti-inflammatory cytokines, such as adiponectin and TNF-α, in response to the exercise test is another limitation of the current study.
4.1. Conclusions
In summary, findings of this study showed that although relatively long-term moderate-intensity aerobic exercise leads to acute increase in IL-6 levels in adult male smokers and nonsmokers, a significant decrease was observed in anti-inflammatory effects of exercise from one-hour to 24 hours after exercise. A general conclusion is needed for measuring other indicative markers of inflammatory profile. Therefore, future studies should focus on regarding markers of inflammatory profile.
Acknowledgements
References
-
1.
Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34(3):J258-65. doi: 10.1016/j.jaut.2009.12.003. [PubMed: 20042314].
-
2.
Baraz S, Miladinia M, Mosavinouri E. A comparison of quality of life between adolescences with beta thalassemia major and their healthy peers. Int J Pediatr. 2016;4(1):1195-204.
-
3.
Karadag F, Kirdar S, Karul AB, Ceylan E. The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med. 2008;19(2):104-8. doi: 10.1016/j.ejim.2007.04.026. [PubMed: 18249305].
-
4.
Orosz Z, Csiszar A, Labinskyy N, Smith K, Kaminski PM, Ferdinandy P, et al. Cigarette smoke-induced proinflammatory alterations in the endothelial phenotype: role of NAD(P)H oxidase activation. Am J Physiol Heart Circ Physiol. 2007;292(1):H130-9. doi: 10.1152/ajpheart.00599.2006. [PubMed: 17213480].
-
5.
Helmersson J, Larsson A, Vessby B, Basu S. Active smoking and a history of smoking are associated with enhanced prostaglandin F2α, interleukin-6 and F2-isoprostane formation in elderly men. Atherosclerosis. 2005;181(1):201-7. doi: 10.1016/j.atherosclerosis.2004.11.026.
-
6.
Woodward M, Rumley A, Tunstall-Pedoe H, Lowe GD. Associations of blood rheology and interleukin-6 with cardiovascular risk factors and prevalent cardiovascular disease. Br J Haematol. 1999;104(2):246-57. [PubMed: 10050704].
-
7.
de Salles BF, Simão R, Fleck SJ, Dias I, Kraemer-Aguiar LG, Bouskela E. Effects of Resistance Training on Cytokines. Int J Sports Med. 2010;31(7):441-50. doi: 10.1055/s-0030-1251994.
-
8.
Marcell TJ, McAuley KA, Traustadottir T, Reaven PD. Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism. 2005;54(4):533-41. doi: 10.1016/j.metabol.2004.11.008.
-
9.
Frohlich M, Sund M, Lowel H, Imhof A, Hoffmeister A, Koenig W. Independent association of various smoking characteristics with markers of systemic inflammation in men. Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95). Eur Heart J. 2003;24(14):1365-72. [PubMed: 12871694].
-
10.
Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80(3):1055-81. doi: 10.1152/physrev.2000.80.3.1055. [PubMed: 10893431].
-
11.
Gleeson M. Immune function in sport and exercise. J Appl Physiol (1985). 2007;103(2):693-9. doi: 10.1152/japplphysiol.00008.2007. [PubMed: 17303714].
-
12.
Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607-15. doi: 10.1038/nri3041. [PubMed: 21818123].
-
13.
Behboudi L, Eizadi M. The Modifying Impact of Long-Term Endurance Training on Inflammatory Cytokine IL-1B Level and VO2max in Premenopausal Women with Abdominal Obesity. Jundishapur J Chron Dis Care. 2017;(In Press).
-
14.
Cullen T, Thomas AW, Webb R, Hughes MG. Interleukin-6 and associated cytokine responses to an acute bout of high-intensity interval exercise: the effect of exercise intensity and volume. Appl Physiol Nutr Metab. 2016;41(8):803-8. doi: 10.1139/apnm-2015-0640. [PubMed: 27377137].
-
15.
Rabinovich RA, Figueras M, Ardite E, Carbo N, Troosters T, Filella X, et al. Increased tumour necrosis factor-alpha plasma levels during moderate-intensity exercise in COPD patients. Eur Respir J. 2003;21(5):789-94. [PubMed: 12765422].
-
16.
Monazamnezhad A, Habibi A, shakeriyan S, Majdinasab N, Ghalvand A. The Effects of Aerobic Exercise on Lipid Profile and Body Composition in Women With Multiple Sclerosis. Jundishapur J Chron Dis Care. 2015;4(1). doi: 10.5812/jjcdc.26619.
-
17.
Heydarpour P, Fayazi S, Haghighi S. Resistance Training Effect on Lipid Profile and Body Fat Percentage of Premenopausal Women. Jundishapur J Chron Dis Care. 2015;4(2). doi: 10.5812/jjcdc.28339.
-
18.
Lopes WA, Leite N, da Silva LR, Brunelli DT, Gaspari AF, Radominski RB, et al. Effects of 12 weeks of combined training without caloric restriction on inflammatory markers in overweight girls. J Sports Sci. 2016;34(20):1902-12. doi: 10.1080/02640414.2016.1142107. [PubMed: 26852885].
-
19.
Gondim OS, de Camargo VT, Gutierrez FA, Martins PF, Passos ME, Momesso CM, et al. Benefits of Regular Exercise on Inflammatory and Cardiovascular Risk Markers in Normal Weight, Overweight and Obese Adults. PLoS One. 2015;10(10). e0140596. doi: 10.1371/journal.pone.0140596. [PubMed: 26474157].
-
20.
Tibuakuu M, Kamimura D, Kianoush S, DeFilippis AP, Al Rifai M, Reynolds LM, et al. The association between cigarette smoking and inflammation: The Genetic Epidemiology Network of Arteriopathy (GENOA) study. PLoS One. 2017;12(9). e0184914. doi: 10.1371/journal.pone.0184914. [PubMed: 28922371].
-
21.
Zhao J, Li X, Xie F, Yang Z, Pan X, Zhu M, et al. Immunomodulatory effects of cigarette smoke condensate in mouse macrophage cell line. Int J Immunopathol Pharmacol. 2017;30(3):315-21. doi: 10.1177/0394632017716370.
-
22.
Ulven SM, Foss SS, Skjolsvik AM, Stadheim HK, Myhrstad MC, Raael E, et al. An acute bout of exercise modulate the inflammatory response in peripheral blood mononuclear cells in healthy young men. Arch Physiol Biochem. 2015;121(2):41-9. doi: 10.3109/13813455.2014.1003566. [PubMed: 25720858].
-
23.
Benini R, Prado Nunes PR, Orsatti CL, Barcelos LC, Orsatti FL. Effects of acute total body resistance exercise on hormonal and cytokines changes in men and women. J Sports Med Phys Fitness. 2015;55(4):337-44. [PubMed: 25853878].
-
24.
Domagala-Kulawik J. Effects of cigarette smoke on the lung and systemic immunity. J Physiol Pharmacol. 2008;59 Suppl 6:19-34. [PubMed: 19218630].
-
25.
Tracy RP, Psaty BM, Macy E, Bovill EG, Cushman M, Cornell ES, et al. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler Thromb Vasc Biol. 1997;17(10):2167-76. [PubMed: 9351386].
-
26.
Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131(5):1557-66. doi: 10.1378/chest.06-2179. [PubMed: 17494805].
-
27.
Thatcher TH, McHugh NA, Egan RW, Chapman RW, Hey JA, Turner CK, et al. Role of CXCR2 in cigarette smoke-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2005;289(2):L322-8. doi: 10.1152/ajplung.00039.2005. [PubMed: 15833762].
-
28.
Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154-62. doi: 10.1152/japplphysiol.00164.2004.
-
29.
Aarnio M, Kujala UM, Kaprio J. Associations of health-related behaviors, school type and health status to physical activity patterns in 16 year old boys and girls. Scand J Soc Med. 1997;25(3):156-67. [PubMed: 9360271].
-
30.
Mehrotra PK, Varma N, Tiwari S, Kumar P. Pulmonary functions in Indian sportsmen playing different sports. Indian J Physiol Pharmacol. 1998;42(3):412-6. [PubMed: 9741658].
-
31.
Twisk JW, Staal BJ, Brinkman MN, Kemper HC, van Mechelen W. Tracking of lung function parameters and the longitudinal relationship with lifestyle. Eur Respir J. 1998;12(3):627-34. [PubMed: 9762791].
-
32.
Lakhera SC, Kain TC, Bandopadhyay P. Changes in lung function during adolescence in athletes and non-athletes. J Sports Med Phys Fitness. 1994;34(3):258-62. [PubMed: 7830389].
-
33.
De AK, Tripathi MM. Smoking and lung functions in sportsmen. Br J Sports Med. 1988;22(2):61-3. [PubMed: 3167504].
-
34.
Zaccaria M, Ermolao A, Roi GS, Englaro P, Tegon G, Varnier M. Leptin reduction after endurance races differing in duration and energy expenditure. Eur J Appl Physiol. 2002;87(2):108-11. doi: 10.1007/s00421-002-0606-4. [PubMed: 12070619].
-
35.
Hammett CJ, Prapavessis H, Baldi JC, Varo N, Schoenbeck U, Ameratunga R, et al. Effects of exercise training on 5 inflammatory markers associated with cardiovascular risk. Am Heart J. 2006;151(2):367 e7-367 e16. doi: 10.1016/j.ahj.2005.08.009. [PubMed: 16442901].
-
36.
Kastelein TE, Donges CE, Mendham AE, Duffield R. The Acute Exercise-Induced Inflammatory Response: A Comparison of Young-Adult Smokers and Nonsmokers. Res Q Exerc Sport. 2017;88(1):15-25. doi: 10.1080/02701367.2016.1239012. [PubMed: 27805476].
-
37.
Duran AT, Judelson DA, Clark SJ, Gertz E, Haqq AM, Tsang KW, et al. Cytokine Responses to Acute Intermittent Aerobic Exercise in Children with Prader-Willi Syndrome and Nonsyndromic Obesity. Pediatr Exerc Sci. 2015;27(4):525-34. doi: 10.1123/pes.2015-0050.
